Abstract
Parallel increases in strength and rate of force development (RFD) are well-known outcomes from the initial phase of resistance training. However, it is unknown whether neural adaptations with training contribute to improvements of both factors. The aim of this study was to examine whether changes in H-reflex amplitude with resistance training can explain the gain in strength or rather be associated with RFD. Twelve subjects carried out 3 weeks of isometric maximal plantarflexion training, whereas 12 subjects functioned as contr ols. H-reflexes were elicited in the soleus muscle during rest and sub-maximal contractions at 20 and 60% of maximal voluntary contraction (MVC). In addition, surface electromyography (sEMG) was recorded from the soleus, gastrocnemius and tibialis anterior muscles during MVC. The resistance training provided increases in maximal force of 18%, RFD of 28% and H-reflex amplitude during voluntary contractions of 17 and 15% while no changes occurred in the control group. In contrast, the maximal M-wave, the maximal H-reflex to maximal M-wave ratio during rest and sEMG during MVC did not change with training. There was a positive correlation between percentage changes in H-reflex amplitude and RFD with training (r = 0.59), while significant association between percentage changes in H-reflex amplitude and maximal force was not found. These findings indicate the occurrence of changed motoneuron excitability or presynaptic inhibition during the initial phase of resistance training. This is the first study to document that increased RFD with resistance training is associated with changes in reflex excitability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Resistance training has frequently been reported to lead to improvements of both maximal force and rate of force development (RFD) (Aagaard et al. 2002a; Holtermann et al. 2005; Hakkinen et al. 1985; Rich and Cafarelli 2000; Van Cutsem et al. 1998; Holtermann et al. 2007). More specific, a few weeks of resistance training can cause increases in isometric single-joint tasks of up to 30% in maximal force (Carolan and Cafarelli 1992; Moritani and deVries 1979; Pucci et al. 2006; Rabita et al. 2000; Rich and Cafarelli 2000; Thorstensson et al. 1976; Yue and Cole 1992) and RFD (Behm and Sale 1993; Suetta et al. 2004). Even 5 days of maximal isometric dorsiflexion training of the ankle joint can cause increases in maximal force and RFD of more than 15% (Holtermann et al. 2005, 2007).
A scientific focus has been to elucidate the initial increases in maximal force with resistance training. However, the increases in RFD with resistance training have attained less attention. The initial strength gain with resistance training is frequently explained to be due to neural adaptations (Enoka 1997; Gabriel et al. 2006; Griffin and Cafarelli 2005; Moritani 1993; Sale et al. 1992). The “neural training hypothesis” implies that the initial strength gain is primarily mediated by adaptations within the central nervous system resulting in recruitment of additional motor units and/or increased discharge rate (Aagaard et al. 2002b; Carroll et al. 2002; Moritani and de Vries 1979; Sale et al. 1983; Enoka 1988). More specific, the modulation of motor unit recruitment and discharge rate with training must involve an enhanced net excitatory post-synaptic input to the motoneuron pool or increased motoneuron excitability.
The main reported neural adaptations with resistance training are increases in doublet discharges (Van Cutsem et al. 1998), discharge rate (Patten et al. 2001), surface electromyographical (sEMG) amplitude (Moritani and deVries 1979; Hakkinen et al. 1985; Hakkinen and Komi 1986; Yue and Cole 1992) and decreased recruitment threshold (Keen et al. 1994). However, some researchers have suggested that these neural adaptations might have a larger impact on RFD than maximal force generation (Binder-Macleod and Barrish 1992; Holtermann et al. 2007; Miller et al. 1981; Moritani 2002).
An argument for a profound impact on RFD from enhanced excitatory post-synaptic input or motoneuron excitability with training is that high-threshold motor units could be recruited earlier in a MVC increasing the RFD, whereas recruitment of additional motor units ends before maximal tension (Kukulka and Clamann 1981). Therefore, increased excitatory input or excitability is more likely to enhance RFD than maximal force by recruitment of motor units. Another argument is that the upper limit of discharge rate contributing to force enhancement is quite low compared to its contribution to RFD (Buller and Lewis 1965). As a result, increases in maximal discharge rate from normally 50 Hz in young adults to even higher levels with resistance training (Patten et al. 2001) is likely to have larger impact on RFD than maximal force.
Changes in net synaptic excitatory input and excitability of the motoneuron pool can be detected with H-reflex recordings (Misiaszek 2003). The studies applying H-reflex recordings to examine changes in net synaptic excitatory input and excitability with training have either used animals (Almeida-Silveira et al. 1996), applied cross-sectional designs (Casabona et al. 1990; Earles et al. 2002; Koceja and Kamen 1992; Nielsen et al. 1993) or did not considered whether changes in H-reflex were related to gain in maximal force or RFD (Aagaard et al. 2002b; Lagerquist et al. 2006).
The aim of this study was to examine whether changes in H-reflex amplitude are related to the increase in maximal force and/or RFD in the initial phase of resistance training. H-reflex recordings were obtained in physically active young adults divided in a control and an experimental group performing 3 weeks of resistance training.
Methods
Subjects
Twenty-five male subjects volunteered to participate in the study. In one subject, the H-reflex could not be obtained. Therefore 24 subjects completed the experiment. All subjects were familiar with resistance training in general and regularly participated in different exercises and sports 2–5 days per week. The subjects were divided into an experimental group of 12 subjects (21.7 ± 2.2) and a control group of 12 subjects (21.9 ± 2.0) matched for their maximal force generation at the familiarization session prior to the pre-test. All subjects gave written informed consent to participate in the study. The Regional Committee for medical science and ethics, University hospital, NTNU, Trondheim, Norway approved the study.
