Abstract
Purpose
The aim of this study was to determine cancer morbidity amongst Swedish iron foundry workers with special reference to quartz exposure. In addition to respirable dust and quartz, phenol, formaldehyde, furfuryl alcohols, polycyclic aromatic hydrocarbons (PAHs), carbon black, isocyanates and asbestos are used or generated by foundry production techniques and exposure to any of these substances could have potentially carcinogenic effects.
Methods
Cancer morbidity between 1958 and 2004 was evaluated in a cohort of 3,045 male foundry workers employed for >1 year between 1913 and 2005. Standardised incidence ratios (SIRs) with 95 % confidence intervals (95 % CI) were determined by comparing observed numbers of incident cancers with frequencies in the Swedish cancer register. Exposure measures were assessed using information from the personal files of employees and modelling quartz measurement based on a database of 1,667 quartz measurements. Dose responses for lung cancer were determined for duration of employment and cumulative quartz exposure for latency periods >20 years.
Results
Overall cancer morbidity was not increased amongst the foundry workers (SIR 1.00; 95 % CI, 0.90–1.11), but the incidence of lung cancer was significantly elevated (SIR 1.61; 95 % CI, 1.20–2.12). A non-significant negative dose response was determined using external comparison with a latency period of >20 years (SIR 2.05, 1.72 1.26 for the low, medium and high exposure groups), supported by internal comparison data (hazard ratios 1, 1.01, 0.78) for the corresponding groups. For cancers at sites with at least five observed cases and a SIR > 1.25, non-significant risks with SIRs > 1.5 were determined for cancers of the liver, larynx, testis, connective muscle tissue, multiple myeloma plasmacytoma and lymphatic leukaemia.
Conclusions
A significant overall risk of lung cancer was determined, but using external and internal comparison groups could not confirm any dose response at our cumulative quartz dose levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the 1980s, the International Agency for Research on Cancer (IARC) concluded that employment in iron and steel foundries could be correlated with increased incidence of cancer, mainly due to a doubled risk of lung cancer and increased rates of cancer of the digestive system and stomach. This was not unexpected since the foundry environment entails exposures to a large number of carcinogens, including quartz, polycyclic aromatic hydrocarbons (PAHs), formaldehyde, aromatic amines, benzene and asbestos (IARC 1984, 1987, 1997). Increased risks of lung cancer were determined for iron foundry workers in the USA (Andjelkovich et al. 1990) and Denmark (Sherson et al. 1991), as well as for Spanish (Rodriguez et al. 2000) and German foundry workers (Adzersen et al. 2003). Studies showing increased risks of cancer of the bladder (Hansen 1991), upper digestive tract (Adzersen et al. 2003), stomach and prostate (Rotimi et al. 1993) are also available.
Dose–response relationships for an overall increase in the risk of lung cancer and cumulative exposures to total dust, quartz and benzo(a)pyrene were determined amongst Chinese foundry workers (Xu et al. 1996a, b). However, the dose–response findings regarding quartz exposures could not be confirmed in the previously mentioned US cohort (Andjelkovich et al. 1990).
Despite knowledge gathered from previous studies, there are still uncertainties regarding cancer risks, in particular related to well-known exposures and suspected carcinogens such as quartz in the iron foundry environment. Consequently, we have investigated the exposure and cancer risks associated with working in an iron foundry environment in an epidemiological and exposure survey focusing on respirable quartz in Swedish iron foundries. We present here the results of this cancer morbidity study, with special reference to a dose–response analysis of quartz exposure and lung cancer.
Methods
Description of the cohort
Data from ten iron foundries where complete lists of employees were available were analysed in this study. These foundries utilised a mixture of manual and mechanised (old and new) moulding and casting production techniques and were chosen to represent foundries with wide ranges of sizes, numbers of employees and production volumes (Andersson et al. 2009). Company personnel records of the foundries were used to identify workers whose employment began before 2005, providing an initial cohort of 3,996 employees. Of these, 951 subjects were excluded, including those who died before 1 January 1958 (n = 4) and were employed for less than 1 year (n = 676), whose identities were uncertain (n = 4), or for whom employment information was inadequate (n = 33). Data for female subjects (n = 234) were also excluded, giving a final set of 3,045 quartz-exposed male workers employed for more than 1 year and who were both alive and had not emigrated when the follow-up started. The mean and median durations of employment of this cohort were 13 and 9 years, respectively, and their total employment comprised 70,388 person-years, with a distribution of person-years of 33 % for individuals born before 1930, 54 % for subjects born between 1930 and 1959, and 13 % for individuals born after 1960.
