Abstract
This paper reports the connections between red blood cells abnormality risk of petrochemical workers and their exposure to airborne polycyclic aromatic hydrocarbons (PAHs). Urinary 1-hydroxypyrene (1-OHP), as the biomarker of PAHs exposure, was adopted to assess the exposure risk of the petrochemical workers to PAHs in Xigu, the west suburb of Lanzhou where petrochemical industries are located. Fifty-three workers, sub-grouped to 36 petrochemical workers and 17 office workers, participated in this investigation. Logistic regression model and spearman correlation analysis were performed to estimate the associations between PAHs exposure levels and red blood cells abnormality risk of petrochemical workers. Strong associations between some red cell indices (MCH, MCHC, RDW) and 1-OHP concentration were found. Results also show that the red blood cells abnormality risk increased with increasing PAHs exposure level. Compared with office workers, risk level of red blood cells abnormality in petrochemical workers was higher by 41.7 % (OR, 1.417; 95 % CI: 0.368–5.456) than that in office workers. This result was verified by the tissue-to-human blood partition coefficient for pyrene and 1-OHP. The quantitative assessments of the potential health risk through inhalation exposure to PAHs were conducted using the Incremental Lifetime Cancer Risk (ILCR) model. It was found the ILCR from inhalation exposure to PAHs for the petrochemical workers ranged from 10−5 to 10−4 with 95 % probability, indicating that petrochemical plant workers were under a high potential cancer risk level.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) have been listed by the United Nations Economic Commission for Europe (UNECE) Convention on Long-range Transboundary Air Pollution (CLRTAP, http://www.unece.org/env/lrtap/) as a class of persistent organic pollutants (POPs). PAHs are generated by incomplete combustion of organic compound (IARC 1983). They are mutagenic and carcinogenic and cause adverse health effects on human being, including hemolytic anemia, immunosuppression, and liver and kidney damage (Sanctucci and Shah 2000; Peterson et al. 2015; Block and Calderon-Garciduenas 2009). Hemolytic anemia is a form of anemia due to hemolysis which can lead to the abnormal breakdown of red blood cells (Gehrs and Friedberg 2002). Red blood cells are responsible for taking up oxygen in the lungs or gills and release it into tissues. The abnormality occurring in red blood cells can induce many hematologic diseases (Kim et al. 2014). A population-based study in Taiwan revealed that the increase in the number of white blood cells and red blood cells increased the odds ratio (OR) of clustering of cardiometabolic risk factors (Wang et al. 2004). Blood cancer incidences have been reported to be steadily increasing in recent years. Such type of cancers has a common characteristic that the normal blood cell development process is interrupted by uncontrolled growth of an abnormal type of blood cell (Mcphee et al. 2006). While the concern has been raised in the association between occupational PAHs exposure of petrochemical workers and their abnormality risk in red blood cells, quantitative cancer risk assessments for petrochemical workers via their exposure to airborne PAHs are still lacking due to scarce in-situ measurement data.
The main routes of human body exposure to PAHs include primarily respiratory, digestion, and skin contact. It is not straight-forward to assess precisely individual’s actual exposure levels by a direct sampling analysis. Since the 1980s, a number of studies have used PAHs metabolites as the biomarkers to assess human exposure to PAHs (Jongeneelen 2001). The concentration level of PAHs biomarkers in human tissues or body fluids can be regarded as indicators of PAHs exposure level and used to evaluate human exposure risks. Given that urine samples are readily available, non-destructive, and can effectively reflect recent exposure to PAHs, the urine sampling method has been widely used in cancer risk assessment (Strickland and Kang 1999). Pyrene is a main specie composition of PAHs in the environment. The urinary 1-hydroxypyrene (1-OHP) is the metabolite of pyrene and considered as one of qualitative biological indicators. The urinary 1-OHP has been demonstrated to be a useful and effective biomarker for human exposure to PAHs (Jongeneelen 2001). Upon exposure to PAHs, individuals would uptake and absorb PAHs via different routes, leading to the metabolism and distribution of these contaminants in various human tissues. Toxic effects on these tissues may occur. Since it is not always feasible to measure target tissue concentration of a toxic chemical via various exposure routes, physiologically based toxicokinetic (PBTK) and incremental lifetime cancer risks (ILCR) models are often employed in PAHs cancer risk assessments (Chiu et al. 2007; Liu et al. 2015). Jongeneelen and Berge (2011) simulated the human experimental blood–air partition coefficients of VOCs using the PBTK model and found that the modeled blood–air partition coefficient agreed well with measured coefficient. Xia et al. (2010, 2013) used ILCR model to investigate the cancer risk of children, adolescents, and adults through dietary PAHs intake. Their modeling study was able to quantify the inhalation exposure risk of these population groups to PAHs.
In several case studies, Yang et al. (2002) connected nine serious air pollution events in Taiwan with air contaminants releases from petrochemical industries in several municipalities between 1971 and 1990. Vainio et al. (1992) investigated PAHs released from petrochemical industries as environmental carcinogens and their health influence. Results from these studies suggested that petrochemical workers were particularly under a high exposure risk to air pollution in their workplaces because they would receive much higher doses of pollutants than others.
The present study aims to investigate the linkage between red blood cells abnormality risk of petrochemical workers and their exposure to PAHs in a large-scale petrochemical plant in Lanzhou, northwest China. The PBTK model was used to predict tissue-to-human blood partition coefficients of pyrene and 1-OHP, thereby discriminating the relationship between red blood cells abnormality risk and PAHs exposure of petrochemical plant workers. The ILCR model was applied to predict the incremental lifetime cancer risk of petrochemical workers via exposure to PAHs and to determine averaging contribution variance of occupational exposure PAHs concentration (Co) to workers’ inhalation incremental lifetime cancer risk.