Protocol and force recordings
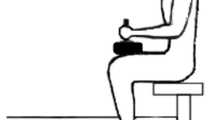
The subject was positioned in a chair (rigid steel frame with seat and back support in wood) with both legs in a custom-built device with the ankle fixed at ∼10° plantarflexion from a neutral (standing) position and the knee joints fixed at a position of ∼80° of flexion from a complete extended knee position. The device consisted of one pedal for each leg, in which the center of rotation could be aligned with the center of rotation of the talocrural joint in the ankle. The foot was strapped to the pedal with non-elastic straps pulled tightly across the foot, just below the metatarsal-phalangeal joints. To avoid movement of the center of rotation of the talocrural joint during contraction, the lower limb was stabilized with a knee attachment with non-elastic straps attached to the rotation point of the device. In addition, the hip and back of the subject was strapped to the chair with broad non-elastic bands. By this means, the center of rotation of the talocrural joint could not be moved, insuring that the force cell only recorded plantarflexion force. The force-cell was attached with 90° alignment to the pedal recording plantar and dorsiflexion force of the ankle joint only. For accuracy purposes, the force-cell was attached through rotating joints at the fixations, minimization of oblique forces on the force-cell with small deformations of the equipment under loading. The force was recorded with a force cell (SM-2000N, Interface MFG, Scottsdale, AZ, USA). The sensitivity of the force cell was 2 mV N−1. The signal was recorded using a Bagnoli EMG system (Delsys, Boston, MA, USA) and sampled at 1,000 Hz. The signal was analog to digital converted with DAQCard-6036E (16bit multifunction, National Instruments), and low-pass filtered at 20 Hz with an eight order zero phase lag Butterworth filter. The force was multiplied with the moment arm (0.23 m) to attain torque.
To get familiar with the experimental task, the subject participated in three familiarization sessions prior to the pre-training test. In the first session, the dominant leg was determined with a behavioral inventory of foot preference (Chapman et al. 1987). Three subjects were left footed and the remaining subjects were right footed. In addition, the subjects carried out three isometric maximal plantarflexion contractions of the dominant leg followed by 8 minute lasting submaximal plantarflexions. In the subsequent two familiarization sessions, the subjects performed sub-maximal contractions below 40% MVC only. Visual feedback of the target and generated force was given on a monitor straight in front of the subject.
During the pre- and post-training tests, the subject was given the instruction to generate three MVC (maximal force as fast as possible) of plantarflexion using the dominant leg. The subjects rested 1 min between each contraction. The subject performed 3 MVC with dorsiflexion of the dominant ankle. In addition, percutaneous stimulation for H-reflex recordings during rest and sub-maximal contractions at 20 and 60% MVC were elicited (see percutanous stimulation).
Training
The resistance training consisted of 3 weeks with three sessions per week. Because neural adaptations are task specific (Wolpaw and Tennissen 2001), evaluation of neural adaptations to training should be performed under the same conditions as training (Zehr 2002). Therefore, the training was carried out in the same device and under similar conditions as testing. In each session, the subject performed five series, each consisting of ten trials. In each trial, the subject performed an isometric plantarflexion MVC of the dominant leg lasting 4 s. A 10 s rest period was allowed between trials, and 3 min rest period between series. The instruction to the subjects was to generate maximal force in each trial. The subject received instant feedback of the generated plantarflexion force from a monitor.
Electromyographical recordings
sEMG recordings were made during the MVC and during the H-reflex investigations.
During MVC, sEMG recordings were performed to examine changes in muscle activation and net excitatory input with resistance training. Bipolar sEMG recordings were made using two 1 ×10 mm electrodes with 10 mm inter-electrode distance (Delsys, DE-2.1 Single Differential Detection EMG sensors) and collected with a sampling frequency of 1,000 Hz and amplified with a gain of 1,000 (Bagnoli-16 EMG system, Delsys, Boston, MA, USA, bandwidth 20–450 Hz). Electrode pairs were placed on the soleus (3–5 cm below the distal end of the gastrocnemius muscle), gastrocnemius (middle of medial and lateral muscle bulks), and the tibialis anterior (8 cm below the caput fibulae in the middle of the muscle bulk). The bipolar electrodes were aligned with the assumed muscle fibre direction.
For the investigation of the H-reflex, monopolar electrode configurations were used, since H-reflex amplitudes are larger and variation is smaller during voluntary effort with monopolar compared to bipolar electrode configuration (Gerilovsky et al. 1989; Gerilovsky et al. 1985). Therefore, a single monopolar surface electrode (Pre-gelled, silver-silverchloride recording surface, recording area 9 ×6 mm, Medtronic) was placed 6–7 cm below the distal end of the gastrocnemius muscle (Gerilovsky et al. 1989; Mineva et al. 1993). The reference electrode was placed on the Achilles tendon, with the ground electrode on the proximal part of the gastrocnemius. The monopolar sEMG signals from the soleus muscle were amplified with a gain of 1,000 (Viking Select, Nicolet Biomedical INC, Madison, Winsconsin, USA, bandwidth 2 Hz–5 kHz).
Modulation in muscle activation of both agonistic and antagonistic muscles can influence the changes in H-reflex amplitude (Nielsen and Kagamihara 1993; Schieppati 1987). To evaluate whether the percutanous stimulation occurred under the same muscle activation levels before and after the training period, sEMG was also recording with bipolar electrodes placed on the soleus, gastrocnemius and tibialis anterior. For this purpose, the same equipment was used as for the MVC trials. To ensure similar conditions for recordings pre and post tests, all electrode positions were precisely marked on the skin with a waterproof pen.
