Abstract
Purpose
To examine the association between elevated blood cadmium (Cd) and lead (Pb) concentrations and increased risk of gestational diabetes mellitus (GDM).
Methods
This cross-sectional study included pregnant women (n = 16,955) enrolled in the Japan Environment and Children’s Study. Concentrations of Cd and Pb in blood samples collected at 22–28 weeks’ gestation were measured by inductively coupled plasma mass spectrometry. GDM was diagnosed according to the 2011 Japan Society of Obstetrics and Gynecology and Japan Association of Obstetricians and Gynecologists criteria. Adjusted odds ratios (OR) and 95% confidence intervals (CI) were estimated using logistic regression analysis.
Results
Blood Cd and Pb concentrations were slightly higher among women with GDM than among those without GDM; however, these differences were not statistically significant. Elevated blood Cd and Pb concentrations were not associated with increased GDM risk in the nulliparous group (Cd OR 0.76; 95% CI 0.28–2.08 for high vs low category; Pb OR 2.51; 95% CI 0.72–8.72) or the parous group (Cd OR 0.64; 95% CI 0.29–1.44; Pb OR 0.31; 95% CI 0.04–2.29).
Conclusions
This study demonstrates that Cd and Pb exposure, in the range of blood levels observed, has no significant relationship with the development of GDM. Further prospective studies would be valuable to confirm these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gestational diabetes mellitus (GDM) occurs when resistance to circulating insulin leads to hyperglycemia, with this impaired glucose metabolism being first detected during pregnancy. The estimated incidence of GDM [defined according to the Japan Diabetes Society (JDS) diagnostic criteria] is 7–14% (Morikawa et al. 2010; Kodama et al. 2013). GDM increases the lifetime risk of developing type 2 diabetes mellitus (DM), obesity, and metabolic syndrome for both the mothers and their children (Buchanan et al. 2012; Yessoufou and Moutairou 2011). Several key risk factors for GDM have been identified, primarily obesity, family history of type 2 DM, GDM history, and older maternal age (JSDP, 2015); however, little is known about the environmental risk factors.
It has been suggested that environmental contaminants can affect processes that are related to type 2 DM (Hectors et al. 2011). Toxic metals, such as cadmium (Cd), lead (Pb), mercury (Hg), and inorganic arsenic (i-As), can damage cellular components as a result of elevated levels of oxidative stress (Jomova and Valko 2011; Chen et al. 2009). During long-term exposure, Cd initially accumulates in the kidney, liver, and, to a lesser extent, the pancreas. Animal studies show that Cd can induce hyperglycemia and pancreatic toxicity (Edwards and Prozialeck 2009), and that increased Cd concentrations in the pancreas are related to a reduction in serum insulin (Edwards and Prozialeck 2009). This is thought to be the consequence of beta-cell dysfunction or death as a result of increased oxidative stress (Chang et al. 2013). Pb exposure induces hyperglycemia and glucose intolerance in rat models and may stimulate hepatic gluconeogenic gene expression (Tyrrell et al. 2017), thereby potentially increasing the risk of developing DM. Therefore, further evaluation is required to understand the relevance of dose levels and the mechanism of action in the context of human exposure.
Several studies have suggested a possible association between Cd or Pb and DM (Schwartz et al. 2003; Afridi et al. 2008; Edwards and Prozialeck 2009; Nie et al. 2016; Li et al. 2017). However, a number of other studies have shown no association between DM and Cd and Pb exposure, measured in blood and urine samples (Moon 2013; Barregard et al. 2013; Kuo et al. 2013; Borné et al. 2014). Therefore, evidence of the relationship between Cd and Pb exposure and DM is not consistent. In addition, exposure to these heavy metals during pregnancy may affect the sensitive hormonal activity during gestation and eventually increase the risk of GDM by eliciting a shift towards DM, i.e., the diabetogenic environment of pregnancy (Ma and Chan 2009). However, extensive review of the literature shows that despite GDM being a major potential complication of pregnancy that is associated with significant adverse effects, such as DM and metabolic syndrome in both mothers and infants, few studies have investigated the association between Cd or Pb exposure and GDM risk. In this study, we have conducted a cross-sectional analysis to evaluate the association between elevated blood Cd and Pb concentrations and increased levels of GDM.
