Abstract
Although reductions in resting H-reflex responses and maximal firing frequency suggest that reduced efferent drive may limit muscle strength in elderly, there are currently no reports of V-wave measurements in elderly, reflecting the magnitude of efferent output to the muscle during maximal contraction. Furthermore, it is uncertain whether potential age-related neural deficiencies can be restored by resistance training. We assessed evoked reflex recordings in the triceps surae muscles during rest and maximal voluntary contraction (MVC), rate of force development (RFD), and muscle mass in seven elderly (74 ± 6 years) males before and after 8 weeks of heavy resistance training, contrasted by seven young (24 ± 4 years) male controls. At baseline, m. soleus (SOL) V/M ratio (0.124 ± 0.082 vs. 0.465 ± 0.197, p < 0.05) and H/M ratio (0.379 ± 0.044 vs. 0.486 ± 0.101 p = 0.07) were attenuated in elderly compared to young. Also, SOL H-reflex latency (33.29 ± 2.41 vs. 30.29 ± 0.67 ms, p < 0.05) was longer in elderly. The reduced neural drive was, despite similar leg muscle mass (10.7 ± 1.2 vs. 11.5 ± 1.4 kg), mirrored by lower MVC (158 ± 48 vs. 240 ± 54 Nm, p < 0.05) and RFD (294 ± 126 vs. 533 ± 123 Nm s−1, p < 0.05) in elderly. In response to training SOL V/M ratio (0.184 ± 0.092, p < 0.05) increased in the elderly, yet only to a level ∼40 % of the young. This was accompanied by increased MVC (190 ± 70 Nm, p < 0.05) and RFD (401 ± 147 Nm⋅s−1, p < 0.05) to levels of ∼80 % and ∼75 % of the young. H/M ratio remained unchanged. These findings suggest that changes in supraspinal activation play a significant role in the age-related changes in muscle strength. Furthermore, this motor system impairment can to some extent be improved by heavy resistance training.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although a decline in both endurance and muscular strength capacities may be delayed by an active lifestyle (Aagaard et al. 2010; Wang et al. 2014), increasing age is associated with impaired muscle force characteristics. On average, maximal muscle strength falls steadily from the fifth decade (Larsson et al. 1979; Hughes et al. 1995; Lindle et al. 1997; Narici and Maffulli 2010) and accelerates from the seventh decade (Larsson et al. 1979; Hughes et al. 1995; Kosek et al. 2006). This age-related decline in muscular strength is associated with a reduction in general motor function, poor balance, increased risk of falls, and fractures (Campbell et al. 1989; Hyatt et al. 1990; Sillanpaa et al. 2014) and may ultimately challenge independent living and quality of life at old age (Hyatt et al. 1990; Geirsdottir et al. 2012). In contrast, heavy resistance training (∼80 % of 1RM) is shown to effectively restore muscle strength in moderately old (60–75 years) subjects to a similar level as active young after only 3–4 months (Kosek et al. 2006). Although the typical age-related alterations in muscle strength may partly be explained by changes in muscle mass, there are indications that neural properties may also play a conspicuous role (Young et al. 1985; Klitgaard et al. 1990; Kamen et al. 1995; Jubrias et al. 1997; Roos et al. 1997; Morse et al. 2004; Kennis et al. 2014), both in the typically observed decline with age and as a part of training-induced strength adaptations. However, evidence regarding the role of the neural drive, especially involving supraspinal factors, is limited during maximal muscle contractions.
Previously, elderly are documented to have reduced maximal motor unit discharging frequency (Kamen et al. 1995; Klass et al. 2008), and interpolated twitch studies have revealed that maximal muscle activation may also be incomplete (Winegard et al. 1996; Harridge et al. 1999; Scaglioni et al. 2002; Morse et al. 2004; Mau-Moeller et al. 2013). Interestingly, this diminished maximal muscle function is accompanied by reports of impaired spinal and cortical excitability in elderly (Scaglioni et al. 2002; Kido et al. 2004; McGinley et al. 2010). Although measured at rest, the total amplitude of the H-reflex normalized to the maximal M-wave is documented to be lower in elderly subjects than in young (Sabbahi and Sedgwick 1982; deVries et al. 1985; Vandervoort and Hayes 1989; Scaglioni et al. 2002; Scaglioni et al. 2003), which may indicate changes in motoneuron excitability and/or pre/postsynaptic inhibition (Aagaard et al. 2002; Zehr 2002; Aagaard 2003). However, to our knowledge, there has not been a study that have investigated how supraspinal descending pathways are affected by healthy aging, and how this may affect the efferent drive to the muscle, or its plasticity following heavy resistance training, during strong maximal muscle contractions.
Although several methodologies have been used to estimate human motoneuron excitability, few of them are applicable to detect age-related differences during strong maximal muscle contractions (McNeil et al. 2013). Indeed, this is an important aspect since it also reflects the changes of the fast twitch muscle fibers, which are closely linked to balance adjustments and fall prevention (Hvid et al. 2010). The V-wave methodology, an electrophysiological variant of the H-reflex technique, evoked in the triceps surae muscles during maximal muscle contraction has been applied for this frame of testing in several studies (Upton et al. 1971; Aagaard et al. 2002; Duclay and Martin 2005; Fimland et al. 2009; Ekblom 2010; Fimland et al. 2010; Fimland et al. 2011; Vila-Cha et al. 2012). The V-wave is typically normalized to the maximal M-wave (V/M ratio), and its magnitude is suggested to reflect neural drive to the muscle, as well as spinal excitability and pre/postsynaptic inhibition (Upton et al. 1971; Aagaard et al. 2002; Aagaard 2003). Although it may be difficult to identify the exact site of the motor system responsible for a difference in V-wave amplitude, it may be used in combination with the H-reflex technique to more specifically determine the sites and mechanisms of neural changes (Aagaard et al. 2002; Vila-Cha et al. 2012), since the H-reflex is more dependent on motoneuron excitability (Misiaszek 2003; Ekblom 2010; Vila-Cha et al. 2012), whereas the V-wave is more sensitive to supraspinal activation of the motoneuron pool. Several studies have, using the V-wave methodology, demonstrated increased descending drive in response to heavy resistance training in young populations (Aagaard et al. 2002; Del Balso and Cafarelli 2007; Fimland et al. 2009; Ekblom 2010). Interestingly, the V-wave method has to our knowledge not previously been applied in elderly populations. Hence, it is uncertain whether these strength training-induced increases in efferent neural drive in young may be reflected also in a training intervention with elderly subjects.