Percutaneous stimulation
The H-reflex was evoked in the soleus muscle by percutaneous stimulation with a bipolar configuration (6 mm diameter of the electrodes and 30 mm inter-electrode distance) oriented longitudinally over the posterior tibial nerve in the popliteal fossa with the cathode proximal to the anode (Scaglioni et al. 2002). The optimum site of stimulation was first located by hand-held stimulation and thereafter the probe was attached to this site with rigid straps and taping to ensure a constant pressure and position. Constant-current square pulses of 1 ms duration were delivered by the stimulator (Viking Select, Nicolet Biomedical INC, Madison, WI, USA).
First, a maximal M-wave was generated during rest by gradually increasing the stimuli intensity to no further enhancement in M-wave could be visually observed, with a subsequent significant increase in stimulation intensity (between 5 and 10 mA) to ensure acquiring of the M max. Subsequently, an M-wave to H-reflex calibration curve was created during rest by varying the stimulation intensity from the visual observation of an H-wave to M-max with the use of at least 32 intensity levels. Since post-activation depression of the H-reflex amplitude during rest can occur with inter-pulse intervals shorter than 10 s (Gollhofer et al. 1998), the stimulation rate was 0.1 Hz.
Subsequently, the H-reflex amplitude was recorded during sub-maximal voluntary contractions of 20% MVC and 60% MVC, respectively. Because the soleus H-reflex amplitude does not increase during percutanous stimulation at higher voluntary contraction levels than 60% MVC, H-reflex recording during higher force levels is an inaccurate measure of motoneuron pool excitability (Butler et al. 1993). The subject received a target force of 20 or 60% MVC on a screen, and was instructed to generate a constant force of similar magnitude. Since presynaptic influences on the H-reflex amplitude are not changed after keeping a constant force level for 400 ms (Meunier and Pierrot-Deseilligny 1989), the first stimulus was given at least 1 s after the force target was reached. It is critical in an experiment with repeated testing of H-reflex that the synaptic input received by the alpha motoneurons is constant (Zehr 2002). Therefore, the stimulus intensity was adjusted to provide an M-wave of a constant percentage of maximal M-wave (Capaday 1997) so that the actual M-wave amplitude (displayed on a computer screen) was within the range of 20 ± 2.5% of M max (Aagaard et al. 2002b; Simonsen and Dyhre-Poulsen 1999). Since a reliable H-reflex measure requires an average of a few responses (Hopkins et al. 2000), 12 stimuli were applied to obtain enough registrations within 20 ± 2.5% of M max attained during rest in each sub-maximal contraction. Because H-reflex amplitude is not influenced by post-contraction depression under voluntarily active contractions (Burke et al. 1989), a stimulation rate of 0.2 Hz was used at the contraction of 20% MVC. To avoid fatigue and achieve a correct force level during the percutaneous stimulation at 60% MVC, the stimulations were elicited in non-recurrent mode with irregular and reduced inter stimulation intervals.
Because the task-dependent change in H-reflex depends on motor activity (Capaday 1997), the muscle activation must be similar and controlled during stimulation at pre and post tests. Since the sEMG – torque relationship is quite linear during isometric contractions (Milner-Brown and Stein 1975), to obtain similar motor activity during the sub-maximal voluntary contractions with percutaneous stimulation at pre and post test, we performed the post test at two force levels: (1) a force normalized to the maximal force from the post test (MVCpost) and (2) a force normalized to maximal force from pre test (MVCpre). The recorded muscle EMG activity of the involved muscles in plantar flexion of the ankle during stimulation was controlled off-line. Due to the influence on H-reflex amplitude of position changes in body (Goulart et al. 2000), hip (Knikou and Rymer 2002), head (Hayes and Sullivan 1976) and contra-lateral leg (Robinson et al. 1979), the subject was instructed to stay in a standardized and constant body and head position with folded arms and relaxed contra-lateral leg during stimulation.
Analyses
All data were analyzed with Matlab software (The MathWorks, Natick, MA, USA) version 7.0.
Force data
The maximal force was calculated as the average value of a period of 0.25 s around the recorded peak force. The trial with highest force during MVC was considered as maximal force (F max).
The rate of force development was calculated from the force-time slope normalized relative to F max for each trial respectively, defined as RFD (%F max s−1) (Aagaard et al. 2002a; Holtermann et al. 2007). This normalization of the force-time slope before calculation of RFD is necessary to attain a measure of the change in RFD independent of the change in F max with resistance training. The RFD was calculated from contraction onset to 300 ms. The contraction onset was set to the time epoch when the force exceeded the baseline by 2.5% of the difference between baseline and F max. The time epoch of 300 ms was chosen because longer time is needed to attained F max (Thorstensson et al. 1976), and a few subjects attained F max shortly after this time epoch. The sub-maximal force during stimulation was normalized relative to F max of the same session.
Bipolar sEMG data
The digital bipolar sEMG signals of all channels were band-passed filtered at 20–300 Hz. Root mean square (RMS) was calculated using a sliding time window of 0.25 s duration from the start to the end of all active voluntary contractions. The time epoch with peak RMS during MVC of plantarflexion for the triceps surae muscles and dorsiflexion for the tibialis anterior muscle was considered as maximal RMS, and used for normalization of the RMS from the sub-maximal contractions during the percutaneous stimulation. The mean RMS from the total recording time of the sub-maximal contractions was calculated from each muscle.
Data from percutaneous stimulation
The peak-to-peak amplitude from the first positive phase (lowest value) to the first negative phase (highest value) of the M-wave and H-reflex was calculated from all percutanous stimulations. The M-wave amplitude attained from the maximal stimulation during rest was considered as the maximal M-wave response (M max). The peak H-reflex amplitude from the recruitment curve during rest was considered as maximal H-reflex response (H max). While the H max contains information of the maximal number of recruited motor units, the M max gives information of the absolute number of motor units, therefore the H max to M max ratio (H max/M max) provides information of the motoneurone reflex excitability. The M-wave amplitude for the 12 responses was calculated during voluntary sub-maximal muscle contractions at 20 and 60% MVC. Only H-reflexes with preceding M-waves within the range of 20 ± 2.5% M max were included in the further analyzes. The mean M-waves and H-reflexes from the 12 percutaneous stimulations from each contraction were calculated from the included data.