Methods
Study population
This cross-sectional study included pregnant women enrolled in the Japan Environment and Children’s Study (JECS). Details of the prospective cohort have been published previously (Michikawa et al. 2018). Briefly, 103,099 pregnant women from 15 Regional Centers throughout Japan who were enrolled in the JCES from January 2011 to March 2014 participated in the study. During their pregnancy, all participants underwent three medical record transcriptions, two self-administered questionnaire surveys, and maternal blood sampling. Participants provided written consent to participate in the JECS after being informed of the purpose of the study and the associated procedures. All study protocols were approved by the ethics committees of the Japan Ministry of the Environment, National Institute for Environmental Studies, and the universities and hospitals in the relevant Regional Centers.
Measurement of blood Cd and Pb concentrations
Maternal blood samples were collected from 22 to 28 (27.5 ± 3.2) weeks of gestation and analyzed for Cd and Pb concentrations. Whole blood was collected in a Vacutainer containing an anti-coagulant; 1.5 mL was separated into 2 mL cryogenic biobanking tubes (Greiner Bio-One international GmbH, Kremsmünster, Austria) and stored at − 80 °C until analysis.
After thawing the frozen blood sample at 5 °C, a 200-µL aliquot was diluted 1:19 (v/v) with the dilution solution (2% v/v butan-1-ol, 0.1% tetramethylammonium hydroxide, 0.5 g/L polyoxyethylene (10) octylphenyl ether, and 0.5 g/L sodium ethylenediaminetetraacetic acid) before analysis. The diluted samples were analyzed by inductively coupled plasma mass spectrometry (ICP-MS; Agilent 7700, Agilent Technologies Inc., Tokyo, Japan). Cd and Pb concentrations were calculated using external calibration with indium and thallium, respectively, as the internal standard. The method detection limits for blood Cd and Pb concentrations were 0.0234 and 0.129 ng/g, respectively.
Diagnosis of GDM
Information on the diagnosis of GDM was collected from hospital medical records. Pregnant women were diagnosed with GDM if the results of a 75 g, 2 h oral glucose tolerance test (OGTT) exceeded the 2011 Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) criteria: fasting ≥ 92 mg/dL (5.1 mmol/L); 1 h ≥ 180 mg/dL (10.0 mmol/L); and 2 h ≥ 153 mg/dL (8.5 mmol/L) (Minakami et al. 2011). For the OGTT, subjects were required to fast from midnight on the day before the test was conducted.
Data collection and setting
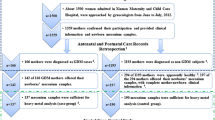
This study used two JECS datasets: (1) jecs-ag-20160424, which was released in June 2016 and revised in October for health outcomes; and (2) jecs-mtl-ai-20170403 for metal measurements. The jecs-ag-20160424 dataset consisted of 103,099 pregnancies. After excluding those with multiple pregnancies (n = 991) and withdrawals (n = 29), 102,079 women were included in the dataset. The jecs-mtl-ai-20170403 dataset consisted of Cd and Pb concentrations in maternal whole blood (n = 18,169), which were randomly selected from the blood samples of all participants at 22–28 weeks of gestation. Of the participants whose blood Cd and Pb data were obtained, 17,718 had complete medical records.
We conducted a cross-sectional study within the JECS, which included 17,718 pregnant women; those who had independent glucose intolerance factors were excluded for the following reasons: 22 had a history of type 1 DM, 47 had received insulin treatment, and 6 had used hypoglycemic agents during pregnancy. A total of 553 women had used steroids during pregnancy and were, therefore, excluded; steroid use is a known independent glucose intolerance factor aside from Cd or Pb exposure (Hwang and Weiss 2014). Given that GDM was defined as glucose intolerance with onset or first diagnosis during pregnancy, and GDM does not include overt DM in pregnancy and pregestational DM, 204 women with overt type 2 DM during pregnancy were excluded. After exclusions, the subjects included in the analysis comprised 16,955 pregnant women (Fig. 1): 339 with GDM and 16,616 without GDM (Table 1).
Statistical analysis
Statistical analyses were performed using JMP 13.0.0 software (SAS Institute Japan Ltd, Tokyo, Japan). We assessed the association between blood Cd and Pb concentrations and GDM by logistic regression analysis with a forward stepwise variable selection (Pin = 0.2 and Pout = 0.2) and results were verified using a forced-entry method. Our models were fitted using subjects with no missing data (complete case analysis). The maternal whole blood Cd or Pb concentration and Cd and Pb were independent variables, and Cd and Pb concentrations (categorized as 0.50 ng/g or 5 ng/g) were entered in the regression models. The medical diagnosis of GDM was used as the dependent variable. Characteristics of the subjects were examined using Pearson’s Chi square, Fisher’s exact, or Wilcoxon rank-sum tests, as appropriate (Table 1).