Materials and methods
Targeted population
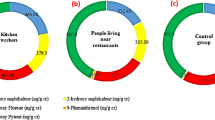
We collected morning urine samples from 53 employees in Lanzhou petrochemical plant. Based on different working environment, subjects were classified into two subgroups. One subgroup included 36 petrochemical workers, and the other consisted of 17 office workers. These participants have been working in the same plant and living in Xigu district of Lanzhou, the capital city of Gansu province. The city, with 3.2 million population and the elevation of 1600 m above the sea level, has been ranked as the mostly polluted city in China by the World Health Organization (WHO). Xigu district, located in the west suburb of Lanzhou, is the home of one of major petrochemical industries in China where the first photochemical smog event was detected in China in 1974 (Wang et al. 2009). Surrounded by mountains, the climate of Lanzhou-Xigu is characterized by very stable atmospheric stratification with strong atmospheric inversion extending to the afternoon which hinders the diffuse out of air pollutants. Heavy PAHs emissions have been occurring in Xigu (Zhang et al. 2000; Pan et al. 2010; Gao et al. 2007; Tao et al. 2012). It has been reported that cancer-standardized rate of local population in Xigu was the highest (192.84/10) in Lanzhou (Zhang et al. 2011).
All participants were healthy and agreed to be involved in this investigation, where all participants worked or were exposed 8 h/day and 6 days/week over the last decade.
All participants completed a detailed questionnaire (including the job category, age, weight, sex, alcohol consumption, and cigarette smoking). The questionnaires were developed based on previous studies (Lee et al. 2007; Chen et al. 2007). The data collected from the questionnaires are presented in Table S1 in Supporting Information. Here, we defined those individuals with smoking history over 3 months as smokers. Those who drink at least three times a week with a drinking history over 1 year were defined as drinkers (Shi et al. 2013; Wang et al. 2015).
Urine samples collection and urinary 1-OHP analysis
The morning urinary 1-OHP concentrations have been used as a representative of PAHs exposures instead of 24-h urinary 1-OHP concentrations (Jongeneelen 1997). We collected morning urine samples (25 ml) from each participant. Ten milliliters was separated into another tube to determine the urinary creatinine by the Jaffe reaction to normalize the urinary concentration of 1-OHP (Taussky 1954). Urinary creatinine concentrations were then calculated to adjust the varying degree of dilution in urine samples. This is done by dividing 1-OHP concentrations by urinary creatinine concentrations (Han et al. 2008). The remaining spot urine sample (15 mL) was stored in plastic bottles and stored at the temperature of −20 °C until analysis.
All urine samples were pretreated following Zhang et al. (2010) and Yang et al. (2009). 1-OHP in urine was determined by synchronous fluorescence spectrometry using a constant-wavelength scan technique. All fluorescence spectra were measured with RF 5301 spectrofluorimeter (Japan). The slit widths of both excitation and emission were kept at 5 nm. Excitation and emission wavelengths were 354 and 388 nm, respectively, yielding a constant wavelength difference between the excitation and emission Δλ = Δλem − Δλex = 34 nm.
Blood routine examination
Participants’ blood samples were collected on the same day during their routine health examination. From each participant, we collected venous blood 0.5–1.0 ml. The blood samples were put in specialized anticoagulant tubes of ethylene diamine tetra acetic acid (EDTA). Methods automatic blood cell analyzer was used for routine blood analysis. The red blood cell indices estimated in this study included the assay of mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean cell hemoglobin concentration (MCHC), red blood cell count (RBC), hemoglobin (Hgb), hematocrit (Hct), and red cell volume distribution width (RDW) (Singh and Srivastava 2010). Among these indices, MCH quantifies the amount of hemoglobin per red blood cell, MCHC measures the amount of hemoglobin per unit volume, MCV defines the average volume of red blood cells, and RDW represents the coefficient of variation of the red blood cell volume distribution (size) (Singh and Srivastava 2010). Overall, these red blood cell indices measure the size and oxygen-carrying protein (hemoglobin) content of red blood cells, taken as part of the blood cell test. They are used to diagnose the cause of anemia and provide information about the hemoglobin content and size of red blood cells (Kim et al. 2014). Values of these indices have their individual standard range (critical value), and those values beyond their critical values are regarded as abnormality. In other words, if one red cell index abnormality was determined, this abnormality could be identified as blood cell test abnormality (Liu 2009). The values of the standard ranges of these indices are presented in Table S7.
Prediction of tissue–human blood partition coefficients of PAHs
A partition coefficient is the ratio of the concentration of a chemical between two phases in thermodynamic equilibrium. The tissue–blood partition coefficients can be used to simulate blood distribution in a human body (Jongeneelen and Berge 2011). In the present study, we used the PBTK model (Jongeneelen 2012) (IndusChemFate model 2.0, http://www.cefic-lri.org/lri-toolbox/induschemfate) to predict tissue–blood partition coefficients. The blood–tissue partitioning can be estimated by the quantitative–structure property relationship (QSPR) algorithm in the PBTK (DeJongh et al. 1997). The PBTK model incorporates the QSPR algorithm in blood–tissue partitioning subject to structural fragments (Beliveau and Krishnan 2005). The model consists of 11 tissue compartments (Table S2). Some compartments defined in terms of the lipid fraction of the tissues can use the same partitioning algorithm to assess the partitioning between different compartments (Jongeneelen and Berge 2011; Woodard and White 1986; DeJongh et al. 1997). The model parameters are presented in Table S3 of Supporting Information.
Model parameters such as partitioning between air, blood, and tissue compartments were predicted by the QSPRs (Table S4 in Supporting Information) subject to physical–chemical properties of a targeted chemical, such as the octanol–water partition coefficient (logKow), vapor pressure, molecular weight, density, and water solubility. Metabolic rate was calculated by the Michaelis-Menten kinetics equation (Jongeneelen 2011).
Table S5 in Supporting Information presents the physical–chemical properties of pyrene and its metabolites used in the PBTK model (Jongeneelen 2012). In PBTK modeling, we have assumed that the workers participated in this investigation were exposed 8 h per day. As a major metabolite of pyrene (Jongeneelen 2012; Keimig et al. 1983), 1-OHP was then conjugated and excreted in urine as a glucuronideconjugate (1-OHP-gluc).