Not only is it unclear to what extent neural factors may contribute to the observed age-related decline in maximal strength, it is also unknown how supraspinal drive, assessed by voluntary V-waves during maximal muscle contractions, may or may not respond to heavy resistance training in elderly. Therefore, the aim of this study was to examine the efferent descending drive to the muscle in healthy old males and explore whether a possible diminished neural function could be restored by a heavy resistance training intervention. We hypothesized that (1) descending neural drive would be lower in elderly compared to young, and (2) heavy resistance training with emphasis on intended velocity in the concentric phase would increase the descending neural drive.
Methods
Ethical approval
The study was approved by the local regional ethical committee (the Regional Committee for Medical and Health Research Ethics in Central Norway), and carried out in accordance with the latest revision of the Declaration of Helsinki. All subjects gave written informed consents prior to inclusion in the study.
Subjects
Seventeen healthy (nine elderly; eight young) nonobese (BMI < 30) and nonsmoking male subjects were included into an elderly training group and a young control group (Table 1). Elderly subjects between 65 and 85 years of age were recruited through local senior societies, while young subjects between 20 and 30 years old were recruited among university students in Trondheim, Norway. The elderly subjects reported to engage in typical aerobic endurance-related activities with moderate or low intensity, such as hiking and golf, one to three times a week. Exclusion criteria were participation in regular strength training more than one time/week during the last 5 years, cardio-respiratory or musculoskeletal disease during the last 5 years, and not being able to carry out the testing or failure to participate in at least 85 % of the resistance training sessions. Two of the elderly and one of the young subjects withdrew due to traveling abroad, leaving seven subjects in each group.
Study design
All subjects conducted the testing procedures before and after the training period. The elderly group performed strength training three times a week throughout the training intervention, while the young group received no training and served as a reference group at baseline as well as a time control from pretest to posttest.
Testing and experimental procedure
Within 1 week prior to the experimental testing, each subject participated in a familiarization session to minimize influence from the testing procedure on the pretraining to posttraining effects. During the testing, measurements of isometric strength parameters and evoked reflex recordings were obtained simultaneously and were performed using the right leg. First, reflex recordings in the calf muscles, isometric strength, and isometric RFD were obtained. After a 15-min break, to assess if the isometric measurements were mirrored by functional relevant dynamic strength, the testing continued with 1RM measurements of dynamic leg press and plantar flexion, as well as dynamic RFD. After 2–3 days, the participants returned to the laboratory for dual X-ray absorptiometry (Hologic Discovery, S/N 83817) assessment of body composition. All body composition measurements were performed by a certified technician at the Department of Endocrinology at St. Olav’s Hospital.
Measurements of muscular strength
Isometric maximal voluntary contraction (MVC) was carried out in a fixed custom-made plantar flexion apparatus (Fig. 1). Each subject was seated with the right foot placed on a shelf with an ankle joint angle of 90°. The knee was held in position by a cushioned board, with a knee joint angle also of 90°. The left foot was placed on the floor. The participants performed eight plantar flexion MVCs, and importantly, emphasis was made on instruction to contract as rapid and forcefully as possible. Each MVC was separated by a 1-min resting period. Maximal isometric strength and RFD measurements were recorded through a force transducer (model 620-1000M-F, Vishay Tedea-Huntleigh load cell, Israel), attached to the custom-made plantar flexion apparatus. Force was multiplied by lever arm to attain torque. The highest recorded torque was set to MVC. Isometric RFD was calculated as Δ torque/Δ time in the time frames 0–30, 50, 100, 150, and 200 ms of the contraction.
Additionally, 1RM in leg press and dynamic plantar flexion were determined. These tests were performed bilaterally, and for the leg press exercise, the knee joint angle was set to 90°, while the plantar flexion was performed from an ankle joint angle of ∼20° dorsiflexion up to ∼30° plantar flexion. After two warm-up sets (four to six repetitions on light loads), several lifts were carried out, with increasing loads of 5–10 kg until the subjects were unable to complete the lift. 1RM was achieved within four to seven trials after the warm-up sets. Dynamic RFD was recorded during a maximal contraction in leg press, using a force platform (9286AA, Kistler, Switzerland) at 2000 Hz, attached to the leg press apparatus. The measurement was carried out at a load set to 80 % of the pretest 1RM from a 90° knee angle (Mosti et al. 2013). The highest recorded concentric force throughout the range of motion was set to peak force. RFD was defined as Δ force/Δ time in the time frames 0–30, 0–50, 0–100, 0–150, and 0–200 ms of the contraction. Data were analyzed using BioWare v.3.06 (Kistler, Switzerland).