Covariates included in the multiple regression models were determined on the basis of the following factors: maternal age at birth (years) and pre-pregnancy body mass index (BMI < 18.5, 18.5–24.9, or ≥ 25 kg/m2), which were included as independent variables in all models as they are fundamental biological attributes. Moreover, other maternal physical and lifestyle factors were included in the model if the factor was significantly associated with the dependent variables in the bivariate analyses (p < 0.001). These factors included history of GDM (yes/no), pregnancy-induced hypertension (yes/no) (Bryson et al. 2003), carbohydrate intake (g/kcal/day) (Schulze et al. 2004; Villegas et al. 2007) as a continuous variable, alcohol consumption during pregnancy (yes/no) (Ettinger et al. 2014), and dyslipidemia (yes/no) (Nie et al. 2016). Dyslipidemia was defined as triglyceride levels > 250 mg/dL or high-density lipoprotein < 35 mg/dL (JDS, 2013). The evaluation of the association between dependent variables and history of GDM was performed in parous groups. In addition, we considered the effect factor of Cd intestinal absorption as a covariate, along with daily calcium (mg/day) and zinc (mg/day) intake as a continuous variable, hypochromic anemia (yes/no), and administration of iron (Horiguchi et al. 2004; Kippler et al. 2009). Given that tobacco smoking is one of the potential sources of Cd exposure in the general Japanese population (Watanabe et al. 1983), we considered smoking status as self-reported smoking history (current smokers/former smokers/nonsmokers) and pack-years (py). Smoking pack-years were calculated by multiplying the number of packs of cigarettes smoked per day by the number of years the person has smoked, assuming 20 cigarettes per pack. Subjects were then categorized as follows: never smokers (< 5 py), smoker with 5–9 py, smoker with 10–19 py, or smoker with ≥ 20 py (Adams et al. 2011).
Subjects for the logistic regression analysis were categorized into nulliparous and parous groups, and logistic regression models were adjusted for the following covariates: maternal age at birth, pre-pregnancy BMI, history of GDM (parous group only), pregnancy-induced hypertension, and smoking pack-years. For the nulliparous group analysis, we chose not to include history of GDM as a covariate in this model.
Results
Characteristics of the study subjects according to GDM status
Of the subjects included in the analysis (n = 16,955), 339 women (2.0%) were diagnosed with GDM (Table 1). Subjects with GDM were older (33.2 ± 5.0 vs 31.1 ± 5.0 years) and had a higher pre-pregnancy BMI (23.2 ± 5.0 vs 21.2 ± 3.2 kg/m2) than those without GDM (p < 0.001 for each). Subjects with GDM were more likely to have a history of GDM (4.7% vs 0.39%, p < 0.001) and to be diagnosed with pregnancy-induced hypertension (5.3% vs 2.1%, p < 0.001). In addition, subjects with GDM had a greater number of smoking pack-years compared with the non-GDM group (data for ≥ 20, 10–19, or 5–9 py were 2.5% vs 0.82%, 7.0% vs 5.7%, and 15.9% vs 11.6%, respectively; p < 0.001). There were no significant differences between women with/without GDM in terms of parity (p = 1.000), daily carbohydrate intake per energy (p = 0.072), alcohol consumption (p = 0.528), dyslipidemia (p = 0.032), daily calcium intake (p = 0.194), daily zinc intake (p = 0.114), hypochromic anemia (p = 0.492), administration of iron (p = 0.245), smoking history (p = 0.509), or gestational week during blood collection (p = 0.259, Table 1). Missing values were included in the analyzed subjects (n = 879, 5%).