Incremental lifetime cancer risks model
The incremental lifetime cancer risks (ILCR) was often used as a metric in the evaluation of human cancer exposure risk to PAHs. This model was employed here to quantify the lifetime cancer risk of the petrochemical workers. The ILCR of the petrochemical workers in Xigu by PAHs inhalation exposure was calculated based on occupational exposure incremental lifetime cancer risk (ILCRo) and general exposure incremental lifetime cancer risk (ILCRg), defined by:
where CSF is the inhalation carcinogenic slope factor, and C is equivalent BaP concentration calculated by urinary 1-OHP concentration (Duan et al. 2011). C o is occupational exposure concentration, and C g is general exposure concentration. In our study, we defined the exposure level of office workers as the general exposure. t o and t g in Eqs. (1) and (2) are the time for occupational exposure and general exposure, respectively. IRo and IRg are respiratory rate, EFo and EFg are the exposure frequency (365 days/year), and EDo and EDg are the exposure duration (year) for occupational exposure and general exposure, respectively. AT in Eqs. (2) and (3) is the averaging time for carcinogenic (day), BW is the body weight (kg), and cf is the conversion factor (10−6). To evaluate model uncertainties, we have applied Monte Carlo method in Crystal Ball software to run the ILCR model 10,000 times to ensure the stability of the modeling result. Details of the uncertainty analysis are presented in Table S6 of Supporting Information.
Statistical analysis
Statistical analysis was performed using SPSS software version 19.0. The normality test of the 1-OHP concentrations was conducted by using the kurtosis and skewness coefficients of 1-OHP concentrations. Descriptive statistics were calculated, mostly in the mean, median, and standard deviation (SD) of 1-OHP concentration data. T test and Mann–Whitney U test were used to compare the difference of exposure data. T test is often used to determine significant difference between two dataset, which is most commonly applied in statistic test of a normally distributed dataset. In our statistical analysis, when the normality assumption did not hold, T test was then replaced by the non-parametric (Mann–Whitney) U test. The U test has been often applied in two samples from the same population against an alternative hypothesis. In our case, the normality test showed that the raw 1-OHP concentration data displayed an abnormal distribution but normal distribution after logarithmic transformation of the raw data. We used T test to compare personal covariates and urinary 1-OHP concentrations between petrochemical workers and office workers. Non-parametric Mann–Whitney U test was applied to compare the differences of urinary 1-OHP concentrations between different categories in the same group of worker (in petrochemical workers or in office workers). Spearman correlation analysis was then carried out to examine the associations between urinary 1-OHP concentration and red cell indices. The statistical significance was set at the 0.05 level. Logistic regression was used to estimate the odds ratios (OR) and to assess the risk of red blood cells abnormality.
Results
Urinary 1-OHP concentration levels
Characteristics of the subjects in investigation
As shown in Table S1 of Supporting Information, 53 employees completed the questionnaires. In the petrochemical workers, mean urinary 1-OHP concentration in male was higher than that in female. However, the mean urinary 1-OHP concentration in female office workers was higher than male office workers. Among those smokers and nonsmokers, 1-OHP concentrations were higher in smokers than nonsmokers in both petrochemical workers and office workers. This result also applied in drinkers. Overall, urinary 1-OHP concentrations in the petrochemical workers were higher than that in the office workers.
Urinary 1-OHP concentration levels
Summary statistics of urinary 1-OHP concentration are presented in Table 1. We used creatinine as a clearance protein to unify the differences of urine concentrations in urine samples. Creatinine correction was carried out for all samples (n = 53). The mean unadjusted urinary 1-OHP concentration was 6.28 μg/L (range: 2.5–21.5 μg/L). Mean adjusted urinary 1-OHP concentration was 1.96 μmol/mol creatinine (range: 0.6–13.96 μmol/mol creatinine).
Significant differences of urinary 1-OHP levels between the petrochemical workers and office workers were identified (Table 2). Result showed considerably higher (p = 0.023) mean concentrations [95 % confidence interval (CI: 1.52, 3.08)] of 1-OHP in the petrochemical workers than the office workers [95 % confidence interval (CI: 1.05, 1.47)].
Effect of subject characteristics on urinary 1-OHP concentration
Multiple linear regression of urinary 1-OHP influence factors (Table 3) between the log-transformed urinary 1-OHP concentration (dependent variable) and job category, age, gender, weight, working history, smoking and drinking habits (independent variables) was estimated. Results showed that the job category in the multivariate regression model was the sole variable having statistical significant influence on the urinary 1-OHP concentration. Other factors failed to pass statistical test in the regression model and hence were neglected.
Comparing urinary 1-OHP concentration in subjects with 1-OHP biological exposure limits
Jongeneelen (2014) proposed a “no observed genotoxic effect level” (NOGEL) and a “lowest observed genotoxic effect level” (LOGEL) as the baseline values for the cancer risk assessment of the workers exposing to PAH. The NOGEL represents the highest level of a hazard substance in an experiment which does not result in a genotoxic effect. On the other hand, the LOGEL denotes the lowest observed genotoxic level in the workers exposing to PAH. Jongeneelen proposed the NOGEL at 1.0 μmol/mol creatinine and LOGEL at 1.9 μmol/mol creatinine of 1-OHP concentration in occupational exposure population respectively.
We compared urinary 1-OHP concentration with NOGEL and LOGEL to determine whether the genotoxic effect existed in these petrochemical workers. Results are illustrated in Fig. 1. As expected, mean 1-OHP concentrations in petrochemical workers were higher than that in office workers. Among petrochemical workers, 1-OHP concentrations in smokers (2.87 umol/mol creatinine) were much higher than the LOGEL (1.9 umol/mol creatinine) and 1-OHP concentrations in non-smokers (1.59 umol/mol creatinine). In general, the mean 1-OHP concentrations in both petrochemical workers and office workers were higher than the NOGEL (1.0 umol/mol creatinine).
Effects of PAHs exposure on changes in red blood cells
Indices of red blood cells
Summary statistics of the red blood cell index for petrochemical workers and office workers are shown in Table S7 of Supporting Information. In general, comparing with blood routine standard range (Table S7) of Lanzhou Petrochemical Hospital, all red cell indices of the petrochemical workers did not fall into the normal range.