The cancer morbidity analyses covered the period from 1958 (when the Swedish Cancer registry started) through to 2004. We obtained information on vital status and date of emigration as of 31 December 2004, from the Swedish population registry. The cancer diagnosis was coded according to the International Classification of Disease (ICD-7). The study was approved by the Ethical Committee in Uppsala, Sweden.
Exposure measurements and database
Respirable dust and quartz exposure measurement data were collected from each company participating in the study, but no area measurements were included. Recent exposures for the 10 foundries were determined from 340 personal full-shift exposure measurements taken between April 2005 and May 2006 (Andersson et al. 2009). Historical measurement data were also available from compulsory measurements performed by the 10 foundries and national exposure surveys from the 1960s; the latter provided by the Swedish Work Environment Authority. The resulting measurement database compiled from these two sources contained 1,667 air concentration measurements of respirable quartz. For each measurement data in our database, the specific foundry, job title and time period was specified in our measurement database.
Exposure modelling
A mixed model was used to calculate (from data compiled in our measurement database) quartz exposure concentrations for workers in four time periods (1968–1979, 1980–1989, 1990–1999 and 2000–2006), with different job titles (11 categories) in each of the 10 foundries, that is, to estimate the individual workers’ quartz exposure intensity for their entire work history. The exposure measurement data were found to be skewed, so natural logarithm transformation was applied. Estimates from the model allowed us to identify factors affecting quartz concentration levels, which were used to calculate the surrogate quartz dose for each individual in the cohort (Brown and Prescott 1999). The exposure measurement database, exposure modelling and the calculation of individual cumulative quartz exposures are presented in detail elsewhere (Andersson et al. 2012).
Exposure measures
Exposure of the subjects to quartz was classified in terms of three measures: ever/never exposed, duration of employment and cumulative quartz exposure. Latency time was recorded as the time between the first exposure and cancer diagnosis. Exposure to respirable quartz was defined as cumulative exposure in units of mg m−3 year (i.e. exposure level exposure time) and categorised into three dose groups: <1, 1–2 and >2 mg m−3 year. These classes correspond to <25, 25–49 and >50 %, respectively, of the maximum allowed lifetime exposure of 4 mg quartz (equivalent to 40 years of exposure at the present annual Swedish Occupational Exposure Limit for quartz of 0.1 mg m−3).
Smoking habits
Individual smoking data were unavailable for the whole cohort. To illustrate differences in smoking habits in the cohort, a questionnaire was sent to 500 randomly selected persons born before 1980. The smoking habits were registered as smoker or never smoker (including ex smoker) and were used to illustrate the smoking habits amongst the low-, medium- and high-exposed groups.
Statistical methods
Cause-specific Standardised Incidence Ratios (SIRs), obtained by comparing the cancer incidence of the cohort with that of the general population of Sweden, were expressed as ratios derived from numbers of observed and expected cancer morbidity. The expected numbers of cancer cases were calculated by multiplying the person-years with the specific cancer rates for the general population of Sweden corresponding to gender, five-year age groups, calendar year and cause. SIRs were also calculated by exposure status. These computations were carried out using Stata Statistical Software, Release 12 (StataCorp. 2011. College Station, TX, USA). The Swedish Cancer Registry was established in 1958, and the observation period was from 1958 to 2004. The 95 % confidence intervals (95 % CI) of the SIRs were computed assuming a Poisson distribution of the observed numbers of cancer cases. We performed a dose–response analysis by duration of exposure for cancers at sites with at least five observed cases and an increased cancer incidence of 25 % or higher (SIR > 1.25), as well as for some tumours of particular interest.
Unadjusted Kaplan–Meier survival estimate plots were used to illustrate morbidity. Cox proportional hazard regression (Stata Statistical Software, release 12) was used for internal comparison between lung cancer and cumulative quartz exposure in three dose groups adjusted by age at diagnosis categorised in 5-year age bands, with first category younger than 20 years and last category older than 55 years, and modelled as a categorical variable.