Evoked potentials
While seated in the plantar flexion apparatus, H-reflexes and V-waves were evoked in the tibial nerve, in the popliteal fossa, using a current stimulator (DS7AH, Digitimer, Welwyn Garden City, UK). A 1-ms square wave stimulus were given by gel-coated (Lectron 2 conductive gel, Pharmaceutical Innovations Inc., Newark, NJ, USA) bipolar felt pad electrodes, 8 mm in diameter, 25 mm between the tips (Digitimer, Welwyn Garden City, UK). The pad electrodes were placed at the position evoking the largest H-reflex amplitude relative to the M-wave amplitude. Electric potentials were recorded from the m. soleus, m. gastrocnemius medialis (GM), m. gastrocnemius lateralis (GL), and m. tibialis anterior (TA), using self-adhesive AG/AgCI electrodes (Ambu, M-00-S/50, Ballerup, Denmark) placed as recommended by SENIAM (Hermens et al. 2000). Measuring tape and anatomical landmarks were used to identify the appropriate location of electrode placement, and pictures were taken of the relevant leg to ensure identical placement of the electrodes at pretest and posttest. Before placement of the electrodes, the skin was carefully shaved, rubbed with skin prep gel (Nuprep, Weaver and company, Aurora, CO, USA), and wiped clean with alcohol. The preparation procedure was included to ensure minimal resistance in the skin; the maximal interelectrode impedance level was set to 5 kΩ. Data were obtained using Megawin software 700,046 version 3.0, by using the ME6000 Biomonitor (Mega Electronics LTD, Kuopio, Finland) at 2 kHz, common mode rejection ratio 110 dB, amplified, and bandpass filtered (8–500 Hz).
H-reflex measurements were taken at rest, seated in the exact same position as during the MVCs. In order to ensure similar head and eye movement during testing, the subjects were instructed to focus their vision at a specific marked point on the wall, horizontally positioned, in front of them. The current intensity was gradually increased by 1 mA searching for the maximal H-reflex amplitude (Hmax) and further by 2–5 mA searching for the maximal M-wave. Hmax was normalized to the maximal M-wave obtained at rest (Mmax). When the assumed Mmax was recorded, three supramaximal stimuli at 150 % of the stimulus needed to evoke Mmax were given to ensure that the true Mmax was reached. Eight V-wave recordings were performed. The V-wave responses were evoked during MVC, by delivering a supramaximal stimulus to the tibial nerve after a plateau of torque was observed. One-minute rest periods were given between each MVC. The V-waves, where the amplitude of the M-wave was ≥95 % of Msup and the torque was ≥95 % of MVC, were used for analysis. V-waves were normalized to the maximal M-wave obtained during MVC (Msup). V-waves were analyzed for m. soleus, GL, and GM, while H-reflex recordings were analyzed only for m. soleus. H-reflex latency was determined as the time interval from the point of the electrical stimulus to the first detection of the H-reflex. In agreement with Sale and coworkers (Sale et al. 1983), our laboratory has reported substantial test-retest reliability of the V-wave technique (intraclass correlation coefficient = 0.86) (Solstad et al. 2011).
Training intervention
The elderly group attended 8 weeks of supervised heavy resistance training, three sessions per week. All training sessions were carried out in a laboratory setting, and each participant was given individual guidance. The training consisted of three exercises for the lower limbs; functionally relevant bilateral dynamic leg press and plantar flexion and test-specific unilateral isometric plantar flexion. The intensity was set to 75–80 % of 1RM, and the two dynamic exercises were organized in four sets of 12 repetitions, in which the ∼2–3 last repetitions of the final set were forced repetitions assisted by the supervisor. When the subject was able to complete the last set without assistance, the load was increased by 5 kg in leg press and 2.5 kg in dynamic plantar flexion. Importantly, the dynamic exercises were carried out with a controlled eccentric phase of the movement, a short stop, and maximal mobilization of force in the concentric phase. This movement pattern has been frequently used in our laboratory to facilitate neuromuscular adaptations to resistance training (Hoff et al. 2007; Storen et al. 2008; Fimland et al. 2009, 2010). To facilitate maximal intended velocity, 3-min resting periods were applied between each set, while each exercise was separated by 5-min rest. Additionally, the participants performed ten maximal isometric plantar flexion contractions for each leg, also with emphasis on rapid force development. Each repetition was separated by a 30-s rest period. The young control group received no training and served as a time control and as a reference for the elderly training group.
Statistical analysis
All statistical analyses were performed using the software IBM SPSS Statistics 21 (Chicago, IL, USA). Figures were made using GraphPad Prism 5 (San Diego, USA). Wilcoxon signed-rank tests were used to detect within group differences from pretest to posttest, and Mann-Whitney U tests were used to assess differences between groups. Effect sizes (Cohen’s d) are presented along with training-induced changes. The statistical level of significance was set to p < 0.05. For descriptive purposes, data are presented as mean ± SD, unless otherwise noted.
Results
Subjects
Seven subjects from the elderly group and seven subjects from the young control group completed the study. The elderly group had a compliance rate of 21 ± 1 out of 24 planned sessions, in addition to the pretest and posttests.
Evoked potentials
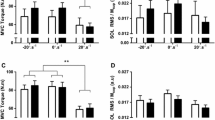
At baseline, the soleus, GM, and GL V/M ratio were significantly lower (p < 0.05) in the elderly group compared to the young. This age-related difference was, despite 8 weeks of supervised heavy resistance training, still evident at posttest with the elderly exhibiting V/M ratios between 30 and 40 % of the young (Table 2). As a consequence of training, soleus V/M ratio increased 72 ± 73 % (Cohen’s d 0.70) (Fig. 2). A similar increase was apparent for the GL V/M ratio, increasing 75 ± 84 % (Cohen’s d 0.41) from pretest to posttest, whereas the GM V/M ratio did not display a significant change following the training period. No change was observed in TA activity.
a Plantar flexor maximal isometric voluntary contraction (MVC), b m. soleus V/M ratio, and c right leg lean mass before and after 8 weeks of heavy resistance training. Data are presented as mean ± SE.*p < 0.05, significant difference between pre and post; #p < 0.05, significant difference between elderly and young at baseline; +p < 0.05, significant difference between elderly and young at posttest
As for the supramaximally evoked potentials during MVC, soleus H/M ratio tended to be lower (p = 0.07) in the elderly group at baseline (Table 3). However, in contrast to the V-wave measurements, no changes were observed in the H/M ratio following training. The soleus H-reflex latency also displayed a ∼10 % longer latency in elderly compared to young, and this difference was not altered by the heavy resistance training (Table 3).
