Abstract
The “living high–training low” model (LHTL), i.e., training in normoxia but sleeping/living in hypoxia, is designed to improve the athletes performance. However, LHTL efficacy still remains controversial and also little is known about the duration of its potential benefit. This study tested whether LHTL enhances aerobic performance in athletes, and if any positive effect may last for up to 2 weeks after LHTL intervention. Eighteen swimmers trained for 13 days at 1,200 m while sleeping/living at 1,200 m in ambient air (control, n=9) or in hypoxic rooms (LHTL, n=9, 5 days at simulated altitude of 2,500 m followed by 8 days at simulated altitude of 3,000 m, 16 h day−1). Measures were done before 1–2 days (POST-1) and 2 weeks after intervention (POST-15). Aerobic performance was assessed from two swimming trials, exploring \( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} \) and endurance performance (2,000-m time trial), respectively. Reticulocyte, serum EPO and soluble transferrin receptor responses were not altered by LHTL, whereas reticulocytes decreased in controls. In POST-1 (vs. before): red blood cell volume increased in LHTL only (+8.5%, P=0.03), \( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} \) tended to increase more in LHTL (+8.1%, P=0.09) than in controls (+2.5%, P=0.21) without any difference between groups (P=0.42) and 2,000-m performance was unchanged with LHTL. In POST-15, both performance and hematological parameters were similar to initial levels. Our results indicate that LHTL may stimulate red cell production, without any concurrent amelioration of aerobic performance. The absence of any prolonged benefit after LHTL suggests that this LHTL model cannot be recommended for long-term purposes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Altitude training—i.e., live and train at moderate altitude—has been used for decades by athletes to improve their physical performance after return to sea level. The classic underlying hypothesis is that prolonged exposure to altitude during the training camp may improve red blood cell volume, oxygen carrying capacity of the blood, and therefore, maximal oxygen transport (Klausen et al. 1966). However, in practice, the management of the training process in the hypoxic environment remains complex, which may explain why the efficacy of this method is still very controversial (Gore et al. 1998; Levine 2002).
An alternative method of discontinuous exposure to hypoxia, namely “living high–training low” (LHTL), was first proposed by Levine et al. (1991). These authors originally hypothesized that LHTL improves sea-level aerobic performance, both because resting hypoxic residence increases erythropoiesis and red blood cell volume, and because training performed at or near sea level preserves training intensities, O2 flux and thus muscle function. This training method—which is now popular among athletes and coaches—was found to improve maximal oxygen transport, as well as field aerobic performance (Levine and Stray-Gundersen 1997; Stray-Gundersen et al. 2001). These authors showed that the main mechanism accounting for this improvement was the LHTL-induced increase in red blood cell volume. However, this hypothesis for “central” performance enhancement (systemic changes in hematology and O2 transport) is challenged by other reports showing that LHTL neither increases red blood cell volume (Ashenden et al. 1999a, b), nor improves (Clark et al. 2004; Roberts et al. 2003) or even decreases maximal O2 transport (Gore et al. 2001). Such findings rather support a “peripheral” hypothesis (local changes in skeletal muscle) for explaining the small improvement in performance detected with LHTL (Hahn and Gore 2001). With respect to muscle function, LHTL may either be advantageous through an increase in muscle buffer capacity (Gore et al. 2001), or disadvantageous because hypoxia is known to decrease Na+–K+–ATPase activity (Green et al. 2000), therefore accelerating muscle fatigue. However, depressed Na+–K+–ATPase activity during LHTL would not adversely affect performance (Aughey et al. 2005). In summary, both the main adaptive response to LHTL (central or peripheral) and its outcome (beneficial or not) are still a matter of debate.
Assuming that LHTL enhances performance, a second question arises, as to how long the benefit may last. Indeed, most of the previous works focused on the short-time (2–4 days post LHTL) effects of LHTL (Gore et al. 2001; Roberts et al. 2003; Stray-Gundersen et al. 2001), whereas athletes and coaches also expect sustained LHTL-related effects (weeks). Thus, the persistent improvement in aerobic performance shown over the first 3 weeks after LHTL (Levine and Stray-Gundersen 1997) has an interesting practical implication.
What is the rationale for any prolonged enhancement of performance after LHTL? One often cited reason—although not verified—is that LHTL increases red blood cell volume and aerobic power, which in turn allows the athletes to improve their training load for a few weeks after the return to sea level. Thus, the performance enhancement could be maintained after the hematological modifications had disappeared. However, the only available data suggests that the opposite, i.e., reducing training during return from altitude, is also compatible with a persistent gain in aerobic performance (Levine and Stray-Gundersen 1997). Alternatively, these data support the idea that some robust central and/or peripheral adaptations may also account for the observed prolonged LHTL benefit.
The present study tested the hypothesis that LHTL (normobaric hypoxia) increases aerobic performance in a team of national-level swimmers. This question was addressed in relation to the erythropoietic changes associated with LHTL. Furthermore, we evaluated whether any positive effect of LHTL could last for up to 2 weeks after the intervention.