Results
The overall cancer morbidity (Table 1) for the sample cohort, based on 347 observed cases, was close to the expected value derived from the general population, with an SIR of 1.00 (95 % CI, 0.90–1.11). Significantly, increased risks were noted only for cancer of the lung and pleura (SIR, 1.58; 95 % CI, 1.18–2.06) and for bronchus and lung cancers (SIR, 1.61; 95 % CI, 1.20–2.12). For cancers at sites with at least five observed cases and an increased incidence of ≥25 % (SIR > 1.25), non-significant enhanced risks with SIRs > 1.5 were determined for cancers of the liver, larynx, testis, connective muscle tissue, multiple myeloma plasmacytoma and lymphatic leukaemia.
For all cancers analysed by standard measures of exposure, a weak but non-significant trend was observed for the duration of employment, with the SIR increasing from 0.85 (95 % CI, 0.56–1.22) to 1.04 (95 % CI, 0.88–1.22) (Table 2). No increased risk was observed for short-term employees (<2 years) (SIR 0.85, 95 % CI 0.56–1.22). For lung cancer incidence, as analysed by duration of exposure, the SIRs of the different exposure groups were non-significant, ranging from 2.05 (95 % CI, 0.75–4.47) for the short-term workers to 1.58 (95 % CI 0.99–2.40) for the long-term exposure group exposed for >20 years. For liver and bladder cancers, the SIRs for the long-term exposed groups were 2.11 (95 % CI, 0.85–4.36) and 1.45 (95 % CI, 0.77–2.48), respectively.
Analysis of lung cancer incidence by duration of employment and latency time (years) showed no significantly enhanced risk of lung cancer amongst the 10–19 and >20 year exposure groups for latency times of less than 20 years. In fact, negative dose-responses, with SIRs ranging from 1.61 (95 % CI, 0.04–8.98) to 0.35 (95 % CI, 0.01–1.93), were found for these groups (Table 3). However, for the group with >20 year latencies, the SIRs reached 2.18 (95 % CI, 0.71–5.08), 1.55 (95 % CI, 0.67–3.06), 2.35 (95 % CI, 1.12–4.31) and 1.72 (95 % CI, 1.08–2.61) for workers employed in the foundries for <2, 2–9, 10–19 and >20 years, respectively, with significantly increased risks for workers employed for 10–19 and >20 years.
Using specific measures of exposure and cumulative exposure to crystalline quartz (Table 4), no significantly increased risk was observed for any of the quartz exposure groups when the latency time was less than 20 years. However, when the latency time was >20 years, significant SIRs were noted for both the 1–2 mg m−3 years exposure group (SIR, 1.72; 95 % CI, 1.00–2.75) and the <1 mg m−3 years group (SIR, 2.05; 95 % CI, 1.32–3.02). Nevertheless, no clear dose–response relationship was noted. Significantly, increased risks were found at dose levels corresponding to air concentrations of respirable quartz of 0.025–0.05 and <0.025 mg m−3.
A dose–response analysis based on internal comparison between lung cancer and cumulative quartz exposure using Cox regression analysis was undertaken (Table 5). The hazard ratios for the medium exposed group were 1.01 (95 % CI, 0.55–1.84),
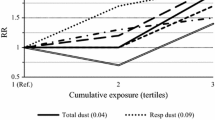
and for the high-exposed group 0.78 (95 % CI, 0.24–2.57). Data were also illustrated through a Kaplan–Meier survival estimates figure (Fig. 1). Thus, no dose–response pattern could be seen using internal comparison within the cohort.
It should also be noted that only one case of malignant pleural mesothelioma (ICD-7, 162.2) was detected in our cohort, compared to expected 1.3 cases in the general population, and one case of silicosis (ICD-10, 62.8) was also detected amongst the lung cancer cases in our cohort.
In our questionnaire regarding smoking habits, the response rate was 60 %. Amongst these answers, ex smokers and smokers represented in the low-, medium- and high-exposed group were 68, 65 and 84 % for our foundry workers.
Discussion
In this study of Swedish iron foundries, we investigated the dose–response relationship between different exposure measures and cancer morbidity by comparing cancer incidence of foundry workers with those of the general population obtained from national databases. An overall increased risk of lung cancer was determined amongst the foundry workers, but when latency time and exposure measures such as duration of employment and cumulative quartz exposure were applied, no clear dose–response pattern was noted. Internal comparison based on different exposure groups showed the similar results. No significant excess risks were identified for other cancer diseases, but non-significantly enhanced risks with SIRs > 1.5 were determined for cancers of the liver, larynx, testis, connective muscle tissue, multiple myeloma plasmacytoma and lymphatic leukaemia.