Leg muscle mass
The heavy resistance training induced a significant increase (p < 0.05) in the right leg (5.6 ± 4.8 %) (Cohen’s d 0.45) and combined legs (4.4 ± 3.5 %) (Cohen’s d 0.35) lean mass (Table 4). This was accompanied by a tendency (p = 0.07) of the total body mass to increase from pretest to posttest (Table 4). However, these increases did not result in any differences between the groups in muscle mass at the combined legs, or the right leg, at pretest or posttest (Table 4). Whole body fat percentage, combined leg fat percentage and right leg fat percentage, were higher in the elderly group (p < 0.05) and thus accounted for most of the difference in weight between elderly and young (Table 4).
Maximal strength and rate of force development
Plantar flexion MVC was 34 % lower (p < 0.05) in the elderly than in the young at baseline (Fig. 2). Although this difference was still apparent (21 % lower (p < 0.05)) after the 8-week training period, plantar flexion MVC increased from 158 ± 48 Nm at pretest to 190 ± 70 Nm (18.7 ± 14.5 %) (Cohen’s d 0.53) at posttest in the elderly group (Fig. 2). Particularly two of the elderly subjects revealed low MVC baselines (90 and 123 Nm). These two subjects also exhibited the largest training-induced improvements in MVC (45 and 29 %). As for the MVC, isometric plantar flexion RFD (0–200 ms) was lower (p < 0.05) in the elderly at baseline (294 ± 126 Nm s−1 (elderly); 533 ± 123 Nm s−1 (young)), and increased following the training period by 58 ± 63 % (p < 0.05) (Cohen’s d 0.78) but was still 27 % lower (p < 0.05) compared to young controls (401 ± 147 Nm s−1 (elderly); 546 ± 115 Nm s−1 (young)). The same two subjects that exhibited a low maximal strength at baseline also displayed a low RFD (117 and 131 Nm s−1). These two subjects’ training-induced percentage improvements were 75 and 98 %, respectively. Isometric plantar flexion RFD in the time frames 0–30, 0–50, 0–100, 0–150, and 0–200 ms of the contraction are presented in Table 5.
Maximal dynamic strength reflected the results found during the isometric contractions. The elderly exhibited a lower (p < 0.05) 1RM at baseline for both plantar flexion (84 ± 16 kg (elderly); 130 ± 26 kg (young)) and leg press (157 ± 50 kg (elderly); 207 ± 27 kg (young)), and these parameters were improved (p < 0.05) following training to 108 ± 23 kg (+28 ± 9 %) (Cohen’s d 1.20) in plantar flexion and 196 ± 55 kg (26 ± 8 %) (Cohen’s d 0.73) in leg press. The elderly training group also displayed a concomitant dynamic leg press RFD (0–200 ms) and peak force improvement (p < 0.05) of 108 ± 61 % (Cohen’s d 1.43) and 8 ± 6 % (Cohen’s d 0.24), respectively. No changes were observed in the young control group in any of these parameters.
Discussion
Since it is not fully understood how the efferent neural drive to the muscle is affected by healthy aging, and whether the neural function would demonstrate plasticity following heavy resistance training, we sought to investigate the differences in evoked reflex potentials between elderly and young subjects during maximal muscle contraction, as well as the exercise-induced response in elderly. The main findings were that (1) V/M ratio was strongly reduced in the elderly group compared to young at baseline (2); the elderly group improved the V/M ratio in response to heavy resistance training, but even after training the V/M ratio of the elderly was still ∼60 % lower than the young; (3) despite similar level of leg muscle mass, muscle strength, and RFD were lower in the elderly both before and after training.
Reductions in descending neural drive with age
As expected, the elderly in this study displayed the well-known age-related reduction in maximal strength. The ∼25–35 % reductions in isometric and dynamic strength for the 74 ± 6-year-old participants in the current study were in line with previous, typically ∼1 % decline/year from the fifth decade, observations (Larsson et al. 1979; Lindle et al. 1997; Macaluso and De Vito 2004). The reduction in maximal strength was mirrored by a clear tendency of a similarly reduced soleus H/M ratio and an increased soleus H-reflex latency time of ∼10 % compared to the young controls, also in accordance with previous reports for elderly populations (Scaglioni et al. 2002; Scaglioni et al. 2003). Although not all studies have managed to statistically discriminate H/M ratio between elderly and young (Scaglioni et al. 2003), there is general agreement that H/M ratio is lower in elderly subjects compared to young (Koceja et al. 1995; Scaglioni et al. 2002; Kido et al. 2004). The slower conduction velocity is previously suggested to originate from reduced myelination and internodal length (Metter et al. 1998; Scaglioni et al. 2002; Aagaard et al. 2010), but also, the typically observed age-related shift toward slow twitch muscle fibers may influence conduction properties, because loss of the fastest conducting axonal fibers would decrease conduction velocity (Aagaard et al. 2010).