Methods
Subjects
The study was approved by the Necker Hospital Ethics Committee (Paris, France). After a complete medical examination and an echocardiography, written informed consent was obtained from the 18 subjects involved in the study. All the athletes were low altitude residents and were not acclimatized to altitude prior to the study.
Eighteen swimmers (2 women, 16 men) were recruited from the national team of the French swimming federation. Sixteen of the swimmers were middle-distance specialists and two of them were long-distance specialists. All athletes were involved in international swimming competitions during the year of this study. After the determination of maximal oxygen uptake \( (\ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} ) \) at the altitude of 1,200 m, the subjects were ranked and paired according to their \( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} . \) Then each pair \( {\text{(of similar}}\;\ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} ) \) was randomly assigned to two fitness-matched groups: a LHTL group (1 woman, 8 men, n=9), or a control group (1 woman, 8 men, n=9). The characteristics (mean ± SD) of the swimmers were: age 17±0.5 years, height 180±7 cm, weight 68±6 kg and \( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} \) 58.5±5.7 ml min−1 kg−1 (Control); and age 20±3 years, height 179±5 cm, weight 71±9 kg and \( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} \) 57.9±5.6 ml min−1 kg−1 (LHTL).
It has to be pointed out that our endurance-trained swimmers displayed relatively low basal \( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} . \) This particular feature of elite swimmers is classically reported in the literature (Cerretelli 2001). Furthermore, all the exercise procedures were performed at the altitude of 1,200 m, which is known to reduce sea-level \( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} \) by 5–6 % (Robergs et al. 1998) or more, particularly in athletes (Gore et al. 1996). Thus, it is not unreasonable to estimate a mean \( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} \) at sea level around 63–65 ml min−1 kg−1 in these subjects.
Experimental design
All parts of the experiment, i.e., testing procedures, training sessions and residence, were conducted at the same location, namely the National Center for Cross-country Ski, Prémanon, French Jura, located at an altitude of 1,200 m (barometric pressure, P B=674 mmHg). This choice was made to spare the athletes the trouble of traveling frequently between different places. For this reason, no measurements were performed at sea-level in this study. The successive phases of the experiment are shown in Fig. 1. After an initial 4-day testing period (PRE), all the swimmers underwent a 13-day period of supervised training, while sleeping at 1,200 m, either in ambient air (Control) or in hypoxic rooms (LHTL). Normobaric hypoxia in the rooms was obtained through an oxygen extraction system (OBS, Husøysund, Norway). For safety reasons, air composition (O2 and CO2) of each hypoxic room was continuously monitored by O2 and CO2 analyzers (OBS, Husøysund, Norway), as well as arterial oxygen saturation of each athlete was measured by finger pulse oximetry (NPB-290, Nellcor Puritan Bennett, Pleasanton, CA, USA). All these data were continuously transmitted to a central control unit in the presence of a medical doctor. The LHTL swimmers spent 16 h day−1 in hypoxia during 13 consecutive days, the first 5 days at simulated altitude of 2,500 m (inspired O2 fraction, FiO2=0.174), then the next 8 days at a simulated altitude of 3,000 m (FiO2=0.164). Within 1–2 days after return from LHTL, the subjects performed a first comparative testing session (POST-1). After having completed the 13-day training camp, all the athletes were allowed to reduce the training stimulus during the consecutive 14-day period of supervised training. Then they performed another comparative testing session during days 15–17 after return from the LHTL intervention (POST-15). The duration of 2 weeks between the end of LHTL and the delayed testing was chosen according to field observations and practical recommendations (Dick 1992), suggesting that a minimum of 2 weeks is required to achieve a subsequent peak in performance after altitude training.
Performance
All the swimming procedures were performed in a 25-m swimming pool with a water temperature of 26°C.
\( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} \)
\( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} \) was measured during an incremental swimming exercise to exhaustion, before (PRE), within 1–2 days (POST-1) and 2 weeks (POST-15) after LHTL intervention. The subjects performed five consecutive 200-m efforts of increasing velocity with a 15-s rest interval in between. The velocity of each swimming stage was determined from each swimmer’s personal best competition time over 200 m, measured in the preceding month. The first 200-m effort was set at the individual best competition time over 200 m plus 30 s. Then the subjects completed the three following 200-m stages, which were set at the best competition time plus 25, 20 and 15 s, respectively. The final 200 m was performed at the maximal velocity, with the subject being instructed to swim as fast as possible. The swimmer’s velocity was controlled with an Aquapacer Solo (Challenge and Response, Inverurie, UK) with the subject adjusting the velocity to auditory signals at 12.5 m intervals, delimited by visual marks along the bottom of the pool. The maximal O2 uptake—as well as the other gas exchange parameters—corresponded to the highest 30-s average value from the breath-by-breath data. The maximal velocity corresponded to the average velocity over the last 200-m swimming bout.