Statistics
The statistical analyses were carried out to test changes induced by training in several variables. Their main outcome variables were: F max, RFD, M max, H max/M max, H-reflex at 20% MVC, H-reflex at 60% MVC. In addition, difference in H-reflex amplitude of the post test recorded during sub-maximal force levels normalized to MVCpost and MVCpre, maximal RMS during MVC and RMS during the sub-maximal contractions from soleus, medial gastrocnemius, lateral gastrocnemius and tibialis anterior was analyzed. General linear models with repeated measurements (group × time) were applied to test interactions between groups and training. Student’s t test for paired samples with Bonferroni correction was applied to test changes from pre to post test for each group. Pearson’s correlation coefficient (r) was used to test the association between percentage change in H-reflex amplitude at 20 and 60% MVC and F max, and RFD from pre to post testing of the subjects of the experimental group. The explained variance was calculated from the second power of the correlation coefficient (r 2). In addition, test-retest reliability of F max, RFD and the bipolar EMG amplitudes from the main muscles influencing plantarflexion torque during MVC, M max and H max/M max recorded during rest, and H-reflex amplitude during stimulation at 20 and 60% MVC was estimated with intra-class correlation coefficient (R). The estimations were based on the subjects of the control group. Statistical analyses were performed on peak values. Average values of the H-reflex amplitudes with stimulation intensities between 20 ± 2.5% M max during the sub-maximal voluntary contractions were used. Within-subject coefficient of variation (CV) of the H-reflex amplitudes with stimulation intensities between 20 ± 2.5% M max from the pre test of all subjects were carried out. CV was defined as SD/mean × 100.
Results
Reliability, as estimated with intra-class correlation coefficients from the pre and post tests of the control group was R = 0.93 for F max, R = 0.88 for RFD, R > 0.76 for the bipolar EMG amplitudes from the main muscles influencing plantarflexion torque during MVC, R = 0.86 for M max, R = 0.81 for H max/M max, R = 0.80 for the H-reflex amplitude recorded during 20% MVC and R = 0.75 when recorded during 60% MVC. The within-subject CV of the H-reflex amplitudes with stimulation intensities between 20 ± 2.5% from the pre test of all subjects was 6.4 (2.9)% for H-reflex amplitudes recorded during 20% MVC and 8.7 (4)% during 60% MVC.
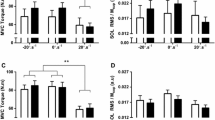
The nine sessions of isometric maximal resistance training provided a significant strength gain of 17.6% in the experimental group (P < 0.01), with a non-significant change in the control group of 4.7% (P = 0.16) (Fig. 1a), in accordance with previous studies (Aagaard et al. 2002a; Behm and Sale 1993; Hakkinen et al. 1985; Hakkinen and Komi 1986).
Changes from pre to post tests of the experimental and control group in a F max and b RFD. The RFD was calculated from contraction onset to 300 ms of the force–time slope normalized relative to F max for each trial, respectively. The change in strength from pre to post test was significantly different between the groups, with a significant increased strength of the experimental group only. General linear models with repeated measurements (group × time) were applied to test interactions between groups and training. Student’s t test for paired samples was applied to test changes from pre to post test for each group. Differences between pre and post tests: *P < 0.05 and **P < 0.01
The change in strength from pre to post test between the groups was significantly different (P < 0.01). In accordance with the increased strength with training, the RFD recorded from contraction onset to 300 ms significantly increased 28.4% (P < 0.01) in the experimental group with a no modification in the control group (P = 0.56) (Fig. 1b). The change in RFD from pre to post test between the groups was significantly different (P < 0.05).
In contrast to the strength gain, the maximal RMS recorded with sEMG during the MVC did not significantly change in any of the main muscles influencing plantarflexion torque in either group (Fig. 2). Similarly, the M max and H max/M max obtained at rest was not significantly modified by resistance training (Fig. 3).
Changes in RMS amplitude recorded with sEMG bipolar electrode configuration of the main muscles involved in plantarflexion of the ankle from pre to post tests of the experimental (a) and control group (b) were all non-significant. The RMS was determined as the epoch with highest value calculated with a 0.25 s sliding time window of the entire contraction
Changes from pre to post tests of the experimental and control group were all non-significant in a M max and b H max/M max. The M max and H max/M max were recorded with the subject seated in the experimental device in a resting state with supra-maximal stimulation and M-wave to H-reflex recruitment curve of at least 32 percutaneous stimulations of the tibial nerve
H-reflex amplitude increased significantly in the experimental group during the sub-maximal voluntary contraction at force levels of 20% by 17.4% MVC post (P < 0.05) and 14.8% MVCpre (P < 0.05) (Fig. 4a). H-reflex amplitude increased with 14.7 % (P < 0.05) at force level of 60% MVCpost and 18.5% (P < 0.05) at force level of 60% MVCpre (Fig. 4b). In contrast, there was no training effect on H-reflex amplitude in the control group during the sub-maximal contraction at 20% MVCpost (P = 0.7) and 20% MVCpre (P = 0.53) (Fig. 4a), or during 60% MVCpost (P = 0.67) and 60% MVCpre (P = 0.79) (Fig. 4b).