In the current study, for the first time, our findings show that the attenuated H-reflex responses are extended to include impairments at supraspinal levels, resulting in attenuated efferent drive to the muscle. At baseline, the V/M ratio of the elderly group was less than one third of that observed for the young (Fig. 3), and this finding was consistent for all three plantar flexors: m. soleus, GM, and GL. The amplitude of the V-wave relies on the probability of antidromic collision, as the removal of antidromic M-wave potentials by efferent descending motor output allows more of the reflex volley to reach the muscle (Upton et al. 1971; Aagaard et al. 2002). Thus, in addition to motoneuron excitability, the maximal firing frequency, as well as the proportion of motoneurons recruited will determine the size of the V-wave, as they will both affect the probability of antidromic collision. Although pre/postsynaptic inhibition may also affect the amplitude of the V-wave, these factors would influence the amplitude of the H-reflex similarly (Zehr 2006; Vila-Cha et al. 2012). Interestingly, the reduction in V/M ratio was more than three times larger than the reduction in H/M ratio. This discrepancy suggests that the difference in V/M-ratio between young and elderly was mainly caused by the magnitude of efferent motor output, likely reflecting a reduced supraspinal descending drive in elderly. This indicates that the neural function may actually be more altered at supraspinal levels than at spinal levels (Aagaard et al. 2002; Vila-Cha et al. 2012). Such a central impairment may not immediately lead to a loss of muscle strength, since a gradual loss of motoneurons may be compensated for through axonal sprouting (McNeil et al. 2005; Aagaard et al. 2010). However, since fast twitch type II motor units are mainly reinnervated by slow twitch motoneurons (Aagaard et al. 2010), it may alter muscle force characteristics because of a shift toward a slower muscle phenotype.
Age and neural plasticity following heavy resistance training
In response to the heavy resistance training intervention, the elderly subjects improved the efferent drive to the muscle. The observed 72 % increase in V/M ratio implies that neural factors not only play a role in the age-related decline in maximal strength but also demonstrate plasticity. Previous findings have been conflicting regarding the role of neural factors following strength training in elderly. Some observations of increased muscle activation (Scaglioni et al. 2002; Suetta et al. 2007) have been contrasted by others that have shown no changes, despite large improvements in muscle strength (Harridge et al. 1999). However, interpolated twitch methods may be insensitive to training-induced changes (Herbert and Gandevia 1999), and muscle activation results may also have been blurred by the involvement of more peripheral neuromuscular factors. Interestingly, in agreement with previous studies (Scaglioni et al. 2002; Del Balso and Cafarelli 2007; Fimland et al. 2009), we observed no strength training-induced changes in the H/M ratio. This implies that the increase in V/M ratio in the current study was mainly a result of increased efferent motor output, rather than changes in motoneuron excitability, likely a result of elevated descending drive from corticospinal pathways. In previous studies with young, elevated maximal firing frequency have primarily been attributed the training-induced increases in efferent drive and V/M ratio (Aagaard et al. 2002). As Kamen and Knight (2004) found that the motoneuron firing frequency in elderly were restored up to the level of young during 6 weeks of heavy resistance training, this explanation may certainly apply also for the elderly subjects in the current study.
Although improvements in efferent drive, to our knowledge, have not been observed in elderly before, young subjects have been documented to exhibit similar V/M ratio improvements of 50–80 % after heavy resistance training (Sale et al. 1983; Aagaard et al. 2002; Del Balso and Cafarelli 2007; Fimland et al. 2009; Ekblom 2010). Importantly, in the current study, all training was performed with emphasis on intended velocity in the concentric phase of the movement. This could have resulted in the large improvements, as this previously has been suggested to play an important role in neural adaptations following heavy resistance training (Behm and Sale 1993). However, despite a clear demonstration of plasticity, the V/M ratio was still ∼60 % lower compared to young after the training period in our study (Fig. 2). Even for the strongest individual in the elderly group, who displayed muscle strength similar to the best among the young, the V/M ratio after training was ∼50 % lower compared to the young average. It cannot be excluded that prolonged training may improve the V/M ratio of the old to a similar level as the young subjects. However, it is previously observed that the most prominent neural adaptations take place during the first 2–4 weeks of a resistance training intervention (Moritani and deVries 1979; Folland and Williams 2007); thus, the conspicuously large discrepancy in V/M-ratio between old and young also at posttest may raise the question whether elderly exhibit neuromuscular deficiencies even in the trained state.
Maximal muscle strength and rate of force development adaptations in elderly
Heavy resistance training is documented to work as a countermeasure for the typically observed age-related fall in maximal muscle strength, muscle mass and nervous function (Aagaard et al. 2010). In our study, MVC and RFD increased by ∼19 and ∼58 %, respectively, following training. These improvements were also reflected by functional dynamic strength improvements of ∼26, ∼28, and ∼108 % in 1RM leg press, 1RM plantar flexion, and leg press RFD, respectively. These robust improvements are in line with previous studies investigating heavy resistance training in elderly (Tracy et al. 1999; Scaglioni et al. 2002; Ferri et al. 2003; Caserotti et al. 2008; Verdijk et al. 2009) and add further weight to the pool of evidence not only showing large beneficial strength gains, but even gains comparable with the young population (Ciolac et al. 2010a; Ciolac et al. 2010b). Particularly two subjects, entering the study at a low fitness level, exhibited very large strength gains during the 8 weeks of training, exemplifying that heavy resistance training is manageable and very effective also for elderly with a low fitness level. If compared to average rates of strength loss (Larsson et al. 1979; Lindle et al. 1997; Macaluso and De Vito 2004), the improvements in the current study correspond to ∼20 years of rejuvenation.