Gas exchange
Gas exchange during the incremental swimming trial was recorded through a K4b2 remote breath-by-breath system (Cosmed, Rome, Italy), which has already been confirmed as a reliable device for measuring expired gas measurements (McLaughlin et al. 2001). The device allowed the continuous determination of minute ventilation \( (\ifmmode\expandafter\dot\else\expandafter\.\fi{V}_{{\text{E}}} ,\;{\text{in l}}\,{\text{min}}^{{{\text{1}}}} {\text{),}} \) oxygen uptake \( (\ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{2} ,\;{\text{in ml}}\,{\text{min}}^{{{\text{1}}}} \,{\text{kg}}^{{{\text{1}}}} {\text{)}} \) and end-tidal CO2 partial pressure (P ETCO2, in mmHg). The K4b2 system was connected to a snorkel (Aquatrainer, Cosmed, Rome, Italy). The Aquatrainer device has been previously validated during exercise (Keskinen et al. 2003). The connection of the inlet and outlet tubes to the K4b2 turbine (50 ml) through a connecting unit (140 ml) allows inspiratory and expiratory gases to mix to a small extent at the beginning of both the expiration and inhalation. The distance between the snorkel mouthpiece and the K4b2 turbine unit was 128 cm and the volumes of both the outlet and inlet tubes were 825 ml. The dead space of the snorkel and valve system was 825 ml. This snorkel allowed the measure of the expired gas during swimming without significantly increasing the forward resistance. Due to the snorkel device, each subject was instructed to perform an “open turn”. In pilot experiments, it was established that the required time to complete this kind of turn, with the snorkel attached, was on average 1.6 s longer than for a standard “tumble turn”. The subjects were trained to swim with the snorkel prior to the tests.
Endurance performance
The subjects performed a 2,000-m freestyle swimming time trial, before (PRE) immediately (POST-1) and 2 weeks (POST-15) after LHTL intervention. Subjects were asked to complete this distance as fast as possible. They were informed of the elapsed distance but received no feedback on time. A 2,000-m trial was chosen because these swimmers were accustomed to perform this distance during their routine testing.
Intra-vascular compartments
Red blood cell volume, blood volume and plasma volume were determined by a carbon monoxide rebreathing method (Circulating Blood Volume System, CBV-Medical, Denmark) (Christensen et al. 1993; Poulsen et al. 1998). Briefly, after a 20-min resting period in a sitting position, the subjects breathed into a closed circuit, including a 2-l rebreathing bag, and a carbon dioxide absorber. Extra oxygen was administrated into the circuit to compensate for oxygen consumption. Rebreathing time was 10 min to ensure that maximal hemoglobin carbon monoxide saturation (COHb, in %) had been reached. In the present study, maximal COHb was ≤6%. From the athlete’s body weight and hemoglobin concentration, a software determined the volume of CO to be administrated, among a set of pre-calibrated syringes (44.42 or 49.34 ml). Before administration of CO and immediately after the completion of the rebreathing period, venous blood samples were collected in triplicate for the measurements of COHb and hemoglobin concentration ([Hb]) by spectrophotometry (OSM 3, Radiometer, Copenhagen, Denmark). Hematocrit (Hct) was measured in triplicate by a microcentrifuge. Total hemoglobin mass (nHb), red blood cell volume (V RBC), plasma volume (PV) and blood volume (BV) were then obtained by calculation (Christensen et al. 1993).
The determination of the intra-vascular compartments was performed before (PRE), and within 1–2 days after LHTL (POST-1). The measurements always took place in the morning, before any experimental intervention. The order among the subjects remained the same throughout the study.
Training
Rationale for the training program
According to the current trends in elite swimming (Avalos et al. 2003), our training program mainly involved aerobic work sessions below or at the onset of blood lactate accumulation (OBLA) corresponding to the 4-mmol l-1 lactate threshold, whereas the high-intensity interval work remains marginal. It is accepted today that high doses of aerobic training per se elicit an improvement in aerobic capacity in athletes (Avalos et al. 2003; Welde et al. 2003). The swimmers performed two high-training-load microcycles during the LHTL period (representative of the highest training stimulus over the annual training cycle among our athletes) in order to maximize the impact of LHTL on the adaptive responses. In an attempt to achieve any overcompensation and thus to show a prolonged effect of LHTL, the athletes were instructed to reduce their training load during return from altitude (Fig. 2). The reduction of the training stimulus after LHTL was chosen according to the findings of the only available report dealing with the prolonged LHTL effects, suggesting that such a training program may induce a persistent gain in performance (Levine and Stray-Gundersen 1997). We are aware neither of any report that re-examined the effect of the same training program, nor of studies in which different training procedures were tested.
Quantification of the training: the left panel depicts the training stimulus (expressed in arbitrary training units (T.U.), see Methods) and the right panel depicts the training time. Each point corresponded to an average over seven consecutive days. R-1 and R-2 are first and second periods of return to low altitude after LHTL, respectively. In the two groups (Control and LHTL), training stimulus, as well as training time, were lower during R-1 or R-2 than those monitored during the 13-day intervention period. Values are means ± SD
Quantification of the training stimulus
The quantification of the training stimulus, in terms of volume and intensity, was determined according to the method of Mujika et al. (1996). Briefly, before the experiments, all the athletes underwent a progressive swimming test until exhaustion (failure to follow the required pace) to determine blood lactate concentration. During the test, each swimmer performed 200-m swims (separated by 1 min of recovery) at a progressively increased percentage of his/her personal best time over 200 m, until exhaustion. Blood lactate concentration was determined from fingertip blood samples (Lactate Pro, Arkray, Japan) during the recovery periods separating the 200-m swims. According to the lactate concentrations measured during the test, the training load was then divided into five intensity levels (I, II, III, IV and V), corresponding to lactate concentrations of ~2, 4, 6, 10 and 16 mmol l−1, respectively. The final weighting coefficients for the five training intensity levels were 1, 2, 3, 5 and 8, respectively. Thus, the total weekly training stimulus (expressed in arbitrary training units, T.U.) was computed as the sum of the training time performed at each training intensity, multiplied by their respective weighting coefficients.
