Abstract
Diabetes represents a major endemic disease throughout the world, and different therapeutic methods are used to treat the disease. Xenotransplantation of pig islet cells is a potential treatment for type 1 diabetes, but studies of protein expression in distinct islet cells are rare. ZnT8, a member of the slc30A gene family, is involved in islet endocrine hormone release and is a diabetes auto-antigen, raising the question of whether ZnT8 expression is regulated similarly in pig and human pancreas. We used nested RT-PCR to detect ZnT8 expression in pig pancreas and polyclonal antibody to examine possible co-localization with other islet hormones. Immunohistochemistry of sequential serial sections as well as double immunostaining of pancreatic tissues with antibodies against ZnT8, insulin, glucagon, and somatostatin shows that pig ZnT8 is exclusively co-expressed in insulin-producing, but not in glucagon- or somatostatin-producing cells. The absence of ZnT8 in glucagon-producing cells in pig islets indicates that zinc homeostasis is mediated by a different cellular mechanism compared with human islet cells. Our findings provide important information about the cell-type-specific expression of ZnT8 in porcine islet cells, which should be taken into account when evaluating different xenotransplantation approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The limited number of human donors for organ transplantation is a primary motivation for investigating animal models that may provide suitable organs. Such models include pigs, old-world primates, and as recently highlighted, new-world primates (Mohanasundaram et al. 2011). Compared with the primate groups, pigs have several advantages. They are easier to handle and breed, possess a high reproductive rate, and are widely used for genetic manipulation and cloning (Renner et al. 2013). Different pig tissues have been tested for their applicability in xenotransplantation. Pancreatic islet cells in particular have been used extensively ever since it was shown that transplanted porcine islet cells have the potential to reverse type 1 diabetes in nonhuman primates (Sun et al. 1996; Cardona et al. 2006, 2007; Hecht et al. 2009). However, the usefulness of organs or specific cell types for xenotransplantation requires cellular compatibility to lower the risk of possible cellular and physiological incompatibilities.
In islet B cells, zinc is needed to crystallize insulin and support the dense packaging of this essential molecule in small vesicles (Dodson and Steiner 1998). The absorption of zinc into islet cells is mediated by special transporters, which belong to the solute carrier group (slc). This group is classified into 51 different families responsible for the transport of multiple solutes, such as charged and uncharged organic molecules as well as inorganic ions (Hediger et al. 2004). Inorganic zinc is transported by two solute carrier families, slc39 and slc30 (Eide 2004; Palmiter and Huang 2004). Both families comprise additional subfamilies but only one is known for mammalian species, denoted as slc39A and slc30A (Eide 2004; Palmiter and Huang 2004). In addition, both groups differ in their structural formation and in physiological effects. The slc39A subfamily controls zinc influx from the extracellular fluid and from intracellular vesicles into the cytoplasm, whereas the slc30A subfamily serves as a regulator of intracellular zinc levels. These processes take place either by efflux from cells or by influx into intracellular vesicles (Gaither and Eide 2001; Eide 2006). Both subfamilies contain a number of different members. There are up to 14 (ZIP1-14) for the slc39A group and 10 (ZnT1-10) for the slc30A group (Cousins et al. 2006). The members of both groups are broadly distributed not only among different cellular compartments such as the plasma membrane, endoplasmic reticulum, Golgi apparatus, and secretory vesicles (Kambe 2011), but also in different mammalian organs. However, data on the slc39A tissue distribution in mammalians are limited to eight members (Zip1-2, Zip4-8, Zip12), as detected via mRNA transcripts (Eide 2004; Kambe et al. 2004). In contrast, slc30A tissue localization has been determined for almost all members (Cousins et al. 2006; Wang and Zhou 2010; Kambe 2011).