The main focus of this study was on iron foundry workers, for whom data were available from a long follow-up period, providing a substantial number of cases for analysis. The total employment of the cohort comprised 70,388 person-years with a mean duration of employment of 13 years. The majority of the cohort members (75 %) were born before 1960, thus providing sufficient exposure and latency time of the cohort to enable robust statistical evaluation of their lung cancer risks. For our study, we were able to make use of the comprehensive records in the Swedish cancer morbidity register. In addition, a foundry-specific measurement database of respirable dust and quartz air concentrations, based on data from our survey and our historical measurement database, was available. We were thus able to calculate cumulative quantitative exposure measures of respirable quartz doses for each individual in addition to standard measures of exposure.
Data for our iron foundry cohort were extracted from registers of a number of Swedish iron foundries, reflecting wide ranges in the scale of production, production techniques, the chemical binders used for cores and moulds and hence possible chemical exposures. In addition to respirable dust and quartz, phenol, formaldehyde, furfuryl alcohols, polycyclic aromatic hydrocarbons (PAHs), carbon black, isocyanates and asbestos are used or generated by foundry production techniques, and exposure to any of these substances could have potentially carcinogenic effects.
Lung cancer
In the IARC monographs addressing iron and steel foundry workers (IARC 1984, 1987), observed elevated risks of lung cancer could not be linked to any specific causative agent, although several suspected carcinogens were mentioned, including PAHs, quartz, metal fumes, formaldehyde and asbestos. Our results indicated an overall increased risk of lung cancer (SIR 1.61) amongst the male iron foundry workers we considered, but no dose–response trends were detected when the data were analysed either by duration of employment or by cumulative quartz exposure. Internal comparison studying dose–response pattern by the low-, medium- and high-exposed groups showed similar results, that is, no excess lung cancer due to lifetime quartz exposure below 0.05 mg m−3.
Other studies have found varying dose–response patterns when using standard exposure measures. However, their overall findings regarding excess lung cancer are in line with our findings. The cohort study of United States foundry workers found an overall increase in lung cancer mortality risk but no clear dose–response, even when cumulative quartz exposure measures were used in a nested case–control study within the cohort (Andjelkovich et al. 1994). Other studies of cancer mortality, that is, a cohort of Chinese iron and steel foundry workers and Danish foundry workers, also detected significant excess risks (Xu et al. 1996b). Similar findings were noted in a recent study of German male iron foundry workers (Adzersen et al. 2003).
Using cumulative quartz exposure measures, the US nested case–control study of grey iron foundry workers failed to determine a dose response (Andjelkovich et al. 1994), based on high, medium and low quartz exposure levels (1.5; 0.55 and 0.05 mg m−3, respectively).The case–control study of Chinese iron foundry workers with cumulative respirable quartz exposures ranging from <3.7 up to >27.7 mg m−3 year established a significant excess risk for all exposure groups (Xu et al. 1996a). Exposure of the low-exposed group would correspond to approximately 0.1 mg m−3 of respirable quartz exposure for a working life of 40 years. In a nested case–control study (Westberg and Bellander 2003) of quartz exposure and lung cancer, Swedish aluminium foundry workers were found to have an excess risk of SIR 1.6 for cumulative quartz exposures up to 1.0 mg m−3 year and SIR 2.6 for exposures exceeding 1.0 mg m−3 year (median 1.4 mg m−3 year); however, these excess risks were not statistically significant. In a recent meta-analysis of associations between quartz exposure and lung cancer (Lacasse et al. 2009), 10 studies, including two foundry studies, were evaluated. Significant relative risks >1 were determined for cumulative quartz doses >1.84 mg m−3 years.
No epidemiological study in the iron foundries has presented dose–response relationships and excess incidence findings for cumulative exposures <2 mg m−3 year, corresponding to 0.05 mg m−3 over a working life of 40 years. Our data from the Swedish iron foundries, including both external and internal comparison analysis, are in line with these findings.