Training, which was performed at 1,200 m, was supervised by the coaches during the entire study. Each swimmer filled a daily training logbook that included duration/kilometers and the intensity of each training session. Heart rate was monitored during training with a heart rate monitor (S810, Polar, Kempele, Finland).
Iron intake
The swimmers did not take oral iron supplementation during the study. Iron intake was determined from the recording of the dietary intake using GENI software (MICRO6, Villers-lès-Nancy, France) with French (REGAL) and German (SOUCI) tables. The dietary intake was evaluated by the estimations of food and beverage intake. The subjects had to record in their notebook all food intake as precisely as possible. All these data were validated using a specific picture book for the estimation of quantities (SU.VI.MAX, Food portions, Polytechnica Editions, Paris, 1994; Le Moulenc et al. 1996).
Blood analyses
Resting venous blood samples were collected in the morning, while the subjects were supine and in an overnight fasted state. Measurements were performed during PRE and POST-15 periods, and also the morning after the last night of each altitude stage, i.e., at simulated altitude of 2,500 m and 3,000 m [within 15 min after the subject had left the hypoxic (LHTL) or normoxic (Control) room] (see Fig. 1). Hemoglobin concentration (Hb), hematocrit and percent reticulocytes were determined by a Pentra 120 analyzer (ABX, Montpellier, France). The serum samples were assayed for their erythropoietin (EPO) level by enzyme-linked immunosorbent assay (ELISA) using human EPO Quantikine IVD from R&D. Soluble transferrin receptor (sTfR) and ferritin levels were determined in sera respectively using the Nichols advantage Soluble transferrin receptor and the Nichols advantage Ferritin reagent cartridges (Nichols Institute Diagnostics, USA). Those tests were fully automated chemiluminescence immunoassay for the quantitative determination of sTfR and ferritin in serum, which were processed on the Nichols Advantage automate. The sTfR and ferritin concentrations were determined by reference to factory-made standard curves of ten known concentrations. Before each run, the calibration curve was readjusted for each cartridge and each test using a two-point recalibration. Each serial of samples was analyzed during the same run. Samples with high levels of sTfR or ferritin were re-analyzed after a manual dilution (1/4th) in a test specific sample diluter.
Statistics
All values are reported as arithmetic means ± SD. A Mann–Whitney U test was used to analyze the effect of the intervention (LHTL vs. control). The effect of time on the different parameters (two or more repeated measures) was evaluated in each group (LHTL or control) with the Friedman test, followed by a Wilcoxon test to determine the location of the significant differences. A Pearson product moment correlation test (r 2, coefficient of determination) was used to analyze the relationship between two quantitative variables. One LHTL swimmer suffered from shoulder pain during POST-1 measurements and, therefore, did not complete any of the two exercise tests. At the same time, gas analysis gave erroneous values for another subject during the incremental swimming test. The comparisons between PRE and POST-1 in the LHTL swimmers were done from n=8 for the 2,000-m test and n=7 for the gas exchange parameters during the \( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} \) test. Statistics were done with the Statview Version 5.0. (SAS Institute, Cary, NC). Differences were considered significant at P<0.05.
Results
Training
The training stimulus, as well as the training duration, were kept equivalent between the two groups, control and LHTL (Fig. 2). Figure 2 also shows that training stimulus/duration was reduced (P<0.05) between the LHTL intervention and the subsequent 2-week period. The mean training profile in the two groups of swimmers was: 85% of training at or below OBLA (intensity I and II), 13% of training above OBLA (mostly intensity III and IV) and 2% of speed/weight-lifting exercises.
Nocturnal oxygen saturation
Mean pulse oxygen saturation (SpO2) over five or eight nights (average over the whole night) in the LHTL group was 92±1% over five nights at simulated altitude of 2,500 m and 92±1% over 8 nights at 3,000 m. Mean SpO2 in the control group was 96±1%.
Sleep disturbance—which was assessed from daily questionnaires—was not different between the control and LHTL groups (results not shown).
Blood analyses
Blood analyses are summarized in Table 1. Hb and Hct remained unchanged both in LHTL and control swimmers. Reticulocytes did not change among LHTL swimmers, but decreased transiently among controls. Serum EPO was transiently depressed during the training camp in both groups, whereas sTfR significantly increased in the two groups. Finally, serum Ferritin was not modified throughout the study.