One slc30A subfamily member, ZnT8, has been detected in only a few mammalian organs, such as adipose tissue (Smidt et al. 2007), blood lymphocytes (Overbeck et al. 2008), the thyroid gland (Murgia et al. 2009), and the pancreas (Chimienti et al. 2004, 2005; Murgia et al. 2009; Mohanasundaram et al. 2011). Most primate and mouse species show localized ZnT8 expression in islet B and A cells, whereas in B cells, ZnT8 functions in the intracellular uptake of zinc into secretory vesicles, modulating insulin biosynthesis and secretion, the function of ZnT8 in A cells remains unclear (Gyulkhandanyan et al. 2008).
The link of ZnT8 to diabetes auto-antigenicity as well as the use of porcine islet cells for xenotransplantation approaches raises the question of how ZnT8 expression is regulated in pig islet cells. We therefore investigated cell-type-specific expression of ZnT8 in pig pancreatic tissue by double immunostaining techniques to assess its possible co-localization with other islet hormones. We found that ZnT8 is expressed in insulin-containing B cells but not in glucagon- or somatostatin-containing cells. Furthermore, RT-PCR experiments confirmed the local expression of ZnT8 in the pancreas.
Materials and methods
Animals and tissue preparation
Six pigs of the German Landrace breed ranging in age from 6 to 10 months were obtained from the experimental station of animal husbandry and small animal breeding of the University of Hohenheim. Pigs were housed and fed under the same conditions and slaughtered at different time points. Immediately after slaughter, pancreatic tissue was removed, sectioned, and divided into two parts. One part was placed in RNAlater (Life technologies, Darmstadt, Germany) before use for RT-PCR analysis, and the other one was immersion-fixed in Bouin’s solution for 48 h and after subsequent dehydration embedded in paraffin according to standard procedures. For immunohistochemical staining, serial sections were cut to a thickness of 3-5 μm and mounted on Superfrost glass slides (R. Langenbrinck, Emmendingen, Germany).
ZnT8 antibody and dot-blot assay
A synthetic peptide sequence corresponding to the N-terminal region of the pig ZnT8 (amino acids 52–67) was synthesized, conjugated to the N-terminus of keyhole limpet hemocyanin (KLH), and used to immunize two rabbits (Charles River, Kissleg, Germany). Antisera titers against the ZnT8 peptide were tested by enzyme-linked immunosorbent assay (ELISA).
Antibody specificity was tested by dot-blot assay as follows: four aliquots (1 μg/μL) of KLH, peptide, pig, and bovine serum albumin (BSA) were spotted on nitrocellulose membranes (Whatman, Dassel, Germany). Two additional aliquots of the primary and secondary antibodies were used as controls. Membranes were blocked for 1 h with blocking solution (5 % nonfat dry milk in PBS buffer, 0.05 % Tween20). After rinsing three times with washing solution (PBS buffer, 0.05 % Tween20), membranes were incubated with primary antiserum (anti-ZnT8) in PBS buffer overnight at three final dilutions (1:600, 1:1,000, and 1:3,000). Membranes were then rinsed again with washing solution and incubated for 1 h in PBS buffer containing peroxidase-conjugated goat anti-rabbit immunoglobulin (IgG) (1:4,000) (Sigma-Aldrich, Taufkirchen, Germany). Thereafter, membranes were rinsed again with washing solution and incubated with developer (3.35 mM 4-chloro-1-napthol solved in 20 % methanol, 80 % TBS-buffer, 0.02 % hydrogen peroxide) to visualize immunoreactive dots. The entire procedure was performed at room temperature.
Immunostaining
Serial sections were prepared for co-localization studies of ZnT8 with insulin, glucagon, and somatostatin, respectively. The primary and secondary antibodies used are listed in Table 1.
