Abstract
Anatomy as a descriptive topic of research and instruction in medicine has been increasingly influenced by discoveries in molecular cell and developmental biology and most recently the advent of human induced pluripotent stem cells and organoids. We summarize here how anatomy has been influenced by developmental and stem cell biologists, and how in vitro modelling of the three-dimensional body environment is emerging to understand structure and function of cells during differentiation processes in development and disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Anatomy is the science of the structure of organisms and their parts. Human anatomy as one of the basic essential topics of research and instruction in medicine can be followed back to ancient Greek and Egyptian times as known from the Edwin Smith Papyrus, dated 1600 BCE, which already described heart, blood vessels, liver, kidney, hypothalamus, uterus and bladder (unknown 1600 BCE). In addition to the essential groundwork of descriptive anatomy on which all surgical disciplines are based also nowadays, it is noteworthy that over the centuries anatomists deeply engaged in the analysis of developmental aspects and used animal models to unravel their details and functions. For more than 3500 years, human anatomy has been mostly descriptive and still is in the medical curricula. However, in order to understand how structure and function are intrinsically and causally related, experiments moved into the focus gradually. Such experiments resulted in pioneering insights into the function of the human body on which a considerable proportion of Western medical therapies are based. Remarkably, many of the experimenting anatomists established or improved embryonic animal models.
Among the descriptive anatomists, Harvey (1578–1657) pioneered the discovery of the circulatory system in avian embryos. After the invention of the first microscopes by the Dutch cloth trader Van Leeuwenhoek (1632–1723), the advent of microscopic anatomy was facilitated by small-scale analyses of tissues and body fluids. Prior to the discovery of the mammalian oocyte, Wolff and Purkinje in 1825 investigated into the avian blastodisc and germinal vesicle. Eventually, the description of the mammalian including the human oocyte by von Baer (1792–1876) was a groundbreaking finding that created the basis for reproductive biology and medicine (von Baer 1827). In Europe, the disciplines comparative anatomy and zoology, and also physiology, were traditionally combined in one department or institute until the 1940s. Hence, the establishment of easily accessible animal models to study reproduction as well as development were in the hands of anatomists. Among them, Haeckel (1834–1919) and Müller (1801–1858), director of the Museum of Natural Sciences in Berlin, ranged among the most prominent researchers of developmental processes. At the end of the nineteenth century, Hertwig achieved a milestone by his observations on sea urchin fertilization and embryogenesis (Hertwig 1876). His publication sets the stage for innumerable studies on early animal development by the forthcoming generations of scientists. The Swiss anatomist and physiologist Wilhelm His (1831–1904) was dedicated to the three-dimensional reconstruction of sectioned embryos, and in particular, he investigated the development of the nervous system and was the first to describe the neuroblast.
One of the most important findings of experimental embryology with an enormous bearing on stem cell biology was derived from the experimental work of Spemann (1869–1941) and his pupils (Spemann and Mangold 1924). The central idea that the genome residing in all somatic cells gradually unfolds during development as a consequence of local signalling between neighbouring structures in the embryo constituted the basis for the notions of totipotency, pluripotency, etc., lineage commitment and determination. It furthermore inaugurated the concept of epigenetics that goes back to the theoretical considerations of Waddington (1905–1975) who designed the famous drawing of the epigenetic landscape that is still used by stem cell researchers today to describe cell commitment and the activation and inactivation of genetic programs related to developmental potencies (Waddington 1957; Takahashi 2012).