Intravascular volumes
Immediately after LHTL intervention, LHTL swimmers experienced an increase (P<0.05) in total hemoglobin mass (Table 2) and in red blood cell volume (from 2.23±0.41 l in PRE to 2.42±0.38 l in POST-1) (Fig. 3). By contrast, neither total hemoglobin mass nor red blood cell volume were modified among control swimmers, red blood cell volume being 2.15±0.32 l in PRE and 2.15±0.29 l in POST-1. In POST-1, red blood cell volume and total hemoglobin mass tended to be higher in LHTL than in control swimmers (P=0.08). Plasma volume and blood volume were altered neither by training, nor by LHTL (Table 2).
Iron intake
Iron intake remained similar between the two groups over the time of the study (Table 3). Total iron intake did not differ significantly between the two groups.
Performance
The change in \( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} \) from PRE to POST-1 among the LHTL swimmers (+8.1%, n=7) did not reach significance (P=0.09). The corresponding change in \( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} \) among the control swimmers (+2.5%) was insignificant (P=0.21) (Table 4; Fig. 4). In POST-1, the level of \( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} \) was found similar between LHTL and control subjects (P=0.42). Two weeks after LHTL intervention (POST-15), \( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} \) was similar to PRE values in the two groups. Ventilation at maximal exercise significantly increased from PRE to POST-1 among LHTL swimmers, whereas it stayed unchanged in control swimmers. In POST-15, this variable was not different from PRE values, both in LHTL and control subjects (Table 4). The performance during the 2,000-m swimming trial was slightly ameliorated among the Control swimmers, both during POST-1 and POST-15 testing, whereas it remained unchanged in the LHTL swimmers throughout the study (Table 4; Fig. 5).
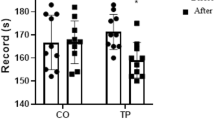
Individual values of maximal oxygen uptake \( (\ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} ), \) in the control (left panel, n=9) and the LHTL subjects (right panel, n=9), before (PRE), 1–2 days after (POST-1) and 2 weeks (POST-15) after a 13-day period of LHTL. Subjects #1–9 were controls; subjects #10–18 were LHTL (see Fig. 3). LHTL sample was n=7 in POST-1. ‡ P=0.09 vs. PRE
Individual values of performance time over a 2,000-m swimming trial, in the control (left panel, n=9) and the LHTL subjects (right panel, n=9), before (PRE), 1–2 days after (POST-1) and 2 weeks (POST-15) after a 13-day period of LHTL. Subjects #1–9 were controls; subjects #10–18 were LHTL (see Fig. 3). LHTL sample was n=8 in POST-1. * P<0.05 vs. PRE
Discussion
The present study demonstrated that 13 days of LHTL were sufficient to elicit an increase in red blood cell volume in highly-trained swimmers. However, this did not involve a concurrent clear improvement in aerobic performance. Furthermore, we failed to detect a persistent effect of LHTL, since neither the level of aerobic performance, nor the range of the measured hematological variables differed from the basal values 2 weeks after the end of the LHTL intervention.
Short-term efficacy of LHTL
Erythropoiesis
The present study indicated that even a short period of LHTL may positively affect red blood cell volume among highly-trained swimmers, thus reflecting an effective erythropoietic stimulation. With the present CO rebreathing method, we were able to obtain a rather low individual variation among our subjects, as suggested by the small variations in the standard deviation (from PRE to POST-1) of the total hemoglobin mass (see Table 2) and the red blood cell volume (see Results). We also acknowledge that large changes in red blood cell volume occurred in two subjects of the control group (#1 and #3) throughout the experimental period, raising the question of a possible methodological error due to the rebreathing technique. We cannot totally exclude the possibility of a leak during the rebreathing period at some point in these subjects, leading to a loss of CO and, therefore an overestimation of their total hemoglobin mass. However, during each measurement, we paid strict attention to avoid any leak or dead space during CO administration and rebreathing. On the other hand, some physiological processes—e.g., hemolysis—could also have accounted for the exaggerated decrease in red blood cell volume observed in these athletes. Nevertheless, if these two subjects are excluded from the statistical analysis, the changes in red blood cell volume among control subjects (n=7) still remain nonsignificant. The higher red blood cell volume observed after LHTL in our swimmers was in agreement with a previous report that demonstrated a 9% increase in red blood cell volume after 28 nights at 2,500 m (Levine and Stray-Gundersen 1997), but differed from other works, showing an absence of change in red blood cell volume after 12 nights at 2,650 m (Ashenden et al. 1999a), 23 nights at 3,000 m (Ashenden et al. 1999b), or 14 nights at 1,956 m (Dehnert et al. 2002). Among the reasons accounting for these conflicting results in the literature, the daily exposure to hypoxia appears to be a critical factor; whereas an exposure of 8–10 h day−1 did not enhance red blood cell volume, independent of the total number of consecutive nights (Ashenden et al. 1999a, b) longer exposure (16–20 h day−1) was associated with a rise in red blood cell volume (Levine 2002). Finally, in spite of a daily exposure to hypoxia of 13 h, Dehnert et al. (2002) did not find an increase in red blood cell volume, probably because the hypoxic stimulus used in that study (1,956 m) was around the threshold required to stimulate erythropoiesis (Ge et al. 2002). Thus, the present study, showing that a 13-day period with a daily exposure to hypoxia of 16 h led to a detectable rise in red blood cell volume, further suggests that, beside other factors (e.g., the level of altitude, the total time spent