Maternal blood Cd and Pb concentrations
The geometric mean (GM) concentrations of Cd and Pb in maternal blood were 0.677 (range 0.0951–4.73) and 6.05 (range 1.50–70.9) ng/g, respectively. Among subjects for whom both Cd and Pb data were available (n = 16,955), blood Cd and Pb concentrations were significantly correlated (Pearson’s test r = 0.241; p < 0.0001). The GM of Cd concentration in maternal blood was slightly higher among women with GDM than those without GDM (0.697 ng/g [geometric standard deviation (GSD), 1.57] versus 0.677 ng/g (1.58, GSD), respectively Table 1), although the difference did not reach statistical significance (p = 0.257). Similarly, the GM concentration of Pb in maternal blood (6.13, GSD 1.45 ng/g) was slightly higher in women with GDM than in those without GDM (6.05, GSD 1.42 ng/g; Table 1), although this difference was not statistically significant (p = 0.703).
Association between maternal blood Cd and Pb concentrations and GDM
Maternal blood Cd and Pb concentrations were not selected in logistic models with stepwise variable selection. Table 2 shows adjusted odd ratios (OR) and 95% confidence intervals (95% CI) for GDM according to Cd and Pb concentrations in maternal whole blood according to models using a forced-entry method in the nulliparous and parous groups. In the models adjusted for maternal age, pre-pregnancy BMI, history of GDM (parous group only), pregnancy-induced hypertension, and smoking pack-years, we found no evidence of an association between blood Cd and Pb concentrations and GDM in the nulliparous group (Cd OR 0.76, 95% CI 0.28–2.08 for high vs low category, p = 0.599; Pb OR 2.51, 95% CI 0.72–8.72, p = 0.149), or in the parous group (Cd OR 0.64, 95% CI 0.29–1.44, p = 0.286; Pb OR 0.31, 95% CI 0.04–2.29, p = 0.252; Table 2). These results were constant for individual Pb or Cd models and for the combined model for both Pb and Cd. Table 3 shows one such example, including covariates in the logistic model.
Discussion
To our knowledge, this is the first population-level survey to investigate the association between Cd and Pb exposure and the incidence of GDM in pregnant women. Assessment of blood Cd and Pb concentrations and GDM-related outcomes showed no correlation in this study population.
Development of GDM or type 2 DM occurs as a result of an interaction between environmental exposure, lifestyle, and genetic factors. Impaired insulin secretion and enhanced insulin resistance have adverse effects in animal models (Chang et al. 2013; Tyrrell et al. 2017). While a thorough literature search revealed no prior epidemiological studies of the association between Cd and GDM, a small number of epidemiological studies have suggested that Cd is associated with type 2 DM (Schwartz et al. 2003; Afridi et al. 2008; Nie et al. 2016; Li et al. 2017). Most of these were cross-sectional studies (Schwartz et al. 2003; Afridi et al. 2008; Nie et al. 2016; Kolachi et al. 2011) or meta-analyses (Li et al. 2017). Despite the lack of prospective studies, previous data suggest a potential association between increasing Cd exposure and a greater risk of type 2 DM. Li et al. (2017) conducted a meta-analysis of data from nine studies, which included 28,691 participants from a variety of populations; the results of the meta-analysis suggest that men and women with type 2 DM have significantly higher concentrations of urinary Cd. In a subgroup analysis, the OR were 1.02 (95% CI 1.00–1.05; I2 = 0.9%) for studies conducted in Asia and 1.11 (95% CI 0.88–1.41; I2 = 86.3%) for studies conducted in USA. Dose-dependent increases in DM risk have also been observed with elevations in urinary Cd (Schwartz et al. 2003). Nie et al. (2016) reported that the likelihood of prediabetes (defined as fasting plasma glucose levels of 5.6–6.9 mmol/L) increased in a dose-dependent manner as blood Cd concentrations increased from ≤ 0.80 to 0.81–2.94 and ≥ 2.95 µg/L (OR 1.19; 95% CI 1.01–1.40, and OR 1.37; 95% CI 1.14–1.63); however, there was no association for DM. Other studies have concluded that Cd exposure does not contribute to DM (Moon 2013; Borné et al. 2014). Overall, however, the literature does appear to suggest that DM is an adverse outcome associated with exposure to high levels of Cd in the blood (≥ 0.5–5 µg/L). In the current study, we found no significant association between blood Cd levels and the development of GDM. This may possibly be due to the low blood Cd concentrations in our study population (0.697 ng/g in patients with GDM).