Surprisingly, despite the lowered maximal strength and the large reduction in RFD, leg muscle mass was similar between young and elderly. Although direct comparison between elderly and young is somewhat hampered by the likely differences in muscle fiber composition and intramuscular fat (Aagaard et al. 2010; Narici and Maffulli 2010), this discrepancy strengthens the argument that neural factors certainly are influential to changes in muscular strength with age. Neural factors are particularly shown to be important for rapid muscle contractions, and thus, it is striking that the most prominent differences between elderly and young were found in RFD. Although the elderly exhibited substantial improvements in RFD (isometric ∼58 %; dynamic ∼108 %), the explosive strength was markedly reduced also after training, a pattern which was mirrored by the V/M ratio.
Heavy resistance training in elderly: clinical implications
While the importance of neural factors in strength training in the elderly population should yet be treated with some caution because of the low sample size and limited muscle groups studied in the current study, recommendations of heavy resistance training with emphasis on maximal mobilization in the concentric phase has provided excellent results not only in this study, but in a wide range of untrained patient populations (de Vos et al. 2005; Hoff et al. 2007; Caserotti et al. 2008; Husby et al. 2009; Fimland et al. 2010; Fimland et al. 2011; Heggelund et al. 2012; Hill et al. 2012; Mosti et al. 2013). Although the isometric training may also account for some of the adaptations, these studies together present mounting evidence that heavy resistance training not only is feasible and effective; additionally, no incidents of injury have been reported. Importantly, the large improvements in RFD in these studies, typically between 50 and 150 %, will imply a clear risk reduction for falls (Hvid et al. 2010). Considering the importance of muscle strength and rapid force development in particular (Fleming et al. 1991; Izquierdo et al. 1999; Suetta et al. 2007; Aagaard et al. 2010), the neuromuscular deterioration certainly represents a challenge in order to maintain high physical functionality at old age. It should be argued that strength training for elderly, in addition to induce muscle growth, should also strive more for neuromuscular adaptations. It is therefore of concern that today’s guidelines for heavy resistance training in elderly suggest that only moderate intensity should be applied and does not include emphasis on the intended velocity of the movement (American College of Sports Medicine 2009).
Conclusion
Elderly and young subjects in the current study exhibited significant difference in efferent descending drive and advocate the importance of neural factors in age-related changes in muscle strength. As a consequence of heavy resistance training the neural drive significantly improved. The increased V/M ratio, accompanied by the unchanged H/M ratio, implies that the improvement was primarily of descending supraspinal origin. These findings imply that neural factors play an important role in age-related changes in muscle force characteristics, and thus should be emphasized in a strength training intervention in elderly.
Abbreviations
- 1RM:
-
One repetition maximum
- GL:
-
Musculus gastrocnemius lateralis
- GM:
-
Musculus gastrocnemius medialis
- Hmax :
-
Maximal H-reflex amplitude
- Mmax :
-
Maximal M-wave amplitude obtained during rest
- Msup :
-
Maximal M-wave amplitude obtained during maximal voluntary contraction
- MVC:
-
Maximal voluntary contraction
- RFD:
-
Rate of force development
- TA:
-
Musculus tibialis anterior
- Vmax :
-
Maximal V-wave amplitude
References
Aagaard P (2003) Training-induced changes in neural function. Exerc Sport Sci Rev 31:61–67
Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P (2002) Neural adaptation to resistance training: changes in evoked V-wave and H-reflex responses. J Appl Physiol (1985) 92:2309–2318
Aagaard P, Suetta C, Caserotti P, Magnusson SP, Kjaer M (2010) Role of the nervous system in sarcopenia and muscle atrophy with aging: strength training as a countermeasure. Scand J Med Sci Spor 20:49–64
(2009). American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc 41, 687–708
Behm DG, Sale DG (1993) Intended rather than actual movement velocity determines velocity-specific training response. J Appl Physiol (1985) 74:359–368
Campbell AJ, Borrie MJ, Spears GF (1989) Risk factors for falls in a community-based prospective study of people 70 years and older. J Gerontol 44:M112–M117
Caserotti P, Aagaard P, Buttrup Larsen J, Puggaard L (2008) Explosive heavy-resistance training in old and very old adults: changes in rapid muscle force, strength and power. Scand J Med Sci Spor 18:773–782
Ciolac EG, Brech GC, Greve JM (2010a) Age does not affect exercise intensity progression among women. J Strength Cond Res 24:3023–3031
Ciolac EG, Garcez-Leme LE, Greve JM (2010b) Resistance exercise intensity progression in older men. Int J Sports Med 31:433–438
de Vos NJ, Singh NA, Ross DA, Stavrinos TM, Orr R, Fiatarone Singh MA (2005) Optimal load for increasing muscle power during explosive resistance training in older adults. J Gerontol A Biol Sci Med Sci 60:638–647
Del Balso C, Cafarelli E (2007) Adaptations in the activation of human skeletal muscle induced by short-term isometric resistance training. J Appl Physiol (1985) 103:402–411
deVries HA, Wiswell RA, Romero GT, Heckathorne E (1985) Changes with age in monosynaptic reflexes elicited by mechanical and electrical stimulation. Am J Phys Med 64:71–81