in hypoxia, or the training), the daily time spent at rest in hypoxia may be a key factor for an efficient stimulation of erythropoiesis. Surprisingly, the hematological parameters measured among our athletes did not clearly depict an accelerated erythropoiesis with LHTL: (1) EPO response was somewhat blunted during the intervention period, even among the LHTL swimmers. This observation could be explained by the well-known downregulation of EPO production during sustained hypoxia (Eckardt et al. 1990), which may have masked a transient increase in EPO following each altitude step; (2) sTfR increased similarly in the control and LHTL swimmers after the training camp (~11%), whereas sTfR (also reflecting erythropoiesis) is known to be up-regulated during sustained hypoxia (Robach et al. 2004; Stray-Gundersen et al. 2001). On one hand, the lack of a LHTL-induced effect on sTfR could be related to our control condition, which involved a concurrent period of training and residence at 1,200 m. Indeed, our control subjects, although exposed to a very mild hypoxic level at 1,200 m during 13 days, could have also experienced—to some extent—a stimulation of erythropoiesis. On the other hand, we acknowledge that the present sTfR response among our LHTL swimmers differed from that previously observed (with the same technique) after a quite similar LHTL procedure in cross-country skiers, i.e., higher sTfR levels in LHTL than in control athletes (Robach et al. 2002). Although the cause of this discrepancy remains unknown, this highlights the possible variability of the sTfR response between collectives of athletes and further suggests that sTfR does not systematically reflect accelerated erythropoiesis during moderate altitude exposure (2,500–3,000 m).
Maximal oxygen uptake
In spite of the increase in red blood cell volume, maximal O2 uptake did not rise immediately after intervention in LHTL swimmers. That the +8.1% change in mean \( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} \) after LHTL (or +5.6% net difference vs. Control) did not reach significance (P=0.09) could be explained by the small study sample, which made the present study prone to a Type II statistical error. However, although an increased red blood cell volume is known to improve the oxygen-carrying capacity of the blood and therefore, maximal oxygen transport (Kanstrup and Ekblom 1984), the insignificant variation seen in \( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} \) after LHTL was not in favor of a consistent enhancement in maximal aerobic power related to an elevated red blood cell volume. Supporting this, we found no correlation between the changes in red blood cell volume and the concurrent changes in \( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} \) (result not shown). A possible mechanism underlying the lack of an increase in \( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} , \) in spite of an elevated red blood cell volume, would refer to the potential local changes in skeletal muscle associated with LHTL. Indeed, LHTL was previously found to depress \( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} \) (Gore et al. 2001), likely because of some disadvantageous effects of hypoxia on muscle function (Green et al. 2000; Aughey et al. 2005). Thus, it is not unreasonable to speculate that in our study, the advantageous increase in red blood cell volume could have been offset by some hypoxia-induced disturbances in muscle function, so that maximal aerobic performance was unchanged after LHTL.
2,000-m swimming time trial
Despite our precaution to propose a field endurance trial the swimmers were accustomed to, a large variability could be observed during the 2,000-m swimming trial, particularly among the LHTL subjects. Thus, the question of the reliability of this field trial may be raised. However, our data, showing that the coefficient of variation remained similar from PRE to POST-1 within a given group (being respectively 2.2 and 3.0% in Controls and 5.5 and 5.7% in LHLT), indicated a rather good reproducibility of the 2,000-m trial. The main result was that endurance performance over 2,000 m was not ameliorated among LHTL swimmers. This finding hardly involved any negative effect of the training, since the concurrent Control swimmers experienced a small increase in 2,000-m performance (+1.6%) at that time, thus showing the adequacy of the training stimulus. This finding differs from previous data, showing that 5,000-m running time (~16-min duration) was enhanced after LHTL, along with the improvement of \( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} \) (Levine and Stray-Gundersen 1997). Even if the present 2,000-m swimming trial (~24 min) undoubtedly involved a lower fraction of \( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} \) utilization, our trial remained strongly dependent on oxygen transport. Thus, the unchanged 2,000-m performance after LHTL could probably be explained because maximal oxygen transport was not modified with LHTL.
Longlasting effects of LHTL
The second finding of the present study was a failure to observe an improvement in aerobic performance 2 weeks after LHTL intervention. At that time, the measured hematological parameters were similar to pre-intervention values. The question, therefore, arises, what factors may account for the absence of any prolonged/delayed benefit after LHTL?
One component that may affect the efficacy of LHTL is the adequacy of training.
Here, the small improvement in performance (velocity at maximal exercise and 2,000-m performance time) seen in the control group from PRE to POST-2 suggests that the present training profile did not induce detraining. Therefore, the lack of any beneficial effect of LHTL on aerobic performance would not be linked to a negative effect of the training. Nevertheless, we failed to show an additional effect of hypoxic residence on the training response, when measured after a 2-week period of deacclimatization.