Few studies have investigated the association between Pb and type 2 DM, but one epidemiological cross-sectional survey has been conducted among 238 males with normal blood sugar levels and 196 male patients with DM living in urban areas of Pakistan (Afridi et al. 2008). This survey showed that the mean Pb levels were significantly higher in blood samples obtained from smokers (339.7–405.6 µg/L) and nonsmokers with DM (256.3–301.7 µg/L) versus controls (223.5–265.7 and 174.4–217.9 µg/L, respectively). However, the results for Pb in our study were similar to those for Cd, and there was no significant relationship between Pb and the development of GDM. As with Cd levels, the blood levels of Pb in the GDM group (6.13 ng/g) were also much lower than those in previous studies. This may account for the lack of correlation seen here.
The traditional reported risk factors for GDM are maternal age, obesity, family history of type 2 DM, and previous GDM (Buchanan and Xiang 2005; Reece et al. 2009). In our logistic regression model, age, pre-pregnancy BMI, and a history of GDM showed a significant positive correlation with GDM and these results are consistent with previous studies (Buchanan and Xiang 2005; Reece et al. 2009). Therefore, obesity and a prior history of GDM can be considered to be strong predictors of increased risk for the development of GDM.
Our study has several noteworthy strengths. A power analysis indicated adequate statistical power for detecting an association between blood Cd concentrations and GDM (e.g., statistical power was 0.99 for the parous group at a significance level of 0.05 and the study sample size of 11,106 subjects). However, the statistical power of blood Pb was modest (0.09 for the parous group). Therefore, further studies are required to evaluate an association between blood Pb concentrations and GDM. Additional strengths include the nature of the study cohort, which comprised a well-characterized population of pregnant women. Blood metal concentrations were assessed by a robust, well-validated, and accurate method (ICP-MS). Structured questionnaires, medical records, and semi-quantitative food frequency questionnaires provided robust covariate data.
Several methodological issues should be highlighted. In our study, blood Cd or Pb concentrations were measured and used in the statistical analysis. Blood Cd concentration is considered to be a valid biomarker of recent exposure, and the half-life shows a fast component of 3–4 months and a slow component of approximately 10 years (Järup and Åkesson 2009). In addition, blood Cd concentration is influenced by the Cd burden of the body, and a history of smoking (even over 5 years ago) increases blood Cd concentrations (Olsson et al. 2002). Whole blood Pb concentration is the most popular indicator of Pb exposure and blood Pb concentration generally reflects recent exposure, i.e., in the 20–30 days prior to measurement (NRC, 1993). As our blood samples were collected at one timepoint during the 22–28 weeks gestation period, Cd and Pb concentrations could have underestimated the cumulative exposure. In addition, we could not measure fasting plasma glucose and insulin levels, which are known indices of insulin resistance and secretion. Therefore, it remains possible that there is a positive relationship between Cd and Pb exposure and impaired glucose tolerance that has not reached the onset of GDM. Since we could not obtain plasma glucose levels after OGTT, a stratified analysis of plasma glucose levels was not conduced. Therefore, the relationship between Cd and Pb and these impaired glucose tolerance levels should be verified in future studies. It should also be noted that the Cd and Pb concentrations in our population were lower than those reported in previous studies that have identified an association between Cd and Pb and type 2 DM (Schwartz et al. 2003; Afridi et al. 2008; Nie et al. 2016; Li et al. 2017).
In conclusion, the current study identified no association between blood Cd and Pb concentrations and GDM-related outcomes. Although it is important to minimize Cd and Pb exposure because of the increased risk of a range of other health effects, our study indicates that Cd and Pb exposure, at the level observed in our population, does not pose a significant GDM risk.