A number of lung carcinogens are present in the foundry environment and could be confounders in our analysis. In particular, exposure to PAHs and asbestos should be addressed. A recent review (Bosetti et al. 2007) of published cohort studies on lung cancer in various occupational settings and exposures to PAHs included several iron and steel foundries, but the excess lung cancer found in the review was not attributed to this exposure. Furthermore, in an earlier review (Boffetta et al. 1997), the excess lung cancer amongst workers in iron foundries potentially due to PAH exposure was considered to be too complex to assess independently because of competing exposures to quartz and asbestos. However, a nested case–control study of Chinese foundry workers has addressed this issue; dose–response patterns were detected for total dust, quartz and benzo(a)pyrene (BaP), and excess risks of lung cancer were found at BaP levels >2 μg m−3 (Xu et al. 1996a). In European iron and steel foundries, PAH concentrations in the range of 0.1–1 μg m−3 have been found (Verma et al. 1982), and measurements of PAHs in aluminium foundries have also been reported, with concentrations ranging from <0.002 to 0.32 μg m−3 (Westberg et al. 2001). Given the relatively low exposure levels generally reported in foundries, the conclusions in the meta-analysis do not support the proposal that PAHs may be a major cause of lung cancer in iron foundries.
Asbestos has been used in iron foundries for insulation purposes, but published measurement data and epidemiological studies focusing on asbestos exposure in iron foundries are rare. In the IARC monograph on cancer risk factors in iron and steel foundries, asbestos was not specifically addressed (IARC 1977), and reported exposure levels in iron foundries were low, ranging from 0.0001 to 0.0033 fibres cm−3 (Gullickson and Doninger 1980), implying that levels of asbestos exposure were low. On the other hand, asbestos-related changes have been observed in X-rays of foundry workers’ lungs (Rosenman and Reilly 1998), and a study of respiratory patients in Lithuania has indicated that a fraction of the lung cancer cases might be attributed to heavy occupational exposure to asbestos (Everatt et al. 2007). Even though the fibre levels would appear to be low, the contribution to cancer incidence of asbestos exposure in iron foundries should not be completely ignored. However, in our study, only one case of mesothelioma was detected amongst the cohort of foundry workers.
No individual data on smoking habits were available for members of our cohort.
We used internal comparison in our dose–response analysis to adjust for differences in smoking habits or other confounders between foundry workers and the general population, and the increased lung cancer risk disappeared when the exposure groups were compared. A slightly higher proportion of smokers were noted amongst the high-exposed group.
The relative incidences of other tobacco smoke-related cancers (IARC 1986), such as cancers of the bladder, larynx and oesophagus, were also evaluated. No elevated risk of oesophagus cancer was noted, but the overall SIR for bladder and larynx cancers was elevated amongst our cohort, supporting our assumptions regarding possible differences in smoking habits between the foundry workers and the general population, or some other occupational exposure.
Bladder cancer
Amongst our cohort, the SIR for bladder cancer was 1.27, and a dose–response effect was noted when the duration of exposure and employment was considered. In line with these findings, a review of 40 cohort and case–control studies of foundry workers (Gaertner and Theriault 2002) reported a summary risk estimate of 1.16 when only studies with good exposure information were considered. However, the cited review also presented dose–response patterns obtained from four studies with exceptionally good exposure information, with increased risk estimates for increased duration of exposure, and our data suggest a similar trend, indicating that the incidence of this cancer differs significantly between long- and short-term employed foundry workers and, thus, that the excess risks could also be due to environmental exposure. An increased risk of bladder cancer, similar to our findings, was also noted in an evaluation of 10 iron and steel foundry cohorts (Bosetti et al. 2007).
An alternative explanation to smoking could be exposure to aromatic amines that are known bladder carcinogens (Gaertner and Theriault 2002) and are formed during casting and shake out from polyurethane binders that have been thermally degraded to aniline and dimethylaniline (MDA) (Renman et al. 1986). During the use of green sand in sand foundries, naphthalene in coal powder additives could also generate naphthylamines (Hansen 1991).