Another component that may affect the efficacy of LHTL is the robustness of acclimatization. Our data, showing no change in hematological parameters 2 week after LHTL, suggest that the actual direct effects of hematological acclimatization had disappeared at that time. Although we did not measure red blood cell volume in swimmers 2 weeks after intervention, we speculate, from separate data showing normal red blood cell volume values 2 weeks after LHTL in cross-country skiers (Robach et al. 2002) and in runners (Brugniaux et al. 2004), that red blood cell volume was also within the basal range among our swimmers at that time. A first explanation could be the duration of our protocol (13 days), which was perhaps not sufficient to induce durable hematological changes. Alternatively, it is not unconceivable that the phenomenon of neocytolysis (rapid and selective degradation of the newly formed red cells) may have accelerated deacclimatization during early return to sea level (Rice et al. 2001). If so, we further speculate that any persistent hematological change after LHTL would be difficult to demonstrate, independently of LHTL duration.
Conclusion
The present study indicates that LHTL had a positive short-term effect on red blood cell volume in athletes. However, this hematological response was not associated with a clear concomitant improvement in aerobic performance. We cannot exclude that another central or peripheral mechanism may have counterbalanced the advantageous rise in red blood cell volume. Secondly, we failed to observe any elevation in aerobic performance 2 weeks after the LHTL intervention. The present results, therefore, suggest that 13 days of LHTL neither adversely affect aerobic performance in highly-trained swimmers, nor confer a big advantage for athletic performance. Nevertheless, we acknowledge that the present study involved small groups of athletes and methods which have inherent errors. Thus, we cannot exclude that LHTL actually induces a small non-detectable advantage which may be relevant for elite sport, where the edge that is being looked for to win is thin.
References
Ashenden MJ, Gore CJ, Martin DT, Dobson GP, Hahn AG (1999a) Effects of a 12-day “live high, train low” camp on reticulocyte production and haemoglobin mass in elite female road cyclists. Eur J Appl Physiol 80:472–478
Ashenden MJ, Gore CJ, Dobson GP, Hahn AG (1999b) “Live high, train low” does not change the total haemoglobin mass of male endurance athletes sleeping at a simulated altitude of 3000 m for 23 nights. Eur J Appl Physiol 80:479–484
Aughey RJ, Gore CJ, Hahn AG, Garnham AP, Clark SA, Petersen AC, Roberts AD, McKenna MJ (2005) Chronic intermittent hypoxia and incremental cycling exercise independently depress muscle in vitro maximal Na+–K+–ATPase activity in well-trained athletes. J Appl Physiol 98:186–192
Avalos M, Hellard P, Chatard JC (2003) Modeling the training-performance relationship using a mixed model in elite swimmers. Med Sci Sports Exerc 35:838–846
Brugniaux JV, Robach P, Schmitt L, Nicolet G, Fouillot JP, Olsen NV, Richalet JP (2004) Living high-training low: effect on red cell mass and aerobic performance in elite middle-distance runners (Abstract). High Alt Med Biol 5:204
Cerretelli P (2001) Fisiologia dell’esercizio: sport, ambiente, età, sesso, 2nd edn. Società Editrice Universo, Roma
Christensen P, Eriksen B, Henneberg SW (1993) Precision of a new bedside method for estimation of the circulating blood volume. Acta Anaesthesiol Scand 37:622–627
Clark SA, Aughey RJ, Gore CJ, Hahn AG, Townsend NE, Kinsman TA, Chow CM, McKenna MJ, Hawley JA (2004) Effects of live high, train low hypoxic exposure on lactate metabolism in trained humans. J Appl Physiol 96:517–525
Dehnert C, Hutler M, Liu Y, Menold E, Netzer C, Schick R, Kubanek B, Lehmann M, Boning D, Steinacker JM (2002) Erythropoiesis and performance after two weeks of living high and training low in well trained triathletes. Int J Sports Med 23:561–566
Dick FW (1992) Training at altitude in practice. Int J Sports Med 13:S203–S206
Eckardt KU, Dittmer J, Neumann R, Bauer C, Kurtz A (1990) Decline of erythropoietin formation at continuous hypoxia is not due to feedback inhibition. Am J Physiol Renal Physiol 258:F1432–F1437
Ge RL, Witkowski S, Zhang Y, Alfrey C, Sivieri M, Karlsen T, Resaland GK, Harber M, Stray-Gundersen J, Levine BD (2002) Determinants of erythropoietin release in response to short-term hypobaric hypoxia. J Appl Physiol 92:2361–2367
Gore CJ, Hahn AG, Scroop GC, Watson DB, Norton KI, Wood RJ, Campbell DP, Emonson DL (1996) Increased arterial desaturation in trained cyclists during maximal exercise at 580 m altitude. J Appl Physiol 80:2204–2210