Changes from pre to post tests of the experimental and control group in H-reflex amplitude recorded with 12 stimulations during sub-maximal contractions at a 20% MVC and b 60% MVC, respectively. The post test was performed at both a relative (percentage of MVC from post test) and absolute force level (percentage of MVC from pre test). The change in H-reflex amplitude from pre to post test was not significantly different between the groups, with a significant increased H-reflex amplitude at both sub-maximal contractions of the experimental group only. Differences between pre and post tests: *P < 0.05
There was no significant difference between the experimental and control group in change of H-reflex amplitude from pre to post tests at the sub-maximal contractions of 20% (MVCpost P = 0.23, MVCpre P = 0.23) or 60% (MVCpost P = 0.22, MVCpre P = 0.11). Force level normalization procedure (MVCpost or MVCpre) did not influence the H-reflex amplitude significantly (P > 0.05).
Figure 5 illustrates a typical example of the monopolar sEMG recording of the soleus (Fig. 5a) and bipolar sEMG recording from the main involved muscles in plantarflexion (Fig. 5b) during percutaneous stimulation during the 60 s sub-maximal contraction at 20% MVC. The muscle EMG activity was not different in the pre compared to the post tests in any of the muscles during the stimulation (P > 0.05) (Table 1). The mean M-wave amplitude, the number of responses within the range of 20 ± 2.5% M max during the sub-maximal contractions at 20 and 60% MVC, and the length of contractions with stimulation in non-recurrent mode at 60% MVC were not different between the experimental and control group (P > 0.05) (Table 2).
A significant positive correlation between the change in normalized RFD and H-reflex amplitude of the experimental group during 20% of MVC (r = 0.59, P < 0.05) was observed (Fig. 6a). Therefore in the experimental group, 35% of the variation in RFD can be accounted for by the variation in H-reflex amplitude recorded during 20% MVC from pre to post testing. In the experimental group, there were no significant correlations between changes in normalized RFD and H-reflex amplitude at 60% of MVC (r = 0.3, P = 0.35), between changes in F max and H-reflex amplitude at 20% of MVC (r = −0.28, P = 0.38) (Fig. 6b), or between changes in F max and H-reflex amplitude at 60% of MVC (r = −0.06, P = 0.85). Correlation analyses were not performed on the control group due to no significant change in H-reflex amplitude from pre to post tests.
Scatter plots of the relations between percentage change from pre to post tests of the the experimental group in a H-reflex amplitude recorded from percutaneous stimulation during 20% MVC and RFD from contraction onset to 300 ms, and b H-reflex amplitude recorded from percutaneous stimulation during 20% MVC and F max. Linear regressions are shown. r 2: squared correlation (explained fraction of the variance)
Discussion
The aim of this study was to examine changes in H-reflex amplitude to get insight into the mechanisms behind the initial increase in strength and RFD with resistance training. Resistance training caused an increased F max and RFD as well as a gain in H-reflex amplitude during voluntary contractions while no change was observed in the control group. H-reflex amplitude gain was positively associated with the increased RFD, but not with the gained Fmax. Neither the M-wave nor H-reflex amplitude recorded during rest nor sEMG recordings from muscles involved in plantarflexion during MVC changed. The implications of these results will be discussed below.
Increased H-reflex with resistance training
Increased H-reflex amplitude during voluntary sub-maximal force levels is in general in accordance with previously reported studies (Aagaard et al. 2002b; Lagerquist et al. 2006). Increased H-reflex amplitude in the initial phase of resistance training (within 3 weeks) is a novel finding. This result indicates that neural adaptations occur during the initial phase of resistance training, supporting the “neural training hypothesis” (Enoka 1988; Sale et al. 1983). Increased H-reflex amplitude following an intervention has been interpreted to be mediated by enhanced excitability of the motoneuron pool (Misiaszek 2003; Pierrot-Deseilligny and Mazevet 2000). However, the H-reflex amplitude can also be modulated by other inputs (e.g., from muscle afferents Schieppati 1987) or pre-synaptic inhibition of the Ia afferent terminals (Rudomin and Schmidt 1999). Therefore, the specific mechanisms behind the increased reflex excitability with resistance training cannot be identified.
Increased H-reflex was associated with increased RFD, but not with maximal force gain
The variation in H-reflex amplitude at 20% MVC accounted for 35% of the variation in RFD. Therefore, changes in H-reflex and RFD after resistance training do probably share common neural mechanisms. The non-significant relation between changes in F max and H-reflex with training indicates that initial strength gain on the other hand may depend more heavily on other mechanisms. Although a statistically non-significant correlation cannot prove that mechanisms differ, it indicates that different neural adaptation mechanisms mediate the initial strength gain and the RFD, respectively.
The often reported parallel increase in strength and RFD with resistance training (Aagaard et al. 2002a; Behm and Sale 1993; Hakkinen et al. 1985; Holtermann et al. 2005; Rich and Cafarelli 2000; Thorstensson et al. 1976) have generated questions regarding the relation between these two aspects of a MVC with resistance training (Andersen and Aagaard 2006; Griffin and Cafarelli 2005; Haff et al. 2005; Mirkov et al. 2004; Holtermann et al. 2007). An enhanced reflex excitability with training such as found in this study indicates an increased net excitatory synaptic input or excitability of the motoneurons. In principle, this can increase performance by recruitment of more motor units earlier in the contraction, an increased number of total recruited motor units, and an increased motor unit discharge rate. Although one could argue for a positive effect from these changes on both RFD and F max, there are several arguments why the observed positive relation between changes in reflex excitability and RFD only is not unexpected. First, recruitment of additional motor units in a MVC is unlikely to contribute to increased F max since motor unit recruitment ends before maximal tension is achieved (Kukulka and Clamann 1981) and the voluntary activation deficit during MVC is very small (Yue et al. 2000), while motor unit recruitment threshold is documented to decrease with training (Van Cutsem et al. 1998) enabling more motor units to be recruited earlier in a MVC enhancing the RFD. In addition, the discharge rate contributing to enhanced tetanic force is much lower than the rate required to obtain a maximal RFD (Buller and Lewis 1965). As a result, increases in maximal discharge rate from high (50 Hz) to very high levels (60 Hz) with resistance training (Patten et al. 2001) is likely to have larger impact on RFD than maximal force. In conclusion, based on present knowledge of motor unit recruitment and discharge rate during MVC, the observed positive relation between changes in reflex excitability and RFD with training makes it more likely that increased net excitatory synaptic input or excitability of the motoneurons with training has a larger impact on RFD than maximal force.