Correlations between urinary 1-OHP concentration and red cell index
Table S8 of Supporting Information presents non-parametric Spearman correlations between urinary 1-OHP concentration and red cell index in the unadjusted-1-OHP and adjusted-1-OHP. Significant correlations were found between 1-OHP concentration and MCH as well as RDW. To further assess the effect of PAH exposure on the abnormality risk of red blood cells, we conducted logistic regression analysis to examine the associations between red blood cells abnormality and job category, sex, smoking habit as well as alcohol drinking habit. We separated the subjects as red blood cells normal group and abnormal group according to the blood cell test abnormality. Table 4 presents the estimated OR with 95 % confidence intervals for job, sex, smoking habit, and alcohol drinking habit with and without taking into consideration of the job category and the subjects’ characteristics. It can be identified that the job category (OR, 1.417; 95 % CI: 0.368–5.456) and smoking (OR, 1.217; 95 % CI: 0.302–4.908) exhibit most strong associations with the risk of red blood cells abnormality. Whereas the ORs for sex and alcohol drinking habit were 0.381 (95 % CI: 0.061–2.390) and 0.252 (95 % CI: 0.072–0.882), respectively, lower considerably than the other two variables. Compared with the office workers, the risk of red blood cells abnormality in petrochemical workers increased 41.7 % (OR, 1.417; 95 % CI: 0.368–5.456). It is also noticed that the risk of red blood cells abnormality in smokers increased 21.7 % (OR, 1.217; 95 % CI: 0.302–4.908) as compared with non-smokers.
Tissue–human blood partition coefficients in prediction of association between urinary 1-OHP and red blood cells abnormality risk
Tissue–human blood partition coefficient of a chemical reveals the distribution and transfer routes of the chemical in a human body. This coefficient also relates to the chemical’s concentration in a target tissue to its concentration in blood under equilibrium conditions (Parham et al. 1997). From these coefficients, we can roughly estimate the chemical entering into the target tissue, causing a certain damage to this tissue. As shown in Table 5, with high lipid solubility, pyrene and 1-OHP adipose tissue–blood partition coefficients were large. Likewise, brain–blood and bone marrow–blood partition coefficients for pyrene (14.4, 14.4) and 1-OHP (11.7, 11.7) were also relatively higher. From these two partition coefficients (brain–blood and bone marrow-blood), we can observe that at the equilibrium the concentrations of pyrene and 1-OHP in brain is about a factor of 14 higher than their concentrations in blood, and about a factor of 11 higher in bone marrow than in the blood. Bone marrow is a target organ for toxicity where pyrene and 1-OHP are more readily accumulated than other tissues, except the adipose tissue and brain. The inability of the bone marrow could increase red blood cell production in response to the red blood cells abnormality. If such the response is inadequate, the red blood cells abnormality condition will progressively worsen (Kaidar et al. 2011; Robinson et al. 1975).
Comparing urinary 1-OHP concentration with urinary 1-OHP genotoxic effect limits
Having demonstrated the associations between PAHs exposure and red blood cells abnormality risk from logistic regression analysis, we further investigated the effects of PAHs exposure on red blood cells. We compared the urinary 1-OHP concentration in red blood cells between normal group and abnormal group with the urinary 1-OHP genotoxic effect limits (Fig. 2). As shown in Fig. 2, for petrochemical workers, mean 1-OHP concentrations were higher than LOGEL (1.9 μmol/mol creatinine) in both red blood cells normal group and abnormal group. For the office workers, however, mean urinary 1-OHP concentrations were lower than LOGEL. For office workers, although mean 1-OHP concentration in red blood cells normal group was 1.12 μmol/mol creatinine which was higher than the lowest NOGEL (1.0 μmol/mol creatinine), this value was almost equivalent to the 2nd lowest NOGEL (1.1 μmol/mol creatinine) reported in literature (Jongeneelen 2014; Van Delft et al. 2001). 1-OHP concentration in red blood cells abnormality group (1.58 μmol/mol creatinine) was lower than LOGEL but higher than the lowest NOGEL (1.0 μmol/mol creatinine). This concentration value was higher than the 2nd and third lowest NOGEL values at 1.1 and 1.3 μmol/mol creatinine from the literature (Jongeneelen 2014; Buchet et al. 1995), respectively.
Quantify cancer risks of petrochemical workers to PAHs
Incremental lifetime cancer risk in petrochemical workers
Figure 3 displays predicted probability density functions of the ILCR of the petrochemical workers. The ILCR between 10−6 and 10−4 indicates potential cancer risk (Chen and Liao 2006). As shown in Fig. 3, the mean ILCR value is 3.9 × 10−4 and median value is 3.7 × 10−4 with mean standard error at 1.4 × 10−6. The confidence level in our simulation is 95 %. This indicates that the petrochemical workers were under the ILCR via the inhalation of PAHs at 95 % probability. The ILCR ranged from 10−5 to 10−4 with the minimum at 8.9 × 10−5 and maximum at 7.7 × 10−4, respectively, suggesting that the petrochemical workers were under high potential cancer risk.
Sensitivity and uncertainty analysis
Sensitivity analysis was carried out to evaluate the influence of input variables and uncertainty of model input parameters in the risk estimate. The influence of the model variables on the model performance were assessed by their respective averaging contributions variance. Results are shown in Fig. 4. As seen, the occupational exposure concentration (Co) was the most influential variable on the workers inhalation ILCR with averaging contribution variance at 58.3 %. There was a negative correlation between body weight (BW) and the workers’ inhalation ILCR.