Duclay J, Martin A (2005) Evoked H-reflex and V-wave responses during maximal isometric, concentric, and eccentric muscle contraction. J Neurophysiol 94:3555–3562
Ekblom MM (2010) Improvements in dynamic plantar flexor strength after resistance training are associated with increased voluntary activation and V-to-M ratio. J Appl Physiol (1985) 109:19–26
Ferri A, Scaglioni G, Pousson M, Capodaglio P, Van Hoecke J, Narici MV (2003) Strength and power changes of the human plantar flexors and knee extensors in response to resistance training in old age. Acta Physiol Scand 177:69–78
Fimland MS, Helgerud J, Gruber M, Leivseth G, Hoff J (2009) Functional maximal strength training induces neural transfer to single-joint tasks. Eur J Appl Physiol 107:21–29
Fimland MS, Helgerud J, Gruber M, Leivseth G, Hoff J (2010) Enhanced neural drive after maximal strength training in multiple sclerosis patients. Eur J Appl Physiol 110:435–443
Fimland MS, Moen PM, Hill T, Gjellesvik TI, Torhaug T, Helgerud J, Hoff J (2011) Neuromuscular performance of paretic versus non-paretic plantar flexors after stroke. Eur J Appl Physiol 111:3041–3049
Fleming BE, Wilson DR, Pendergast DR (1991) A portable, easily performed muscle power test and its association with falls by elderly persons. Arch Phys Med Rehab 72:886–889
Folland JP, Williams AG (2007) The adaptations to strength training : morphological and neurological contributions to increased strength. Sports Med 37:145–168
Geirsdottir OG, Arnarson A, Briem K, Ramel A, Tomasson K, Jonsson PV, Thorsdottir I (2012) Physical function predicts improvement in quality of life in elderly Icelanders after 12 weeks of resistance exercise. J Nutr Health Aging 16:62–66
Harridge SD, Kryger A, Stensgaard A (1999) Knee extensor strength, activation, and size in very elderly people following strength training. Muscle Nerve 22:831–839
Heggelund J, Morken G, Helgerud J, Nilsberg GE, Hoff J (2012) Therapeutic effects of maximal strength training on walking efficiency in patients with schizophrenia - a pilot study. BMC Res Notes 5:344
Herbert RD, Gandevia SC (1999) Twitch interpolation in human muscles: mechanisms and implications for measurement of voluntary activation. J Neurophysiol 82:2271–2283
Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G (2000) Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 10:361–374
Hill TR, Gjellesvik TI, Moen PM, Torhaug T, Fimland MS, Helgerud J, Hoff J (2012) Maximal strength training enhances strength and functional performance in chronic stroke survivors. Am J Phys Med Rehabil 91:393–400
Hoff J, Tjonna AE, Steinshamn S, Hoydal M, Richardson RS, Helgerud J (2007) Maximal strength training of the legs in COPD: a therapy for mechanical inefficiency. Med Sci Sports Exerc 39:220–226
Hughes VA, Frontera WR, Dallal GE, Lutz KJ, Fisher EC, Evans WJ (1995) Muscle strength and body composition: associations with bone density in older subjects. Med Sci Sports Exerc 27:967–974
Husby VS, Helgerud J, Bjorgen S, Husby OS, Benum P, Hoff J (2009) Early maximal strength training is an efficient treatment for patients operated with total hip arthroplasty. Arch Phys Med Rehabil 90:1658–1667
Hvid L, Aagaard P, Justesen L, Bayer ML, Andersen JL, Ortenblad N, Kjaer M, Suetta C (2010) Effects of aging on muscle mechanical function and muscle fiber morphology during short-term immobilization and subsequent retraining. J Appl Physiol (1985) 109:1628–1634
Hyatt RH, Whitelaw MN, Bhat A, Scott S, Maxwell JD (1990) Association of muscle strength with functional status of elderly people. Age Ageing 19:330–336
Izquierdo M, Aguado X, Gonzalez R, Lopez JL, Hakkinen K (1999) Maximal and explosive force production capacity and balance performance in men of different ages. Eur J Appl Physiol O 79:260–267
Jubrias SA, Odderson IR, Esselman PC, Conley KE (1997) Decline in isokinetic force with age: muscle cross-sectional area and specific force. Pflugers Arch 434:246–253
Kamen G, Knight CA (2004) Training-related adaptations in motor unit discharge rate in young and older adults. J Gerontol A Biol Sci Med Sci 59:1334–1338
Kamen G, Sison SV, Du CC, Patten C (1995) Motor unit discharge behavior in older adults during maximal-effort contractions. J Appl Physiol (1985) 79:1908–1913
Kennis E, Verschueren S, Van Roie E, Thomis M, Lefevre J, Delecluse C (2014) Longitudinal impact of aging on muscle quality in middle-aged men. Age (Dordr) 36:9689
Kido A, Tanaka N, Stein RB (2004) Spinal excitation and inhibition decrease as humans age. Can J Physiol Pharmacol 82:238–248
Klass M, Baudry S, Duchateau J (2008) Age-related decline in rate of torque development is accompanied by lower maximal motor unit discharge frequency during fast contractions. J Appl Physiol (1985) 104:739–746
Klitgaard H, Mantoni M, Schiaffino S, Ausoni S, Gorza L, Laurent-Winter C, Schnohr P, Saltin B (1990) Function, morphology and protein expression of ageing skeletal muscle: a cross-sectional study of elderly men with different training backgrounds. Acta Physiol Scand 140:41–54
Koceja DM, Markus CA, Trimble MH (1995) Postural modulation of the soleus H reflex in young and old subjects. Electroencephalogr Clin Neurophysiol 97:387–393
Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM (2006) Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol (1985) 101:531–544
Larsson L, Grimby G, Karlsson J (1979) Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol Respir Environ Exerc Physiol 46:451–456
Lindle RS, Metter EJ, Lynch NA, Fleg JL, Fozard JL, Tobin J, Roy TA, Hurley BF (1997) Age and gender comparisons of muscle strength in 654 women and men aged 20-93 year. J Appl Physiol (1985) 83:1581–1587