Gore CJ, Hahn A, Rice A, Bourdon P, Lawrence S, Walsh C, Stanef T, Barnes P, Parisotto R, Martin D, Pyne D (1998) Altitude training at 2690 m does not increase total haemoglobin mass or sea level \( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} \) in world champion track cyclists. J Sci Med Sport 1:156–170
Gore CJ, Hahn AG, Aughey RJ, Martin DT, Ashenden MJ, Clark SA, Garnham AP, Roberts AD, Slater GJ, McKenna MJ (2001) Live high: train low increases muscle buffer capacity and submaximal cycling efficiency. Acta Physiol Scand 173:275–286
Green H, Roy B, Grant S, Burnett M, Tupling R, Otto C, Pipe A, McKenzie D (2000) Downregulation in muscle Na+–K+–ATPase following a 21-day expedition to 6,194 m. J Appl Physiol 88:634–640
Hahn AG, Gore CJ (2001) The effect of altitude on cycling performance: a challenge to traditional concepts. Sports Med 31:533–557
Kanstrup IL, Ekblom B (1984) Blood volume and hemoglobin concentration as determinants of maximal aerobic power. Med Sci Sports Exerc 16:256–262
Keskinen KL, Rodriguez FA, Keskinen OP (2003) Respiratory snorkel and valve system for breath-by-breath gas analysis in swimming. Scand J Med Sci Sports 13:322–329
Klausen K, Robinson S, Micahel ED, Myhre LG (1966) Effect of high altitude on maximal working capacity. J Appl Physiol 21:1191–1194
Le Moulenc N, Deheeger M, Preziosi P, Monterio P, Valeix P, Rolland-Cachera M.F, Potier de Gourcy G, Christides JP, Galan P, Hercberg S (1996) Validation of the picture book used for the SU.VI.MAX food survey [in French]. Cah Nutr Diet 3:158–164
Levine B, Stray-Gundersen J, Duhaime G, Snell P, Friedman D (1991) “Living high-training low”: the effect of altitude acclimatization/normoxic training in trained runners (Abstract). Med Sci Sports Exerc 23:S25
Levine BD, Stray-Gundersen J (1997) “Living high–training low”: effect of moderate-altitude acclimatization with low-altitude training on performance. J Appl Physiol 83:102–112
Levine BD (2002) Intermittent hypoxic training: fact and fancy. High Alt Med Biol 3:177–193
McLaughlin JE, King GA, Howley ET, Bassett DR Jr, Ainsworth BE (2001) Validation of the Cosmed K4b2 portable metabolic system. Int J Sports Med 22:280–284
Mujika I, Busso T, Lacoste L, Barale F, Geyssant A, Chatard JC (1996) Modeled responses to training and taper in competitive swimmers. Med Sci Sports Exerc 28:251–258
Poulsen TD, Klausen T, Richalet JP, Kanstrup IL, Fogh-Andersen N, Olsen NV (1998) Plasma volume in acute hypoxia: comparison of a carbon monoxide rebreathing method and dye dilution with Evans’ Blue. Eur J Appl Physiol 77:457–461
Rice L, Ruiz W, Driscoll T, Whitley CE, Tapia R, Hachey DL, Gonzales GF, Alfrey CP (2001) Neocytolysis on descent from altitude: a newly recognized mechanism for the control of red cell mass. Ann Intern Med 134:652–656
Robach P, Fulla Y, Westerterp KR, Richalet JP (2004) Comparative response of EPO and soluble transferrin receptor at high altitude. Med Sci Sports Exerc 36:1493–1498
Robach P, Schmitt L, Brugniaux J, Nicolet G, Duvallet A, Fouillot JP, Pialoux V, Moutereau S, Lasne F, Olsen NV, Richalet JP (2002) Living high–training low: effect on erythropoiesis and aerobic performance in highly trained cross-country skiers (Abstract). High Alt Med Biol 3:430
Robergs RA, Quintana R, Parker DL, Frankel CC (1998) Multiple variables explain the variability in the decrement in \( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} \) during acute hypobaric hypoxia. Med Sci Sports Exerc 30:869–879
Roberts AD, Clark SA, Townsend NE, Anderson ME, Gore CJ, Hahn AG (2003) Changes in performance, maximal oxygen uptake and maximal accumulated oxygen deficit after 5, 10 and 15 days of live high: train low altitude exposure. Eur J Appl Physiol 88:390–395
Stray-Gundersen J, Chapman RF, Levine BD (2001) “Living high–training low” altitude training improves sea level performance in male and female elite runners. J Appl Physiol 91:1113–1120
Welde B, Evertsen F, Von Heimburg E, Medbo JI (2003) Energy cost of free technique and classical cross-country skiing at racing speeds. Med Sci Sports Exerc 35:818–825
Acknowledgments
This study was supported by grants from the International Olympic Committee and the French Ministry of Sports. The authors wish to thank the eighteen swimmers for their participation in this study. The skillful assistance of Patrick Bouchet for software development is gratefully acknowledged. We also would like to thank Dr. Poul Christensen for allowing us to use his program and CO-rebreathing device for the determination of intravascular compartments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Robach, P., Schmitt, L., Brugniaux, J.V. et al. Living high–training low: effect on erythropoiesis and aerobic performance in highly-trained swimmers. Eur J Appl Physiol 96, 423–433 (2006). https://doi.org/10.1007/s00421-005-0089-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-005-0089-1