Sensitivity analysis for inhalation ILCR model performance for petrochemical workers. Five model input variables and parameters were examined, including Co (occupational exposure concentration), BW (body weight), Cg (general exposure concentration), IRo, and IRg are respiratory rate under occupational exposure and general exposure, respectively
Discussions
Urinary 1-OHP concentration levels
We applied multiple linear regression and t test to evaluate PAHs exposure levels during the work time of petrochemical workers. Considerably, higher (p = 0.023) mean concentrations [95 % confidence interval (CI: 1.52, 3.08)] of 1-OHP in the petrochemical workers were found by comparing with the office workers [95 % confidence interval (CI: 1.05, 1.47)]. The results from the stepwise regression analysis fitted model showed that only the job category exerted significant effect on the urinary 1-OHP concentration. The effect from other factors (age, gender, weight, working history, smoking habits, drinking habits) appeared negligible (Table 3). Since the largest petrochemical industry in western China is located in Xigu where large amount of PAHs is emitted into atmosphere from chemical production and oil refining, this area has been also contaminated by PAHs. Inhalation became a main route for the participants’ exposure to PAHs. As a result, much higher mean urinary 1-OHP concentration was measured in the petrochemical workers than the office workers. As aforementioned, Jongeneelen (2014) has compared the 1-OHP concentrations in urine samples collected from literature and proposed critical values of the NOGEL at 1.0 μmol/mol creatinine and LOGEL at 1.9 μmol/mol creatinine of 1-OHP concentration in the health assessment of occupational exposure population, respectively. It has been known that the NOGEL represents the highest level of a hazard substance in an experimental dataset which does not result in a genotoxic effect. When the dataset is insufficient to derive the NOGEL, LOGEL, the lowest level of a substance in the dataset, can be taken as an alternative to elucidate the relationship between 1-hydroxypyrene in urine and early genotoxic effects in exposed workers. In those petrochemical workers involved in the present investigation, the mean 1-OHP concentrations in smokers (2.87 μmol/mol creatinine) were much higher than the LOGEL (1.9 μmol/mol creatinine) and non-smokers (1.59 μmol/mol creatinine). However, the difference of 1-OHP concentrations between smokers and non-smokers appeared not significant in terms of Mann–Whitney U test (p = 0.203, z = −1.273). For the office workers, the mean urinary 1-OHP concentrations in smokers and non-smokers were 1.23 and 1.26 μmol/mol creatinine, respectively. The difference between smokers and non-smokers was not significant in terms of Mann–Whitney U test (p = 0.821, z = −0.226). Since cigarette smoking also releases pyrene, smoking is expected to increase the 1-OHP content in urine samples. Previous studies have reported that there was no statistically significant difference of urinary 1-OHP concentrations between smokers with daily consumption of 10∼20 cigarettes and non-smokers (Zhao et al. 1990; Zhao et al. 1992). In the case of occupational exposure, the difference in urinary 1-OHP concentration between smoker and non-smoker is significant only when the background PAHs concentrations are relatively low (Knopp et al. 1999). In our case, however, all participants have been living in Xigu where ambient PAHs levels were high. This would likely overwhelm the influence of smoking on urinary 1-OHP concentrations. Overall, the mean 1-OHP concentrations in the subjects were higher than NOGEL (1.0 μmol/mol creatinine). The results suggest a potential risk of cytogenetic damage to the workers who were exposed to PAH.
Effects of PAHs exposure on changes in red blood cells
Bessman et al. (1983) proposed MCV and RDW as initial classification of anemia based on the heterogeneity of red cell size. In the anemia classification, MCV and RDW were classified as microcytic (MCV ≤ 80 fL), normocytic (MCV: 80–100 fL), or macrocytic (MCV ≥ 100 fL), respectively. A positive correlation was found between urinary 1-OHP concentration and MCV in the present study, suggesting that higher PAH exposure might induce macrocytic anemia (Gehrs and Friedberg 2002). A significant negative correlation between urinary 1-OHP concentration and RDW was also observed. No clinical significance has been reported if the RDW was lower than its normal range (Bessman et al. 1983). However, our statistical analysis revealed a significant correlation between RDW and MCH (r = −0.444, p = 0.001, data not shown). Since early mature red blood cells can be destroyed by PAHs oxidative damage and bone marrow fails to generate and release mature red blood cells on a timely manner, red blood cell distribution is relatively narrow. Hence, along with increasing risks due to red blood cell damage, the red blood cell size could become imbalance and increase RDW value.
The logistic regression analyses showed that smoking could increase the risk of red blood cells abnormality in workers (Table 4). The International Agency for Research on Cancer (IARC) listed at least 35 parent PAHs and a number of methylated PAHs with three or more fused rings formed in cigarette smoking (IARC 1986). Depending on the cigarette brand, smoke type, and filtering, the pyrene generation per cigarette is 50–270 ng in mainstream smoke and 390–1010 ng in sidestream smoke (Li et al. 2000). Some of the enzyme induced constituents of mainstream tobacco smoke, however, may either be poor substrates for cytochrome P450 in the lung or reach the liver through portal blood. It has been also suggested that smoking is not only increasing exposure risk to PAHs from cigarettes, but also affected by other smoke ingredients such as carbolines and even higher chlorinated dioxins (Sherson et al. 1992). This may explain the apparent additive effect of smoking on the risk of red blood cells abnormality. While tissue–blood partition coefficients are employed to measure the route of a contaminant entering into a certain target tissue which causes certain big damage to this tissue, determination of tissue–blood partition coefficient by experimental methods is time-consuming and expensive. As an alternative, the PBTK model has been often applied in the assessments of migration and transformation of contaminants (Cahill et al. 2003). Pyrene is a main composition of PAHs in the environment. It is stable and follows the same route as other PAHs to enter into a human body. Significant correlations between pyrene and total PAHs in air (r = 0.87) and dermal (r = 0.65) samples were reported (McClean et al. 2004). In our case, we found relative higher brain–blood and bone marrow–blood partition coefficients. The brain–blood partition coefficients are often used to measure the ability of PAH compounds to break through the blood brain barrier (Prentis et al. 1988; Cai and Yan 2008). It has been reported that PAHs can move the blood–brain barrier into the brain tissue (Nie et al. 2009). As a result, neural behavior of the occupational workers might be altered by their exposure to PAHs (Persson et al. 2002; Nie et al. 2009). In vitro experiments demonstrated that PAHs could affect multiple tumor types in hematopoietic tissues and reduce the cellularity of the bone marrow which in turn could contribute to impairment of humoral and cell-mediated immunity (Galvan et al. 2006; Heidel et al. 2000). Large values of bone marrow–blood partition coefficients for pyrene and its metabolites 1-OHP predict that PAHs can lead to an impairment of bone marrow hematopoietic cells. Declining hematopoietic function of bone marrow could induce red blood cells abnormality risk.