Percutaneous stimulation during sub-maximal voluntary contractions from 0 to 50% MVC provides a linear relationship between H-reflex amplitude and the generated force, but not above this voluntary force level (Burke et al. 1989). Consequently, H-reflex amplitude recorded during voluntary contractions above 50% MVC might be an inaccurate measure of the excitability of the soleus motoneuron pool. This could have influenced the correlation between changes in RFD and H-reflex amplitude at 60% MVC (r = 0.3, P = 0.35) causing it to not reach significance.
No change in M-max or H max/M max
Several cross-sectional studies have observed a difference in H max/M max between subjects trained for different sports (Earles et al. 2002; Maffiuletti et al. 2001; Nielsen et al. 1993) and between untrained subjects and athletes (Casabona et al. 1990) indicating a change in the maximal number of recruited motor units relative to the absolute number of motor units with training. We observed increased H-reflex amplitude during voluntary activation with training in accordance with previous reports (Aagaard et al. 2002b; Voigt et al. 1998), but we did not find M max nor H max/M max changes in the resting state after resistance training. This might be explained by different mechanisms influence H-reflex amplitude during resting and active state (Capaday and Stein 1987), and that spinal plasticity depends on the task (De Leon et al. 1998; Muir and Steeves 1995). Examination of neural adaptations with H-reflex recordings should accordingly be performed in the same experimental setting during voluntary contractions as during training.
sEMG recording during MVC of agonists
The sEMG amplitude of the agonistic muscles soleus, gastrocnemius medialis and lateralis did not change with resistance training. This finding is in agreement with several resistance training studies (Cannon and Cafarelli 1987; Holtermann et al. 2005; Keen et al. 1994; Rich and Cafarelli 2000). sEMG recordings do seemingly not support the “neural training hypothesis” (Sale et al. 1983; Enoka 1988). However, small but physiological significant changes in muscle activation might be masked by the observed moderate level of reliability in this study (R > 0.76 for the bipolar EMG amplitudes from the main muscles influencing plantarflexion torque during MVC) and the insensitivity of standard bipolar sEMG recordings in general (Farina et al. 2004). As a result, the high sensitivity of H-reflex recordings and its usefulness in intervention studies (controlled stimulation intensity at a constant percentage of maximal M-wave) (Zehr 2002) can have caused the observed increase in H-reflex amplitude and not sEMG amplitude with training.
Antagonistic activation
Increased reciprocal inhibition (Griffin and Cafarelli 2005; Enoka 1997; Gabriel et al. 2006) may cause a decreased antagonistic activation during MVC (Hakkinen et al. 1998; Carolan and Cafarelli 1992). However, the muscle EMG activity of the antagonistic tibialis anterior muscle during MVC did not decrease with training in this study. EMG activity of ankle flexors during MVC can probably not explain the increased strength with resistance training.
Methodological considerations
Non-significant difference between groups in H-reflex amplitude
Many factors influence the H-reflex amplitude. The considerable effort to standardize the experimental setting provided a quite low within-subject variability of the H-reflex amplitudes recorded during the sub-maximal voluntary contractions (CV < 8.7). The variability of the H-reflex amplitudes recorded during the voluntary contractions of 20 and 60% MVC with repeated testing was rather large (R = 0.8 and 0.75). Twelve subjects in each group was therefore insufficient to detect a small difference in H-reflex amplitude between groups.
Conclusion
The findings from this study confirm that neural adaptations occur during the initial phase of resistance training. Present information of motor unit recruitment and discharge rate during MVC supports that enhanced net excitatory synaptic input or excitability of motoneurons with training is more likely to increase RFD than to contribute to gained strength. This is the first study to document that the increased RFD with resistance training is associated to changes in H-reflex excitability. However, the findings of this study can not state that changes in H-reflex with resistance training do not contribute to the observed gain in strength.