Immunostaining of pancreatic tissue was performed as follows. Sections were deparaffinized in clean xylene, rehydrated through a series of graded ethanol solutions, and incubated with 10 % hydrogen peroxide for 10 min to block endogenous peroxidase activity. After rinsing in PBS buffer, specimens were incubated with 10 % normal goat or rabbit serum blocking solution (Dako, Hamburg, Germany) for 30 min to reduce nonspecific immunoreactions. Samples were then incubated overnight with high specificity anti-ZnT8 antibody (1:2,000) in a humidity chamber at 5 °C. After overnight incubation, slides were rinsed and treated with biotinylated secondary goat anti-rabbit IgG (1:400) for 30 min at room temperature. After a final PBS buffer wash and incubation with streptavidin–biotin–horseradish peroxidase (Merck-Millipore, Darmstadt, Germany) for 30 min at RT, the reaction product was visualized with 3, 3′-diaminobenzidine (DAB) chromogen (Biotrend, Cologne, Germany).
Co-localization double immunostaining studies were performed as previously described (Schweiger et al. 2005). In brief, sections were first immunostained for ZnT8 and visualized by DAB reaction (brown). After several rinses in PBS buffer, sections were incubated twice for 2 min with LinBlock (Linaris, Wertheim, Germany). Thereafter, slides were rinsed again in PBS buffer and immunostained for glucagon or somatostatin using the ABC method and visualized by DAB (black).
The specificity of the staining reaction was demonstrated by serial dilution of the primary antigen, which resulted in a gradual decrease in signal intensity. Finally, all specimens were counterstained with hematoxylin, dehydrated, cleared with xylene, and mounted in Entellan (Merck, Darmstadt, Germany) for light microscopy examination.
Immunohistochemical controls
The control experiments were performed as follows: (1) normal goat or rabbit serum was substituted for primary antibody, (2) omitting the primary antibodies and incubating with secondary antibodies alone, and (3) incubation with the DAB reagent alone to exclude the possibility of nonsuppressed endogenous peroxidase activity. No specific staining was observed in control experiments.
RNA extraction
Total RNA was isolated from 15 mg of pancreatic tissue using a RNA isolation kit (Promega, Mannheim, Germany). To rule out contamination by genomic DNA, isolated RNA was treated with RNA-free DNase according to the manufacturer’s protocol (Promega). Thereafter, RNA was quantified by measuring the A260/280. The RNA yields ranged from 1 to 2 μg per extraction. RNA quality was checked by 12.5 % polyacrylamide gel electrophoresis and silver staining (GE Healthcare, Freiburg, Germany).
Primer sequences
Primers specific for insulin and ZnT8 were selected from the NCBI nucleotide database (Table 2). The ZnT8 primer sequence covered the corresponding amino acid sequence of the peptide chosen for anti-ZnT8 polyclonal antibody production.
Performing RT-PCR and nested-PCR
Total RNA (1–2 μg) was denatured with a 500 ng mixture of oligo-dN and oligo-dT primers at 70 °C for 5 min, then slowly chilled to 25 °C and stored on ice. Thereafter, this mixture was used for reverse transcription in a 20 μL volume according to the manufacturer’s protocol (Promega). Reactions were incubated at 40 °C for 45 min followed by an additional 15 min at 70 °C and chilled on ice. After first-strand cDNA synthesis, amplification of insulin was carried out in 50 μL under the following conditions: denaturation at 94 °C for 2 min, followed by 35 cycles denaturation at 94 °C for 45 s, annealing at 63 °C for 45 s, and extension at 72 °C for 1.5 min. The last step was followed by a final extension at 72 °C for 7 min.
For ZnT8, we applied a two-step nested-PCR amplification as described above, with a few alterations. The annealing temperature was 58 °C in both steps, and at the end of the first PCR step, the probes were purified using a PCR purification kit (Macherey–Nagel, Düren, Germany) before using it in the second PCR.
PCR amplification products were visualized after electrophoresis on a 12.5 % polyacrylamide gel and visualized using a silver-staining kit (GE Healthcare).