In contrast to single cells, the formation of tissues and organs involves an additional level of complexity, namely 3D spatial arrangement, resulting in pattern formation. For regenerative medicine, correct organ patterning and stability present the greatest challenge. Historically, the developmental patterning of organs has been experimentally studied in two complementary ways: (1) by grafting of organ anlagen (homotopic and heterotopic) and (2) by cell dissociation and reaggregation experiments (isotypical and heterotypical). Experiments were carried out in different vertebrates, e.g. in the embryonic chicken model, where kidney, liver and skin cells of 8- to 14-day embryos were reaggregated and tracked in 8-day embryos (Weiss and Taylor 1960). In this way, the contributions of embryonic cell populations to adult derivatives or transitory structures, as well as their time of commitment, could be determined. In the absence of reporter genes, the first classical experiments used natural markers, for example grafting from the quail to the chicken embryo. The most well-known results obtained were the multiple derivatives of the neural crest (Le Douarin and Teillet 1974), and the somitic origin of the skeletal muscle in the limbs (Christ et al. 1977; Fig. 1). Interestingly, the migration and spatial arrangement of grafted cells, even after dissociation, followed specific rules also active during normal embryonic development. Cells showed characteristic germ layer preferences (Moscona 1952; Townes and Holtfreter 1955) that set the grounds for the discovery of cell adhesion molecules (Takeichi 1987) and are the basis for our current understanding of embryoid body formation and organoid culture as discussed below.
Somite transplantation experiment from a quail embryo into a chick embryo; Feulgen-staining reveals nucleolus-associated heterochromatin, a natural feature of quail cells which are found in myoblasts of limb muscle blastemas (arrows). Experiment performed by Prof. Bodo Christ (1941–2016) and Dr. Heinz-Jürgen Jacob; original specimen photographed from the embryological collection of the Institute of Anatomy, Ruhr-Universität Bochum
Since the 1960s the disciplines ‘Macroscopic Anatomy’ and ‘Microscopic Anatomy’ have been increasingly complemented by more discoveries in molecular cell and developmental biology, giving rise to a discipline which could be considered as ‘Molecular Anatomy’. In the recent decades, development of tissues and organs could be increasingly studied in animal model systems, which were also amenable to genetic engineering strategies. By merging key findings from developmental biology, reproductive biology and molecular biology including epigenetic gene regulation, a considerable breakthrough for cell differentiation research was brought about during the last decade: the induction of pluripotent stem cells (PSCs) which resulted in awarding the Nobel Prize to Shinya Yamanaka and Sir John Gurdon in 2012. Human PSCs, first derived from blastocysts (Thomson et al. 1998), later by transcription factor—induced reprogramming of human somatic cells (Takahashi et al. 2007), opened a new era to many disciplines including anatomy by allowing to study human development with genuine human in vitro differentiation model systems.
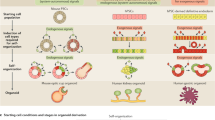
Striking recent achievements explore the differentiation of three-dimensional organ-like tissues, called organoids from human pluripotent stem cells. The term ‘organoid’ refers to ‘resembling an organ’ concerning its cell types, their organization as well as recapitulating their function (Lancaster and Knoblich 2014). Pluripotent stem cells left in suspension culture without the inductive clues to self-renew (e.g. LIF in mouse, bFGF in human) tend to form three-dimensional aggregates termed ‘embryoid bodies’ (EB), which consist of cells of all three germ layers. In principle, the various organoid protocols resemble patterning processes in embryonic development, whereby signal transduction and transcription factor cascades involved in germ layer and lineage specifications (e.g. Hox, Pax, Wnt, Bmp, Fgf, Egf, Tgf) are sequentially applied to differentiating PSCs/EBs in liquid culture systems or embedded in semisolid gels resembling extracellular matrices. Both factors, which induce or inhibit certain developmental signalling cascades as well as surface interactions, support the self-organization process towards a certain tissue structure. The organoid approach can in theory be applied to every human organ system, since protocols to generate all 200 cell types of the human body from pluripotent stem cells can be refined from partially available two-dimensional protocols to organoid aggregation protocols in the years to come.
Most prominent among all organoids might be cerebral organoids, termed ‘mini-brains’, representing different brain regions including Sox2-positive neural progenitor/stem cell layers (Lancaster et al. 2013, Fig. 2). Organoid induction from human PSCs within the ectodermal lineage has also been very successfully applied to the differentiation towards retinal structures (Nakano et al. 2012). PSCs can be patterned towards hindgut endoderm via mesendoderm. Examples among endodermal organoids include modelling of intestinal tissue (Spence et al. 2011), gastric organs (McCracken et al. 2014), liver (Takebe et al. 2013) and lung (Wong et al. 2012; Huang et al. 2014). Mesodermal kidney structures have been modelled as complex multicellular organoids from PSC-derived mesendodermal precursors (Takasato et al. 2015). Heart and skeletal muscle as well as bone organoids from PSCs are, however, still in their infancy.