References
Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P (2002) Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol 93:1318–1326
Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P (2002) Neural adaptation to resistance training: changes in evoked V-wave and H-reflex responses. J Appl Physiol 92:2309–2318
Almeida-Silveira MI, Perot C, Goubel F (1996) Neuromuscular adaptations in rats trained by muscle stretch-shortening. Eur J Appl Physiol Occup Physiol 72:261–266
Andersen LL, Aagaard P (2006) Influence of maximal muscle strength and intrinsic muscle contractile properties on contractile rate of force development. Eur J Appl Physiol 96:46–52
Behm DG, Sale DG (1993) Intended rather than actual movement velocity determines velocity-specific training response. J Appl Physiol 74:359–368
Binder-Macleod SA, Barrish WJ (1992) Force response of rat soleus muscle to variable-frequency train stimulation. J Neurophysiol 68:1068–1078
Buller AJ, Lewis DM (1965) The rate of tension development in isometric tetanic contractions of mammalian fast and slow skeletal muscle. J Physiol 176:337–354
Burke D, Adams RW, Skuse NF (1989) The effects of voluntary contraction on the H reflex of human limb muscles. Brain 112:417–433
Butler AJ, Yue GH, Darling WG (1993) Variations in soleus H-reflexes as a function of plantarflexion torque in man. Brain Res 632:95–104
Cannon RJ, Cafarelli E (1987) Neuromuscular adaptations to training. J Appl Physiol 63:2396–2402
Capaday C (1997) Neurophysiological methods for studies of the motor system in freely moving human subjects. J Neurosci Methods 74:201–218
Capaday C, Stein RB (1987) Difference in the amplitude of the human soleus H reflex during walking and running. J Physiol 392:513–522
Carolan B, Cafarelli E (1992) Adaptations in coactivation after isometric resistance training. J Appl Physiol 73:911–917
Casabona A, Polizzi MC, Perciavalle V (1990) Differences in H-reflex between athletes trained for explosive contractions and non-trained subjects. Eur J Appl Physiol Occup Physiol 61:26–32
Chapman JP, Chapman LJ, Allen JJ (1987) The measurement of foot preference. Neuropsychologia 25:579–584
De Leon RD, Hodgson JA, Roy RR, Edgerton VR (1998) Full weight-bearing hindlimb standing following stand training in the adult spinal cat. J Neurophysiol 80:83–91
Earles DR, Dierking JT, Robertson CT, Koceja DM (2002) Pre- and post-synaptic control of motoneuron excitability in athletes. Med Sci Sports Exerc 34:1766–1772
Enoka RM (1997) Neural adaptations with chronic physical activity. J Biomech 30:447–455
Enoka RM (1988) Muscle strength and its development. New perspectives. Sports Med 6:146–168
Farina D, Merletti R, Enoka RM (2004) The extraction of neural strategies from the surface EMG. J Appl Physiol 96:1486–1495
Gabriel DA, Kamen G, Frost G (2006) Neural adaptations to resistive exercise: mechanisms and recommendations for training practices. Sports Med 36:133–149
Gerilovsky L, Tsvetinov P, Trenkova G (1985) Is the M max/H max ratio a reliable index of the monosynaptic excitability in man? Recruitment curves for H- and M-responses using monopolar and bipolar recording techniques. Acta Physiol Pharmacol Bulgarica 11:42–49
Gerilovsky L, Tsvetinov P, Trenkova G (1989) Peripheral effects on the amplitude of monopolar and bipolar H-reflex potentials from the soleus muscle. Exp Brain Res 76:173–181
Gollhofer A, Schopp A, Rapp W, Stroinik V (1998) Changes in reflex excitability following isometric contraction in humans. Eur J Appl Physiol Occup Physiol 77:89–97
Goulart F, Valls-Sole J, Alvarez R (2000) Posture-related changes of soleus H-reflex excitability. Muscle Nerve 23:925–932
Griffin L, Cafarelli E (2005) Resistance training: cortical, spinal, and motor unit adaptations. Can J Appl Physiol 30:328–340
Haff GG, Carlock JM, Hartman MJ, Kilgore JL, Kawamori N, Jackson JR, Morris RT, Sands WA, Stone MH (2005) Force-time curve characteristics of dynamic and isometric muscle actions of elite women olympic weightlifters. J Strength Condition Res 19:741–748
Hakkinen K, Kallinen M, Izquierdo M, Jokelainen K, Lassila H, Malkia E, Kraemer WJ, Newton RU, Alen M (1998) Changes in agonist-antagonist EMG, muscle CSA, and force during strength training in middle-aged and older people. J Appl Physiol 84:1341–1349
Hakkinen K, Komi PV (1986) Training-induced changes in neuromuscular performance under voluntary and reflex conditions. Eur J Appl Physiol Occup Physiol 55:147–155
Hakkinen K, Komi PV, Alen M (1985) Effect of explosive type strength training on isometric force- and relaxation-time, electromyographic and muscle fibre characteristics of leg extensor muscles. Acta Physiol Scandinavica 125:587–600
Hayes KC, Sullivan J (1976) Tonic neck reflex influence on tendon and Hoffmann reflexes in man. Electromyogr Clin Neurophysiol 16:251–261
Holtermann A, Roeleveld K, Vereijken B, Ettema GJ (2007) The effect of rate of force development on maximal force generation: acute and training-related aspects. Eur J Appl Physiol 99:605–613
Holtermann A, Roeleveld K, Vereijken B, Ettema GJC (2005) Changes in agonist EMG activation level during MVC cannot explain early strength improvement. Eur J Appl Physiol 94:593–601
Hopkins JT, Ingersoll CD, Cordova ML, Edwards JE (2000) Intrasession and intersession reliability of the soleus H-reflex in supine and standing positions. Electromyogr Clin Neurophysiol 40:89–94
Keen DA, Yue GH, Enoka RM (1994) Training-related enhancement in the control of motor output in elderly humans. J Appl Physiol 77:2648–2658
Knikou M, Rymer W (2002) Effects of changes in hip joint angle on H-reflex excitability in humans. Exp Brain Res 143:149–159
Koceja DM, Kamen G (1992) Segmental reflex organization in endurance-trained athletes and untrained subjects. Med Sci Sports Exerc 24:235–241
Kukulka CG, Clamann HP (1981) Comparison of the recruitment and discharge properties of motor units in human brachial biceps and adductor pollicis during isometric contractions. Brain Res 219:45–55