To gain further insight into understanding of the effects of PAHs exposure on red blood cells, we compared the urinary 1-OHP concentration in red blood cells normal group and abnormal group with the urinary 1-OHP genotoxic effect limits. Although genotoxic endpoints of genotoxic effect limits were cytogenetic damage in PAH-exposed workers which were mainly measured in white blood cells (WBC) of workers (Jongeneelen 2014), we found a significant correlation at the 95 % level (p < 0.05) between WBC and Hgb at r = 0.295 (p = 0.032, data not shown). This explains the association between urinary 1-OHP concentration in red blood cells normal and abnormal group and the urinary 1-OHP genotoxic effect limits. We have shown in “Comparing urinary 1-OHP concentration with urinary 1-OHP genotoxic effect limits” that the mean 1-OHP concentrations in petrochemical workers were higher than LOGEL in both red blood cells normal group and abnormal group, but not in office workers. The significant difference in urinary 1-OHP concentration was only found between petrochemical workers and office workers in red blood cells normal group (p = 0.001, data not shown). This suggests that in red blood cells abnormal group, PAH concentrations were high in both petrochemical workers and office workers. In this case, there was no significant difference in urinary 1-OHP concentration between the petrochemical workers and office workers.
ILCR modeling of cancer risks of petrochemical workers from PAHs exposure
Red blood cells abnormality is also a potential biomarker in other diseases, such as lung cancer (Koma et al. 2013), heart failure (Felker et al. 2007), and inflammatory disorders (Song et al. 2012). Our ILCR modeling results showed that the ILCR for the petrochemical workers ranged from 8.9 × 10−5 to 7.7 × 10−4, indicating high cancer risk in the petrochemical workers. Sensitivity analysis in “Sensitivity and uncertainty analysis” yielded the averaging contribution variance of 58.3 % from the occupational exposure concentration (Co) which was the most influential variable on the workers’ inhalation exposure to PAHs. Negative correlation was found between body weight (BW) and workers’ inhalation cancer risk. This is consistent with the result from Chen and Liao (2006). The averaging contributions variance from BW was −26.1 % to the workers inhalation ILCR. It has been demonstrated that people with relatively lower body weight would have relatively high basal metabolism. Such metabolism could accelerate cell division in lung, thereby increasing the cancer risk (Drinkard et al. 1995).
Overall, our investigation was based on a relatively small number of samples with only 53 participants involved in the investigation. The effect of psychophysiological stress and dietary history, which could also affect red blood cells abnormality, was not taken into consideration. Further study is planned. Nevertheless, despite these limitations, our findings provide important information in the deleterious effects of PAHs exposure on red blood cell abnormality in petrochemical workers.
Conclusions
In the present study, we used the biomarker urinary 1-OHP as well as PBTK and ILCR models to assess quantitatively the health exposure risks of occupational workers to PAHs from a petrochemical plant in Xigu, Lanzhou city, northwest China. Results revealed that high PAHs exposure could induce red blood cells abnormality risk of anemia. Compared with the odds ratio (OR) among office workers at 1.0, the OR for petrochemical workers is 1.417, indicating that the risk of red blood cells abnormality in petrochemical workers increased by 41.7 %. PAHs release and levels in the working environment in the petrochemical plant were the major influence factor on the human red blood cells anomalies. The estimated tissue–human blood partition coefficient for pyrene and 1-OHP by the PBTK model supported this finding. The ILCR modeling result further confirmed that the petrochemical workers involved in this investigation were under a high potential cancer risk.
References
Beliveau M, Krishnan K (2005) A spreadsheet program for modeling quantitative structure-pharmacokinetic relationships for inhaled volatile organics in humans. SAR QSAR Environ Res 16:63–77
Bessman JD, Gilmer JP, Gardner FH (1983) Improved classification of anemias by MCV and RDW. Am J Clin Pathol 80(3):322–326
Block ML, Calderon-Garciduenas L (2009) Air pollution mechanisms of neuroinflammation and CNS disease. Trends Neurosci 32(9):506–516
Buchet JP, Ferreira M, Burrion J, Leroy T, Kirsch VM, Van HP et al (1995) Tumor markers in serum, polyamines and modified nucleosides in urine, and cytogenetic aberrations in lymphocytes of workers exposed to polycyclic aromatic hydrocarbons. Am J Ind Med 27(4):523–543
Cahill TM, Cousins I, Mackay D (2003) Development and application of a generalized physiologically based pharmacokinetic model for multiple environmental contaminants. Environ Toxicol Chem 22(1):26–34
Cai Z-Y, Yan A-X (2008) Quantitative structure activity relationships in blood brain partitioning. J Beijing Univ Chem Technol 35(3):65–69 (in Chinese)
Chen SC, Liao CM (2006) Health risk assessment on human exposed to environmental polycyclic aromatic hydrocarbons pollution sources. Sci Total Environ 366(1):112–123
Chen B, Hu Y, Jin T, Zheng L, Wang Q, Shen Y et al (2007) Higher urinary 1-hydroxypyrene concentration is associated with cooking practice in a Chinese population. Toxicol Lett 171(3):119–125
Chiu WA, Barton HA, DeWoskin RS, Schlosser P, Thompson CM, Sonawane B, Lipscomb JC, Krishnan K (2007) Evaluation of physiologically based pharmacokinetic models for use in risk assessment. J Appl Toxicol 27:218–237
DeJongh J, Verhaar HJ, Hermens JL (1997) A quantitative property-property relationship (QPPR) approach to estimate in vitro tissue-blood partition coefficients of organic chemicals in rats and humans. Arch Toxicol 72:17–25
Drinkard CR, Sellers TA, Potter JD, Zheng W, Bostlck RM, Nelson CL et al (1995) Association of body mass index and body fat distribution with risk of lung cancer in older women. Am J Epidemiol 142(6):600–607
Duan XL, Tao S, Xu DQ, Jiang QJ (2011) Exposure measurement and health risk assessment of human exposure to polycyclic aromatic hydrocarbons. China Environmental Science Press
Felker GM, Allen LA, Pocock SJ, Shaw LK, McMurray JJV et al (2007) Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. J Am Coll Cardiol 50:40–47
Galvan N, Page TJ, Czuprynski CJ, Jefcoate CR (2006) Benzo (a) pyrene and 7, 12-dimethylbenz (a) anthrecene differentially affect bone marrow cells of the lymphoid and myeloid lineages. Toxicol Appl Pharmacol 213(2):105–116
Gao H, Ma MQ, Zhou L, Jia RP, Chen XG, Hu ZD (2007) Interaction of DNA with aromatic hydrocarbons fraction in atmospheric particulates of Xigu District of Lanzhou, China. J Environ Sci 19(8):948–954
Gehrs BC, Friedberg RC (2002) Autoimmune hemolytic anemia. Am J Hematol 69:258–271
Han IK, Duan XL, Zhang L, Yang H, Rhoads GG, Wei F, Zhang J (2008) 1-Hydroxypyrene concentrations in first morning voids and 24-h composite urine: intra- and inter-individual comparisons. J Expo Sci Environ Epidemiol 18:477–485
Heidel SM, MacWilliams PS, Baird WM, Dashwood WM, Buters JT, Gonzalez FJ et al (2000) Cytochrome P4501B1 mediates induction of bone marrow cytotoxicity and preleukemia cells in mice treated with 7, 12-dimethylbenz [a] anthracene. Cancer Res 60(13):3454–3460
IARC (1983) Polynuclear aromatic compounds, Part 1, chemical, environmental and experimental data. IARC monographs on the evaluation of the carcinogenicity risk of chemical to humans, Lyon: Int Agency Res Cancer vol 32
International Agency for Research on Cancer (1986) Tobacco smoking. In: IARC monographs on the evaluation of carcinogenic risks of chemicals to humans, vol. 38. Lyon: IARC.