Neural organoid structures differentiated from human induced pluripotent stem cells (day 30 after induction, yellow: neural progenitor marker Sox2, red: neuronal marker tubulin beta III, blue: nucleic DAPI stains) (Figure provided by Prof. Hans Schöler and Dr. Jan Bruder, Max-Planck-Institut für molekulare Biomedizin, Münster)
All current protocols result in organoids with a certain degree of positional randomness, which can partially be attributed to a lack of mimicking embryonic axis formation in the three-dimensional protocols developed so far (Lancaster and Knoblich 2014). Furthermore cell types patterned from PSC within organoids very likely resemble embryonic or foetal phenotypes; this ‘maturation issue’ is also observed in cell types differentiated from PSCs in two-dimensional protocols. Furthermore, to recapitulate organ function, organoid cells also must get a sufficient nutrition supply, which might not be ensured in the inner part of an organoid lacking vascularization. After transplantation, vascularization can be induced by organoid—host cell interactions (Takebe et al. 2013).
As an alternative to patterning organoids from pluripotent stem cells, explanted adult somatic stem cells can be the source for organoid formation, thereby mimicking stem cell niches physiologically (Clevers 2016). This approach has been pioneered by exploring the Wnt pathway in gut stem cells, which are marked by Lgr5 receptor expression. Thereby isolated Lgr5+ intestinal stem cells can be patterned towards crypt-villus structures (‘mini-guts’) (Sato et al. 2009). Further studies used these somatic gut stem cells to differentiate towards stomach, liver and pancreas organoids, which demonstrates that similar organoid structures might either be patterned from PSCs or from explanted adult stem cell populations.
Novel genome editing technologies can be employed in organoid cultures to introduce genetic alterations or phenotypic rescues, as demonstrated for patient-derived intestinal stem cell organoids with mutations at the cystic fibrosis transmembrane conductance regulator (CFTR) locus corrected by CRISPR/Cas9 mediated homologous recombination (Schwank et al. 2013). Modelling and correcting hereditary diseases is just one major aspect to utilize organ-resembling organoids; acquired diseases like pathogen infections as exemplified by challenging human PSC-derived stomach organoids with Helicobacter pylori as well as provision of organoids modelling oncogenesis are targets of these research endeavours (Boj et al. 2015; McCracken et al. 2014).
The science of the structure of organism and their parts has been enriched maybe even transformed by pluripotent stem cell and organoid technology in meaningful ways. In future, it will become increasingly feasible to create novel physiologically complex in vitro models of human diseases with patient-derived organoids to study (developmental) pathogenesis or—for a more translational approach—to do pharmaceutical drug screening, as well as to implement gene and tissue engineering. Organoids will be applied to further develop direct reprogramming strategies, e.g. the conversion of one cell lineage to another via expression of lineage-specific factors within physiological niches, which will lead to novel in vivo reprogramming therapeutic modalities. The developments in molecular cell and developmental biology, we have highlighted here, have tremendous impact on human anatomy as a scientific discipline as well as regenerative medicine in clinical practice.