Liver cancer
We found a non-significantly increased risk of liver cancer amongst our cohort, in comparison with significant findings in a study of US foundry workers (Adzersen et al. 2003). Exposure to nitrosamines formed during core-making, moulding and castings (Wolf et al. 1984), PAHs (Austin et al. 1997; Austin et al. 1987), and both aliphatic and aromatic amines (Lynge 1994) have been discussed as potential causes of liver cancer. Our dose–response data suggest that SIRs increased (non-significantly) with duration of exposure, comparable to the US findings (SMRs ranging from 273.9 to 309) (Adzersen et al. 2003). There are no reports of a significant association between liver cancer and iron/steel foundry work in the early literature (IARC 1987), nor in studies covering more recent exposure situations (Andjelkovich et al. 1990; Sherson et al. 1991; Xu et al. 1996a; Hoshuyama et al. 2006).
The lack of exposure data on nitrosamine and aromatic amines suggests that the level of exposure to aromatic amines in iron foundries should be investigated in future studies for a more detailed analysis of bladder and liver cancer aetiology.
Conclusion
The results of the present study indicate an increased lung cancer risk for Swedish foundry workers exposed to quartz. The overall risk of lung cancer was significant, 1.61, but using external and internal comparison groups could not confirm any dose response at our cumulative quartz dose levels.
References
Adzersen KH, Becker N, Steindorf K, Frentzel-Beyme R (2003) Cancer mortality in a cohort of male German iron foundry workers. Am J Ind Med 43(3):295–305
Andersson L, Bryngelsson IL, Ohlson CG, Naystrom P, Lilja BG, Westberg H (2009) Quartz and dust exposure in Swedish iron foundries. J Occup Environ Hyg 6(1):9–18
Andersson L, Bryngelsson IL, Ngo Y, Ohlson CG, Westberg H (2012) Exposure assessment and modeling of quartz in Swedish iron foundries for a nested case-control study on lung cancer. J Occup Environ Hyg 9(2):110–119. doi:10.1080/15459624.2011.645397
Andjelkovich DA, Mathew RM, Richardson RB, Levine RJ (1990) Mortality of iron foundry workers: I. Overall findings. J Occup Med 32(6):529–540
Andjelkovich DA, Shy CM, Brown MH, Janszen DB, Levine RJ, Richardson RB (1994) Mortality of iron foundry workers. III. Lung cancer case-control study. J Occup Med 36(12):1301–1309
Austin H, Delzell E, Grufferman S, Levine R, Morrison AS, Stolley PD, Cole P (1987) Case-control study of hepatocellular carcinoma, occupation, and chemical exposures. J Occup Med 29(8):665–669
Austin H, Delzell E, Lally C, Rotimi C, Oestenstad K (1997) A case-control study of lung cancer at a foundry and two engine plants. Am J Ind Med 31(4):414–421
Boffetta P, Jourenkova N, Gustavsson P (1997) Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes Control 8(3):444–472
Bosetti C, Boffetta P, La Vecchia C (2007) Occupational exposures to polycyclic aromatic hydrocarbons, and respiratory and urinary tract cancers: a quantitative review to 2005. Ann Oncol 18(3):431–446. doi:10.1093/annonc/mdl172
Brown H, Prescott R (1999) Applied mixed models in medicine. Wiley, Chichester
Everatt RP, Smolianskiene G, Tossavainen A, Cicenas S, Jankauskas R (2007) Occupational asbestos exposure among respiratory cancer patients in Lithuania. Am J Ind Med 50(6):455–463. doi:10.1002/ajim.20467
Gaertner RR, Theriault GP (2002) Risk of bladder cancer in foundry workers: a meta-analysis. Occup Environ Med 59(10):655–663
Gullickson R, Doninger JE (1980) Industrial hygiene aspects of olivine sand use in foundries. Am Foundrymen’s Soc Trans 88:623–630
Hansen ES (1991) Cancer mortality among Danish molders. Am J Ind Med 20(3):401–409
Hoshuyama T, Pan G, Tanaka C, Feng Y, Yu L, Liu T, Liu L, Hanaoka T, Takahashi K (2006) Mortality of iron-steel workers in Anshan, China: a retrospective cohort study. Int J Occup Environ Health 12(3):193–202
IARC (1977) IARC monographs on the evaluation of carcinogenic risk of chemicals to man, vol 14. International Agency for Research on Cancer, IARCPress, Lyon, Asbestos