Lagerquist O, Zehr EP, Docherty D (2006) Increased spinal reflex excitability is not associated with neural plasticity underlying the cross-education effect. J Appl Physiol 100:83–90
Maffiuletti NA, Martin A, Babault N, Pensini M, Lucas B, Schieppati M (2001) Electrical and mechanical H(max)-to-M(max) ratio in power- and endurance-trained athletes. J Appl Physiol 90:3–9
Meunier S, Pierrot-Deseilligny E (1989) Gating of the afferent volley of the monosynaptic stretch reflex during movement in man. J Physiol 419:753–763
Miller RG, Mirka A, Maxfield M (1981) Rate of tension development in isometric contractions of a human hand muscle. Exp Neurol 73:267–285
Milner-Brown HS, Stein RB (1975) The relation between the surface electromyogram and muscular force. J Physiol 246:549–569
Mineva A, Dushanova J, Gerilovsky L (1993) Similarity in shape, timing and amplitude of H- and T-reflex potentials concurrently recorded along the broad skin area over soleus muscle. Electromyogr Clin Neurophysiol 33:235–245
Mirkov DM, Nedeljkovic A, Milanovic S, Jaric S (2004) Muscle strength testing: evaluation of tests of explosive force production. Eur J Appl Physiol 91:147–154
Misiaszek JE (2003) The H-reflex as a tool in neurophysiology: its limitations and uses in understanding nervous system function. Muscle Nerve 28:144–160
Moritani T (2002) Motor unit and motoneurone excitability during explosive movement. In: Komi PV (ed) Strength and power in sport, pp 27–49
Moritani T (1993) Neuromuscular adaptations during the acquisition of muscle strength, power and motor tasks. J Biomech 26:95–107
Moritani T, deVries HA (1979) Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med 58:115–130
Muir GD, Steeves JD (1995) Phasic cutaneous input facilitates locomotor recovery after incomplete spinal injury in the chick. J Neurophysiol 74:358–368
Nielsen J, Crone C, Hultborn H (1993) H-reflexes are smaller in dancers from The Royal Danish Ballet than in well-trained athletes. Eur J Appl Physiol Occup Physiol 66:116–121
Nielsen J, Kagamihara Y (1993) The regulation of presynaptic inhibition during co-contraction of antagonistic muscles in man. J Physiol 464:575–593
Patten C, Kamen G, Rowland DM (2001) Adaptations in maximal motor unit discharge rate to strength training in young and older adults. Muscle Nerve 24:542–550
Pierrot-Deseilligny E, Mazevet D (2000) The monosynaptic reflex: a tool to investigate motor control in humans. Interest and limits. Clin Neurophysiol 30:67–80
Pucci AR, Griffin L, Cafarelli E (2006) Maximal motor unit firing rates during isometric resistance training in men. Exp Physiol 91:171–178
Rabita G, Perot C, Lensel-Corbeil G (2000) Differential effect of knee extension isometric training on the different muscles of the quadriceps femoris in humans. Eur J Appl Physiol 83:531–538
Rich C, Cafarelli E (2000) Submaximal motor unit firing rates after 8 wk of isometric resistance training. Med Sci Sports Exerc 32:190–196
Robinson KL, McIlwain JS, Hayes KC (1979) Effects of H-reflex conditioning upon the contralateral alpha motoneuron pool. Electroencephalogr Clin Neurophysiol 46:65–71
Rudomin P, Schmidt RF (1999) Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res 129:1–37
Sale DG, MacDougall JD, Upton AR, McComas AJ (1983) Effect of strength training upon motoneuron excitability in man. Med Sci Sports Exerc 15:57–62
Sale DG, Martin JE, Moroz DE (1992) Hypertrophy without increased isometric strength after weight training. Eur J Appl Physiol Occup Physiol 64:51–55
Scaglioni G, Ferri A, Minetti AE, Martin A, Van Hoecke J, Capodaglio P, Sartorio A, Narici MV (2002) Plantar flexor activation capacity and H reflex in older adults: adaptations to strength training. J Appl Physiol 92:2292–2302
Schieppati M (1987) The Hoffmann reflex: a means of assessing spinal reflex excitability and its descending control in man. Progr Neurobiol 28:345–376
Simonsen EB, Dyhre-Poulsen P (1999) Amplitude of the human soleus H reflex during walking and running. J Physiol 515:929–939
Suetta C, Aagaard P, Rosted A, Jakobsen AK, Duus B, Kjaer M, Magnusson P (2004) Training-induced changes in muscle CSA, muscle strength, EMG, and rate of force development in elderly subjects after long-term unilateral disuse. J Appl Physiol 7:1954–1961
Thorstensson A, Karlsson J, Viitasalo JH, Luhtanen P, Komi PV (1976) Effect of strength training on EMG of human skeletal muscle. Acta Physiol Scandinavica 98:232–236
Van Cutsem M, Duchateau J, Hainaut K (1998) Changes in single motor unit behaviour contribute to the increase in contraction speed after dynamic training in humans. J Physiol 513:295–305
Voigt M, Chelli F, Frigo C (1998) Changes in the excitability of soleus muscle short latency stretch reflexes during human hopping after 4 weeks of hopping training. Eur J Appl Physiol Occup Physiol 78:522–532
Wolpaw JR, Tennissen AM (2001) Activity-dependent spinal cord plasticity in health and disease. Annu Rev Neurosci 24:807–843
Yue GH, Cole KJ (1992) Strength increases from the motor program: comparison of training with maximal voluntary and imagined muscle contractions. J Neurophysiol 67:1114–1123
Yue GH, Ranganathan VK, Siemionow V, Liu JZ, Sahgal V (2000) Evidence of inability to fully activate human limb muscle. Muscle Nerve 23:376–384
Zehr PE (2002) Considerations for use of the Hoffman reflex in excercise studies. Eur J Appl Physiol 86:455–468
Acknowledgments
We would like to thank Jørgen Ingebrigtsen of Human Movement Sciences program, Norwegian University of Science and Technology, Trondheim, Norway, for his contribution with the data collection and resistance training.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Holtermann, A., Roeleveld, K., Engstrøm, M. et al. Enhanced H-reflex with resistance training is related to increased rate of force development. Eur J Appl Physiol 101, 301–312 (2007). https://doi.org/10.1007/s00421-007-0503-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-007-0503-y