References
Boj SF, Hwang CI, Baker LA et al (2015) Organoid models of human and mouse ductal pancreatic cancer. Cell 160:324–338
Christ B, Jacob HJ, Jacob M (1977) Experimental analysis of the origin of wing muscles from the somites. Anat Embryol 150:171–186
Clevers H (2016) Modeling development and disease with organoids. Cell 165:1586–1597
Hertwig O (1876) Beiträge zur Kenntnis der Bildung, Befruchtung und Theilung des thierischen Eies. Morphologischer Jahrband 1:347–434
Huang SX, Islam MN, O’Neill J, Hu Z, Yang YG, Chen YW, Mumau M, Green MD, Vunjak-Novakovic G, Bhattacharya J, Snoeck HW (2014) Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol 32:84–91
Lancaster MA, Knoblich JA (2014) Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345:1247125
Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA (2013) Cerebral organoids model human brain development and microcephaly. Nature 501:373–379
Le Douarin N, Teillet M (1974) Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neuroectodermal mesenchymal derivatives using a biological cell marking technique. Dev Biol 41:162–184
McCracken KW, Catá EM, Crawford CM, Sinagoga KL, Schumacher M, Rockich BE, Tsai YH, Mayhew CN, Spence JR, Zavros Y, Wells JM (2014) Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516:400–404
Moscona A (1952) Cell suspension from organ rudiments of chick embryos. Exp Cell Res 3:535–539
Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, Saito K, Yonemura S, Eiraku M, Sasai Y (2012) Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10:771–785
Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459:262–265
Schwank G, Koo BK, Sasselli V, Dekkers JF, Heo I, Demircan T, Sasaki N, Boymans S, Cuppen E, van der Ent CK, Nieuwenhuis EE, Beekman JM, Clevers H (2013) Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 13:653–658
Spemann H, Mangold H (1924) Über Induktion von Embryonalanlagen durch Implantation artfremder Organisatoren. Archiv für mikroskopische Anatomie und Entwicklungsmechanik 100:599–638
Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, Wells JM (2011) Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470:105–109
Takahashi K (2012) Cellular reprogramming–lowering gravity on Waddington’s epigenetic landscape. Cell Sci 125:2553–2560
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872
Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, de Sousa Chuva, Lopes SM, Little MH (2015) Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526:564–568
Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N, Aoyama S, Adachi Y, Taniguchi H (2013) Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499:481–484
Takeichi M (1987) Cadherins: a molecular family essential for selective cell-cell adhesion and animal morphogenesis. Trends Genet 3:213–217
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147
Townes PL, Holtfreter J (1955) Directed movements and selective adhesion of embryonic amphibian cells. J Exp Zool 128:53–129
Unknown (1600 BCE) Edwin Smith Papyrus, rare book room, New York Academy of Medicine
von Baer KE (1827) De ovi mammalium et hominis genesi epistolam. Lipsiae Sumptibus L. Vossii, Leipzig
Waddington CH (1957) The strategy of the genes. George Allen and Unwin, London
Weiss P, Taylor AC (1960) Reconstitution of complete organs from single-cell suspensions of chick embryos in advanced stages of differentiation. Proc Natl Acad Sci USA 46:1177–1185
Wong AP, Bear CE, Chin S, Pasceri P, Thompson TO, Huan LJ, Ratjen F, Ellis J, Rossant J (2012) Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat Biotechnol 30:876–882
Acknowledgments
We are acknowledging the continuous financial support by the Medical Faculty, Ruhr-Universität Bochum for our stem cell research and teaching activities. Considering the impact that the above-reviewed developments in stem cell research have on human anatomy, we were encouraged to establish the specialized ‘Master Programme‚ Molecular and Developmental Stem Cell Biology’ headed at the Department of Anatomy and Molecular Embryology at Ruhr-Universität Bochum (iSTEM, http://www.ruhr-uni-bochum.de/istem/index.html.en). The international four semesters course programme towards a ‘Master of Science’ includes lectures and courses on ‘Stem Cell Physiology’, ‘Molecular Genetic Methods’, ‘Tissue Engineering’, ‘Pathology of Degenerative Diseases’ and ‘Advances in Stem Cell Research’.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Dr. med. Detlev Drenckhahn, in appreciation of his constant and outstanding efforts as an Editor-in-Chief of Histochemistry and Cell Biology.
Rights and permissions
About this article
Cite this article
Brand-Saberi, B., Zaehres, H. The development of anatomy: from macroscopic body dissections to stem cell–derived organoids. Histochem Cell Biol 146, 647–650 (2016). https://doi.org/10.1007/s00418-016-1497-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-016-1497-5