IARC (1984) IARC monographs on the evaluation of carcinogenic risk of chemicals to humans. Volume 34 polynuclear aromatic compounds, part 3, industrial exposures in aluminium production, coal gasification, coke production and iron and steel founding. International Agency for Research on Cancer, IARCPress, Lyon
IARC (1986) IARC monographs on the evaluation of the carcinogenic risk of chemicals to human, vol 38. International Agency for Research on Cancer, IARCPress, Lyon, Tobacco smoking
IARC (1987) IARC monographs on the evaluation of carcinogen risk of chemicals to humans, iron and steel founding. Monographs supplement 7. In. International Agency for Research on Cancer, IARCPress, Lyon
IARC (1997) IARC monographs on the evaluation of carcinogenic risks to humans. Volume 68 Silica, some silicates, coal dust and para-aramid fibrils. International Agency for Research on Cancer, IARCPress, Lyon
Lacasse Y, Martin S, Gagne D, Lakhal L (2009) Dose-response meta-analysis of silica and lung cancer. Cancer Causes Control 20(6):925–933. doi:10.1007/s10552-009-9296-0
Lynge E (1994) Danish cancer registry as a resource for occupational research. J Occup Med 36(11):1169–1173
Renman L, Sangö C, Skarping G (1986) Determination of isocyanate and aromatic amine emissions from thermally degraded polyurethanes in foundries. Am Ind Hyg Assoc J 47(10):621–628
Rodriguez V, Tardon A, Kogevinas M, Prieto CS, Cueto A, Garcia M, Menendez IA, Zaplana J (2000) Lung cancer risk in iron and steel foundry workers: a nested case control study in Asturias. Spain. Am J Ind Med 38(6):644–650
Rosenman KD, Reilly MJ (1998) Asbestos-related x-ray changes in foundry workers. Am J Ind Med 34(2):197–201
Rotimi C, Austin H, Delzell E, Day C, Macaluso M, Honda Y (1993) Retrospective follow-up study of foundry and engine plant workers. Am J Ind Med 24(4):485–498
Sherson D, Svane O, Lynge E (1991) Cancer incidence among foundry workers in Denmark. Arch Environ Health 46(2):75–81
Verma DK, Muir DC, Cunliffe S, Julian JA, Vogt JH, Rosenfeld J, Chovil A (1982) Polycyclic aromatic hydrocarbons in Ontario foundry environments. Ann Occup Hyg 25(1):17–25
Westberg HB, Bellander T (2003) Epidemiological adaptation of quartz exposure modeling in Swedish aluminum foundries: nested case-control study on lung cancer. Appl Occup Environ Hyg 18(12):1006–1013
Westberg HB, Selden AI, Bellander T (2001) Exposure to chemical agents in Swedish aluminum foundries and aluminum remelting plants–a comprehensive survey. Appl Occup Environ Hyg 16(1):66–77
Wolf D, Blome H, Schütz A (1984) Measurement problems and evaluation of carcinogenic materials of group I in the air of industrial work places, exemplified in the case of N-nitrosamines. (In German). Staub Reinhaltung der Luft 44:33–37
Xu Z, Brown LM, Pan GW, Liu TF, Gao GS, Stone BJ, Cao RM, Guan DX, Sheng JH, Yan ZS, Dosemeci M, Fraumeni JF Jr, Blot WJ (1996a) Cancer risks among iron and steel workers in Anshan, China, Part II: Case-control studies of lung and stomach cancer. Am J Ind Med 30(1):7–15
Xu Z, Pan GW, Liu LM, Brown LM, Guan DX, Xiu Q, Sheng JH, Stone BJ, Dosemeci M, Fraumeni JF Jr, Blot WJ (1996b) Cancer risks among iron and steel workers in Anshan, China, Part I: proportional mortality ratio analysis. Am J Ind Med 30(1):1–6. doi:10.1002/(SICI)1097-0274(199607)30:1<1:AID-AJIM1>3.0.CO;2-5
Acknowledgments
The authors express their gratitude to Mr. Bengt-Gunnar Lilja and Ms. Marianne Frölander for field measurements and Mr. Lars-Åke Karlsson for collecting cohort data. We are also grateful to the participating companies and Mr. Peter Nayström for valuable support. The project was supported by a grant (no. T-42:03) from AFA, Stockholm.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Westberg, H., Andersson, L., Bryngelsson, IL. et al. Cancer morbidity and quartz exposure in Swedish iron foundries. Int Arch Occup Environ Health 86, 499–507 (2013). https://doi.org/10.1007/s00420-012-0782-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00420-012-0782-4