References
Adams SV, Newcomb PA, Shafer MM, Atkinson C, Bowles EJA, Newton KM et al (2011) Sources of cadmium exposure among healthy premenopausal women. Sci Total Environ 409(9):1632–1637
Afridi HI, Tasneem GK, Kazi N, Jamali MK, Arain MB, Jalbani N et al (2008) Evaluation of status of toxic metals in biological samples of diabetes mellitus patients. Diabetes Res Clin Pract 80(2):280–288
Barregard L, Bergström G, Fagerberg B (2013) Cadmium exposure in relation to insulin production, insulin sensitivity and type 2 diabetes: a cross-sectional and prospective study in women. Environ Res 121:104–109
Borné Y, Fagerberg B, Persson M, Sallsten G, Forsgard N, Hedblad B et al (2014) Cadmium exposure and incidence of diabetes mellitus-results from the Malmö Diet and Cancer study. PLoS One 9(11):e112277
Bryson CL, Ioannou GN, Rulyak SJ, Critchlow C (2003) Association between gestational diabetes and pregnancy-induced hypertension. Am J Epidemiol 158(12):1148–1153
Buchanan TA, Xiang AH (2005) Gestational diabetes mellitus. J Clin Investig 115(3):485–491
Buchanan TA, Xiang AH, Page KA (2012) Gestational diabetes mellitus: risks and management during and after pregnancy. Nat Rev Endocrinol 8(11):639–649
Chang KC, Hsu CC, Liu SH, Su CC, Yen CC, Lee MJ et al (2013) Cadmium induces apoptosis in pancreatic beta-cells through a mitochondria-dependent pathway: the role of oxidative stress-mediated c-Jun N-terminal kinase activation. PLoS One 8(2):e54374
Chen YW, Yang CY, Huang CF, Hung DZ, Leung YM, Liu SH (2009) Heavy metals, islet function and diabetes development. Islets 1(3):169–176
Edwards JR, Prozialeck WC (2009) Cadmium, diabetes and chronic kidney disease. Toxicol Appl Pharmacol 238(3):289–293
Ettinger AS, Bovet P, Plange-Rhule J, Forrester TE, Lambert EV, Lupoli N et al (2014) Distribution of metals exposure and associations with cardiometabolic risk factors in the “Modeling the Epidemiologic Transition Study”. Environ Health 13:90
Hectors TL, Vanparys C, van der Ven K, Martens GA, Jorens PG, Van Gaal LF et al (2011) Environmental pollutants and type 2 diabetes: a review of mechanisms that can disrupt beta cell function. Diabetologia 54(6):1273–1290
Horiguchi H, Oguma E, Sasaki S, Miyamoto K, Ikeda Y, Machida M et al (2004) Comprehensive study of the effects of age, iron deficiency, diabetes mellitus, and cadmium burden on dietary cadmium absorption in cadmium-exposed female Japanese farmers. Toxicol Appl Pharmacol 196(1):114–123
Hwang JL, Weiss RE (2014) Steroid-induced diabetes: a clinical and molecular approach to understanding and treatment. Diabetes Metab Res Rev 30(2):96–102
Järup L, Åkesson A (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238(3):201–208
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283(2–3):65–87
Kippler M, Goessler W, Nermell B, Ekström EC, Lönnerdal B, El Arifeen S et al (2009) Factors influencing intestinal cadmium uptake in pregnant Bangladeshi women—a prospective cohort study. Environ Res 109(7):914–921
Kodama Y, Sameshima H, Ohashi M, Ikenoue T (2013) Impact of new gestational diabetes mellitus criteria on stillbirth: a regional population-based study in Japan. J Obstet Gynaecol Res 39(7):1242–1245
Kolachi NF, Kazi TG, Afridi HI, Kazi N, Khan S, Kandhro GA et al (2011) Status of toxic metals in biological samples of diabetic mothers and their neonates. Biol Trace Elem Res 143(1):196–212
Kuo CC, Moon K, Thayer KA, Navas-Acien A (2013) Environmental chemicals and type 2 diabetes: an updated systematic review of the epidemiologic evidence. Curr Diabetes Rep 13(6):831–849
Li Y, Zhang Y, Wang W, Wu Y (2017) Association of urinary cadmium with risk of diabetes: a meta-analysis. Environ Sci Pollut Res Int 24(11):10083–10090
Ma RC, Chan JC (2009) Pregnancy and diabetes scenario around the world: China. Int J Gynaecol Obstet 104(Suppl 1):S42–S45
Michikawa T, Nitta H, Nakayama SF, Yamazaki S, Isobe T, Tamura K et al (2018) Baseline profile of participants in the Japan Environment and Children’s Study (JECS). J Epidemiol 28:99–104
Minakami H, Hiramatsu Y, Koresawa M, Fujii T, Hamada H, Iitsuka Y et al (2011) Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) 2011 edition. J Obstet Gynaecol Res 37:1174–1197
Moon SS (2013) Association of lead, mercury and cadmium with diabetes in the Korean population: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Diabet Med 30(4):e143–e148