Additionally, to rule out possible contamination by genomic DNA in RNA probes, the RT-PCR was performed without reverse transcriptase enzyme or RNA as well as without cDNA. No spurious DNA fragments were detected. ZnT8 PCR products were purified quantified and sequenced (Eurofins MWG Operon, Ebersberg, Germany).
Results
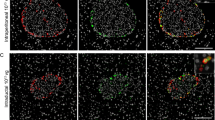
We detected specific immunostaining of ZnT8 in endocrine islet cells in pancreatic paraffin sections (Fig. 1a), with a characteristically strong signal localized to secretory granule structures in the cytoplasm. No immunoreactivity was observed in exocrine or centroacinar cells, fibroblasts, or blood vessels. Based on the number of cells and localization pattern, the data suggested that insulin-producing B cells (Fig. 1b) has the strongest ZnT8 expression. To exclude possible co-expression of ZnT8 in other endocrine cell types, we performed double immunostaining using antibodies against glucagon and somatostatin. These data indicated that both glucagon- and somatostatin-containing cells do not co-express ZnT8 (Fig. 1c, d). Glucagon-containing A cells are in close proximity to B cells but do not show immunoreactivity for ZnT8 (Fig. 1c). The same is true for somatostatin-producing cells, which are fewer in number and located mostly in the periphery of islets (Fig. 1d). Taken together, the double immunostaining data show that ZnT8 expression in porcine islet cells is restricted to insulin-producing B cells and is not co-expressed in other endocrine islet cell types in pig.
Cell-specific immunolocalization of ZnT8 in pig pancreas using serial sections with antibodies against ZnT8 (a) and insulin (b). Double immunostaining with antibodies in (c) against ZnT8 (brown) and glucagon (black) and in (d) against ZnT8 (brown) and somatostatin (black). Comparison of serial sections (a) and (b) clearly demonstrates that B cells are strongly immunostained for ZnT8 antigen. Double immunostaining in (c) and (d) further shows that glucagon- or somatostatin-containing cells do not co-localize with ZnT8 expression. The inserts in (c) and (d) illustrate that ZnT8-expressing cells (brown) are separated from cells expressing glucagon (black) or somatostatin (black)
We also tested antibody specificity in a dot-blot assay with several dilutions. ZnT8 antibody reacted in a dilution of 1:1,000 to the KLH-coupled and KLH-uncoupled peptides but displayed no reactivity to serum albumin proteins (Fig. 2). In addition, antibody pre-absorbed with antigen failed to stain peptide spots.
Dot-blot assays demonstrating the specificity of rabbit antiserum directed against ZnT8 protein at a dilution of 1:1,000. KLH-coupled and uncoupled peptides (Antig.) probes stain positive. Pig serum albumin (PSA) and BSA are negative. Primary antibody (p-AK) and peroxidase-coupled secondary antibody (s-AK) are used as controls
To confirm ZnT8 local expression at the RNA level, we applied the nested RT-PCR technique. By this method, we generate two different PCR products (Fig. 3) whose nucleotide sequence matched the predicted nucleotide sequence of pig ZnT8 (XM_001925124.3) published in the NBCI nucleotide database (Fig. 4).