Macaluso A, De Vito G (2004) Muscle strength, power and adaptations to resistance training in older people. Eur J Appl Physiol 91:450–472
Mau-Moeller A, Behrens M, Lindner T, Bader R, Bruhn S (2013) Age-related changes in neuromuscular function of the quadriceps muscle in physically active adults. J Electromyogr Kinesiol 23:640–648
McGinley M, Hoffman RL, Russ DW, Thomas JS, Clark BC (2010) Older adults exhibit more intracortical inhibition and less intracortical facilitation than young adults. Exp Gerontol 45:671–678
McNeil CJ, Doherty TJ, Stashuk DW, Rice CL (2005) Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve 31:461–467
McNeil CJ, Butler JE, Taylor JL, Gandevia SC (2013) Testing the excitability of human motoneurons. Front Hum Neurosci 7:152
Metter EJ, Conwit R, Metter B, Pacheco T, Tobin J (1998) The relationship of peripheral motor nerve conduction velocity to age-associated loss of grip strength. Aging (Milano) 10:471–478
Misiaszek JE (2003) The H-reflex as a tool in neurophysiology: its limitations and uses in understanding nervous system function. Muscle Nerve 28:144–160
Moritani T, deVries HA (1979) Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med 58:115–130
Morse CI, Thom JM, Davis MG, Fox KR, Birch KM, Narici MV (2004) Reduced plantarflexor specific torque in the elderly is associated with a lower activation capacity. Eur J Appl Physiol 92:219–226
Mosti MP, Kaehler N, Stunes AK, Hoff J, Syversen U (2013) Maximal strength training in postmenopausal women with osteoporosis or osteopenia. J Strength Cond Res 27:2879–2886
Narici MV, Maffulli N (2010) Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull 95:139–159
Roos MR, Rice CL, Vandervoort AA (1997) Age-related changes in motor unit function. Muscle Nerve 20:679–690
Sabbahi MA, Sedgwick EM (1982) Age-related changes in monosynaptic reflex excitability. J Gerontol 37:24–32
Sale DG, Macdougall JD, Upton ARM, Mccomas AJ (1983) Effect of strength training upon motoneuron excitability in Man. Med Sci Sport Exer 15:57–62
Scaglioni G, Ferri A, Minetti AE, Martin A, Van Hoecke J, Capodaglio P, Sartorio A, Narici MV (2002) Plantar flexor activation capacity and H reflex in older adults: adaptations to strength training. J Appl Physiol (1985) 92:2292–2302
Scaglioni G, Narici MV, Maffiuletti NA, Pensini M, Martin A (2003) Effect of ageing on the electrical and mechanical properties of human soleus motor units activated by the H reflex and M wave. J Physiol 548:649–661
Sillanpaa E, Stenroth L, Bijlsma AY, Rantanen T, McPhee JS, Maden-Wilkinson TM, Jones DA, Narici MV, Gapeyeva H, Paasuke M, Barnouin Y, Hogrel JY, Butler-Browne GS, Meskers CG, Maier AB, Tormakangas T, Sipila S (2014) Associations between muscle strength, spirometric pulmonary function and mobility in healthy older adults. Age (Dordr) 36:9667
Solstad GM, Fimland MS, Helgerud J, Iversen VM, Hoff J (2011) Test-retest reliability of v-wave responses in the soleus and gastrocnemius medialis. J Clin Neurophysiol 28:217–221
Storen O, Helgerud J, Stoa EM, Hoff J (2008) Maximal strength training improves running economy in distance runners. Med Sci Sports Exerc 40:1087–1092
Suetta C, Aagaard P, Magnusson SP, Andersen LL, Sipila S, Rosted A, Jakobsen AK, Duus B, Kjaer M (2007) Muscle size, neuromuscular activation, and rapid force characteristics in elderly men and women: effects of unilateral long-term disuse due to hip-osteoarthritis. J Appl Physiol 102:942–948
Tracy BL, Ivey FM, Hurlbut D, Martel GF, Lemmer JT, Siegel EL, Metter EJ, Fozard JL, Fleg JL, Hurley BF (1999) Muscle quality. II. Effects of strength training in 65- to 75-yr-old men and women. J Appl Physiol (1985) 86:195–201
Upton AR, McComas AJ, Sica RE (1971) Potentiation of “late” responses evoked in muscles during effort. J Neurol Neurosurg Psychiatry 34:699–711
Vandervoort AA, Hayes KC (1989) Plantarflexor muscle function in young and elderly women. Eur J Appl Physiol Occup Physiol 58:389–394
Verdijk LB, Gleeson BG, Jonkers RAM, Meijer K, Savelberg HHCM, Dendale P, van Loon LJC (2009) Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J Gerontol A-Biol 64:332–339
Vila-Cha C, Falla D, Correia MV, Farina D (2012) Changes in H reflex and V wave following short-term endurance and strength training. J Appl Physiol (1985) 112:54–63
Wang E, Naess MS, Hoff J, Albert TL, Pham Q, Richardson RS, Helgerud J (2014) Exercise-training-induced changes in metabolic capacity with age: the role of central cardiovascular plasticity. Age (Dordr) 36:665–676
Winegard KJ, Hicks AL, Sale DG, Vandervoort AA (1996) A 12-year follow-up study of ankle muscle function in older adults. J Gerontol A Biol Sci Med Sci 51:B202–B207
Young A, Stokes M, Crowe M (1985) The size and strength of the quadriceps muscles of old and young men. Clin Physiol 5:145–154
Zehr EP (2002) Considerations for use of the Hoffmann reflex in exercise studies. Eur J Appl Physiol 86:455–468
Zehr EP (2006) Training-induced adaptive plasticity in human somatosensory reflex pathways. J Appl Physiol (1985) 101:1783–1794
Acknowledgments
The authors would like to thank the senior citizens who volunteered to participate in this study for their time and efforts.
Conflict of interests
The authors declare that they have no conflict of interest regarding the publication of this paper. The study was funded by the Norwegian University of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Unhjem, R., Lundestad, R., Fimland, M.S. et al. Strength training-induced responses in older adults: attenuation of descending neural drive with age. AGE 37, 47 (2015). https://doi.org/10.1007/s11357-015-9784-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11357-015-9784-y