Morikawa M, Yamada T, Yamada T, Akaishi R, Nishida R, Cho K et al (2010) Change in the number of patients after the adoption of IADPSG criteria for hyperglycemia during pregnancy in Japanese women. Diabetes Res Clin Pract 90(3):339–342
National Research Council (NRC) (1993) Biologic markers of lead toxicity. In: Measuring lead exposure in infants, children, and other sensitive populations. National Academies Press (US), Washington (DC), p 156
Nie X, Wang N, Chen Y, Chen C, Han B, Zhu C et al (2016) Blood cadmium in Chinese adults and its relationships with diabetes and obesity. Environ Sci Pollut Res Int 23(18):18714–18723
Olsson IM, Bensryd I, Lundh T, Ottosson H, Skerfving S, Oskarsson A (2002) Cadmium in blood and urine-impact of sex, age, dietary intake, iron status, and former smoking—association of renal effects. Environ Health Perspect 110(12):1185–1190
Reece EA, Leguizamon G, Wiznitzer A (2009) Gestational diabetes: the need for a common ground. Lancet 373(9677):1789–1797
Schulze MB, Liu S, Rimm EB, Manson JE, Willett WC, Hu FB (2004) Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am J Clin Nutr 80(2):348–356
Schwartz GG, Il’yasova D, Ivanova A (2003) Urinary cadmium, impaired fasting glucose, and diabetes in the NHANES III. Diabetes Care 26(2):468–470
The Japan Diabetes Society (JDS) (2013) Evidence-based practice guideline for the treatment for diabetes in Japan 2013. Nankodo Co., Ltd., Tokyo, p 308
The Japanese Society of Diabetes and Pregnancy (JSDP) (2015) Diagnostic and therapeutic manual of diabetic pregnancy. Medical View Co., Ltd., Tokyo, pp 18–19
Tyrrell JB, Hafida S, Stemmer P, Adhami A, Leff T (2017) Lead (Pb) exposure promotes diabetes in obese rodents. J Trace Elem Med Biol 39:221–226
Villegas R, Liu S, Gao Y-T, Yang G, Li H, Zheng W et al (2007) Prospective study of dietary carbohydrates, glycemic index, glycemic load, and incidence of type 2 diabetes mellitus in middle-aged Chinese women. Arch Intern Med 167(21):2310–2316
Watanabe T, Koizumi A, Fujita H, Kumai M, Ikeda M (1983) Cadmium levels in the blood of inhabitants in nonpolluted areas in Japan with special references to aging and smoking. Environ Res 31(2):472–483
Yessoufou A, Moutairou K (2011) Maternal diabetes in pregnancy: early and long-term outcomes on the offspring and the concept of “metabolic memory”. Exp Diabetes Res 2011:218598
Acknowledgements
We would like to express our gratitude to all of the JECS study participants. We would also like to express our sincere appreciation to the co-operating healthcare providers. The JECS was funded by the Ministry of the Environment, Japan. The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the Ministry of the Environment of the Japanese government. The Members of the JECS group as of April 2017 (principal investigator, Toshihiro Kawamoto): Hirohisa Saito (National Centre for Child Health and Development, Tokyo, Japan), Reiko Kishi (Hokkaido Regional Center for JECS, Hokkaido University, Sapporo, Japan), Nobuo Yaegashi (Tohoku University, Sendai, Japan), Koichi Hashimoto (Fukushima Medical University, Fukushima, Japan), Chisato Mori (Chiba University, Chiba, Japan), Shuichi Ito (Yokohama City University, Yokohama, Japan), Zentaro Yamagata (University of Yamanashi, Chuo, Japan), Hidekuni Inadera (University of Toyama, Toyama, Japan), Michihiro Kamijima (Nagoya City University, Nagoya, Japan), Takeo Nakayama (Kyoto University, Kyoto, Japan), Hiroyasu Iso (Osaka University, Suita, Japan), Masayuki Shima (Hyogo College of Medicine, Nishinomiya, Japan), Yasuaki Hirooka (Tottori University, Yonago, Japan), Narufumi Suganuma (Kochi University, Nankoku, Japan), Koichi Kusuhara (University of Occupational and Environmental Health, Kitakyushu, Japan), and Takahiko Katoh (Kumamoto University, Kumamoto, Japan).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
The members of the Japan Environment and Children’s Study Group are listed in Acknowledgements.
Rights and permissions
About this article
Cite this article
Oguri, T., Ebara, T., Nakayama, S.F. et al. Association between maternal blood cadmium and lead concentrations and gestational diabetes mellitus in the Japan Environment and Children’s Study. Int Arch Occup Environ Health 92, 209–217 (2019). https://doi.org/10.1007/s00420-018-1367-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00420-018-1367-7