Jongeneelen FJ (1997) Methods for routine biological monitoring of carcinogenic PAH-mixtures. Sci Total Environ 199(1):141–149
Jongeneelen FJ (2001) Benchmark guideline for urinary 1-hydroxypyrene as biomarker of occupational exposure to polycyclic aromatic hydrocarbons. Ann Occup Hyg 45(1):3–13
Jongeneelen FJ (2011) A generic, cross-chemical predictive PBTK model with multiple entry routes running as application in MS Excel; design of the model and comparison of predictions with experimental results. Ann Occup Hyg 55(8):841–864
Jongeneelen FJ (2012) Simulation of urinary excretion of 1-hydroxypyrene in various scenarios of exposure to polycyclic aromatic hydrocarbons with a generic, cross-chemical predictive PBTK-model. Int Arch Occup Environ Health 85(6):689–702
Jongeneelen FJ (2014) A guidance value of 1-hydroxypyrene in urine in view of acceptable occupational exposure to polycyclic aromatic hydrocarbons. Toxicol Lett 231(2):239–248
Kaidar PO, Nasrallah H, Haim N, Dann EJ, Bar SG (2011) Disseminated carcinoma diagnosed by bone marrow biopsy in patients with microangiopathic hemolytic anemia and thrombocytopenia: a report of two cases with gastric cancer and a review of the literature. J Gastrointest Cancer 42:123–126
Keimig SD, Kirby KW, Morgan DP, Keiser JE, Hubert TD (1983) Identification of 1-hydroxypyrene as a major metabolite of pyrene in pig urine. Xenobiotica 13:415–420
Kim Y, Shim H, Kim K, Park H, Jang S, Park Y (2014) Profiling individual human red blood cells using common-path diffraction optical tomography. Sci Rep 4:6659
Knopp D, Schedl M, Achatz S, Kettrup A, Niessner R (1999) Immunochemical test to monitor human exposure to polycyclic aromatic hydrocarbons: urine as sample source. Anal Chim Acta 399(1):115–126
Koma Y, Onishi A, Matsuoka H, Oda N, Yokota N, Koyama M et al (2013) Increased red blood cell distribution width associates with cancer stage and prognosis in patients with lung cancer. PLoS One 8(11):1–7
Lee MS, Eum KD, Zoh KD, Kim TS, Pak YS, Paek D (2007) 1-Hydroxypyrene as a biomarker of PAH exposure among subjects living in two separate regions from a steel mill. Int Arch Occup Environ Health 80(8):671–678
Li H, Krieger RI, Li QX (2000) Improved HPLC method for analysis of 1-hydroxypyrene in human urine specimens of cigarette smokers. Sci Total Environ 257:147–153
Liu YY (2009) A study on the influence of asphalt fume over exposed workers. Dissertation, University of Lanzhou
Liu GR, Peng X, Wang RK, Tian YZ, Shi GL, Wu JH, Zhang P, Zhou LD, Feng YC (2015) A new receptor model-incremental lifetime cancer risk method to quantify the carcinogenic risks associated with sources of particle-bound polycyclic aromatic hydrocarbons from Chengdu in China. J Hazard Mater 283:462–468
McClean M, Rinehart R, Ngo L, Eisen E, Kelsey K, Wiencke J et al (2004) Urinary 1-hydroxypyrene and polycyclic aromatic hydrocarbon exposure among asphalt paving workers. Ann Occup Hyg 48(6):565–578
Mcphee SJ, Lingappa VR, Ganong WF (2006) Pathophysiology of disease, 5th edn. Lange Medical Books, New York, pp 106–110
Nie J-S, Sun J-Y, Zhang H-M, Shi Y-T, Wang L-P, Song J et al (2009) Effect of polycyclic aromatic hydrocarbons on peripheral nerve conduction velocity in exposed workers. Chin J Ind Med 2(22):123–125 (in Chinese)
Pan G, Zhang S, Feng Y, Takahashi K, Kagawa J, Yu L et al (2010) Air pollution and children’s respiratory symptoms in six cities of Northern China. Respir Med 104(12):1903–1911
Parham FM, Kohn MC, Matthews HB, DeRosa C, Portier CJ (1997) Using structural information to create physiologically based pharmacokinetic models for all polychlorinated biphenyls. I Tissue: Blood Partition Coefficients. Toxicol Appl Pharmacol 144:340–347
Persson E, Larsson P, Tjalve H (2002) Cellular activation and neuronal transport of intranasally instilled benzo (a) pyrene in the olfactory system of rats. Toxicol Lett 133(2):211–219
Peterson BS, Rauh VA, Bansal R, Hao XJ, Toth Z, Nati G et al (2015) Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA Psychiatry 72(6):531–540
Prentis RA, Lis Y, Walker SR (1988) Pharmaceutical innovation by seven UK owned pharmaceutical companies (1964–1985). Clin Pharmacol 25(3):387–396
Robinson JR, Felton JS, Levitt RC (1975) Relationship between “aromatic hydrocarbon responsiveness” and the survival time in mice treated with various drugs and environmental compounds. Mol Pharmacol 11:850–865
Sanctucci K, Shah B (2000) Association of naphthalene with acute hemolytic anemia. Acad Emerg Med 7(1):42–47
Sherson D, Sigsgaard T, Overgaard E, Loft S, Poulsen HE, Jongeneelen FJ (1992) Interaction of smoking, uptake of polycyclic aromatic hydrocarbons, and cytochrome P450IA2 activity among foundry workers. Br J Ind Med 49:197–202
Shi L, Shu XO, Li H, Cai H, Liu Q, Zheng W et al (2013) Physical activity, smoking, and alcohol consumption in association with incidence of type 2 diabetes among middle-aged and elderly chinese men. PLoS ONE 8(11):1–7
Singh NN, Srivastava AK (2010) Haematological parameters as bioindicators of insecticide exposure in teleosts. Ecotoxicology 19(5):838–854
Song CS, Park DI, Yoon MY, Seok HS, Park JH et al (2012) Association between red cell distribution width and disease activity in patients with inflammatory bowel disease. Dig Dis Sci 57:1033–1038
Strickland P, Kang D (1999) Urinary 1-hydroxypyrene and other PAH metabolites as biomarkers of exposure to environmental PAH in air particulate matter. Toxicol Lett 108(2):191–199
Tao Y, An X, Sun Z, Hou Q, Wang Y (2012) Association between dust weather and number of admissions for patients with respiratory diseases in spring in Lanzhou. Sci Total Environ 423:8–11
Taussky HH (1954) A microcolorimetric determination of creatine in urine by the Jaffe reaction. J Biol Chem 208(2):853–861
Vainio H, Wilbourn J (1992) Identification of carcinogens within the IARC monograph program. Scand J Work Environ Health 18(11):64–73
Van Delft JHM, Steenwinkel MJ, Asten JG, Vogel N, Bruijntjes TC, Schouten T et al (2001) Biological monitoring the exposure to polycyclic aromatic hydrocarbons of coke oven workers in relation to smoking and genetic polymorphisms for GSTM1 and GSTT1. Ann Occup Hyg 45(5):395–408
Wang YY, Lin SY, Liu PH, Cheung BM, Lai WA (2004) Association between hematological parameters and metabolic syndrome components in a Chinese population. J Diabetes Complicat 18:322–327
Wang S, Feng X, Zeng X, Ma Y, Shang K (2009) A study on variations of concentrations of particulate matter with different sizes in Lanzhou, China. Atmos Environ 43:2823–2828
Wang F, Zou Y, Shen Y, Zhong Y, Lv Y, Huang D et al (2015) Synergistic impaired effect between smoking and manganese dust exposure on pulmonary ventilation function in Guangxi manganese-exposed workers healthy cohort (GXMEWHC). PLoS ONE 10(2):1–11
Woodard HQ, White DR (1986) The composition of body tissues. Br J Radiol 59:1209–1218
Xia ZH, Duan XL, Qiu WX, Liu D, Wang B, Tao S, Jiang QJ, Lu B, Song YX, Hu XX (2010) Health risk assessment on dietary exposure to polycyclic aromatic hydrocarbons (PAHs) in Taiyuan, China. Sci Total Environ 408(22):5331–5337
Xia ZH, Duan XL, Qiu WX, Liu D, Wang B, Tao S, Jiang QJ, Lu B, Song YX, Hu XX (2013) Pollution level, inhalation exposure and lung cancer risk of ambient atmospheric polycyclic aromatic hydrocarbons (PAHs) in Taiyuan, China. Environ Pollut 173:150–156
Yang CY, Cheng BH, Hsu TY, Chuang HY, Wu TN, Chen PC (2002) Association between petrochemical air pollution and adverse pregnancy outcomes in Taiwan. Arch Environ Health 57(5):461–465
Yang HM, Wang YS, Li JH, Li GR, Wang Y, Tan X et al (2009) Synchronous fluorescence determination of urinary 1-hydroxypyrene, β-naphthol and 9-hydroxyphenanthrene based on the sensitizing effect of β-cyclodextrin. Anal Chim Acta 636(1):51–57
Zhang L, Chen C-H, Li S-X, Zhang F (2000) Air pollution and potential control schemes in Lanzhou. Res Environ Sci 13:18–21 (in Chinese)
Zhang Y, Ma R, Fan SH (2010) Determination of 1-hydroxyprene in human urine by synchronous fluorescence spectrometry with Tween 20 as enhancer. Chin J Anal Chem 38(7):958–962
Zhang X-D, Zhang S-Q, Liu Y-Q (2011) An analysis of cancer incidence in 2007 in Lanzhou, Gansu. China Cancer 20(11):806–809 (in Chinese)
Zhao ZH, Quan WY, Tian DH (1990) Urinary 1-hydroxypyrene as an indicator of human exposure to ambient polycyclic aromatic hydrocarbons in a coal-burning environment. Sci Total Environ 92:145–154
Zhao ZH, Quan WY, Tian DH (1992) Experiments on the effects of several factors on the 1-hydroxypyrene level in human urine as an indicator of exposure to polycyclic aromatic hydrocarbons. Sci Total Environ 113(3):197–207
Acknowledgments
This study was supported by National Science Foundation of China (Grants 41371453, 41371478, and 40971267). The authors would like to thank the State Key Laboratory of Environmental Chemistry and Ecotoxicology, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences for funding support through the Open Research Project KF2012-03.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 42 kb)
Rights and permissions
About this article
Cite this article
Wang, L., Zhao, Y., Liu, X. et al. Cancer risk of petrochemical workers exposed to airborne PAHs in industrial Lanzhou City, China. Environ Sci Pollut Res 22, 19793–19803 (2015). https://doi.org/10.1007/s11356-015-5203-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5203-2