Nested-PCR was used to detect the presence of ZnT8 in porcine pancreas. Lane 1 molecular size marker (0.5 kb), lane 2 pancreatic cDNA with ZnT8-1 primers, lane 3 no-template control with ZnT8-1 primer pair, lane 4 pancreatic cDNA with ZnT8-2 primers, lane 5 no-template control with ZnT8-2 primer pair, lane 6 pancreatic cDNA with insulin primers, lane 7 no-template control with insulin primer pair. PCR products migrated at the expected sizes, 373, 250, and 111 bp for ZnT8-1, ZnT8-2, and insulin, respectively
Alignment between the predicted ZnT8 (slc30A8) mRNA as published in the NBCI database (XM_001925124.3) and the nucleotide sequences obtained by sequencing of two different PCR products amplified with the ZnT8-1 and ZnT8-2 primer pairs. The alignment demonstrates strong similarity between the nucleotide sequences of the two PCR products with sequence of the predicted nucleotide sequence of pig ZnT8 mRNA
Discussion
Zinc deficiency in islet cells results in dysfunctional islet hormone release. This may be caused by a ZnT8 deficiency or by ZnT8 autoantibodies, as has been discussed in recent studies, to be responsible for development of type 1 or type 2 diabetes (Wenzlau et al. 2008; Lemaire et al. 2009; Nicolson et al. 2009; Pound et al. 2009; Rutter 2010; Wijesekara et al. 2010). In this context, numerous investigations of human, new-world primate, and rodent pancreatic tissue performed by immunohistochemical and molecular biology methods show that ZnT8 is localized not only in islet B cells but also in glucagon-producing A cells (Murgia et al. 2009; Nicolson et al. 2009; Mohanasundaram et al. 2011). In this report, we demonstrate that ZnT8 protein as well as ZnT8-mRNA is expressed in porcine pancreatic tissue. Moreover, we show by immunohistochemical techniques that ZnT8 protein appears to be expressed primarily in insulin-producing B cells. The strong positive immunoreactivity is thereby restricted to small secretory granules localized to B-cell cytoplasm. These subcellular distribution patterns are in accordance with what has been described for other species (Murgia et al. 2009; Nicolson et al. 2009; Mohanasundaram et al. 2011). Interestingly, our double immunostaining experiments did not reveal co-localization of ZnT8 in glucagon- or somatostatin-containing cells. This finding is new and indicates that a different cellular mechanism may exist in porcine endocrine islet A and D cells, regarding zinc uptake and release as compared with other species (Gyulkhandanyan et al. 2008; Murgia et al. 2009; Nicolson et al. 2009; Mohanasundaram et al. 2011). It is therefore thought that additional intracellular localized zinc-binding proteins may control zinc homeostasis in these pig islet cells. Metallothioneins are localized in human B cell as well as in A cell (Tomita and Matsubara 2000). These molecules are known to play an important physiological role in the regulation of intracellular metabolism of metal ions (Maret 2000). However, the possibility cannot be excluded that other members of the slc30A or slc39A gene family, instead of ZnT8, are involved in regulating the uptake, release, and storage of zinc. This seems unlikely, however, since it has been shown in rodents that alternative zinc transporter molecules in islet A cells are expressed at a very low level in comparison with ZnT8 (Nicolson et al. 2009; Zhong et al. 2012). To clarify the manner in which zinc homeostasis is regulated in glucagon- and somatostatin-containing cells of the pig pancreas, further investigations are needed.
Our RT-PCR results indicate that ZnT8 translation may be tightly regulated in the pig pancreas, as we found only local mRNA insulin expression but not for ZnT8 protein, although immunohistochemical detection of ZnT8 showed the same intensity as for insulin. Using the nested-PCR technique, we obtained the expected PCR products for ZnT8, which were verified by sequence analysis. Strong regulation of genomic DNA has been reported for zinc-binding proteins, including zinc transporters (Jackson et al. 2008). For example, their gene expression is controlled by zinc-responsive transcription factors in a zinc-dependent manner (Heuchel et al. 1994; Langmade et al. 2000; Jackson et al. 2007; Coneyworth et al. 2012). In the case of ZnT8, a complex mechanism of zinc-dependent regulation at the genomic DNA level has not yet been confirmed.
In conclusion, the results presented here indicate that pig ZnT8 is expressed in a cell-type-specific manner in insulin-producing B cells. This and the fact that pig ZnT8 is not co-expressed in glucagon- or somatostatin-producing cells as shown in human, new-world primate, and rodent islets are of particular importance regarding xenotransplantation approaches using porcine islet cells.
References
Cardona K, Korbutt GS, Milas Z, Lyon J, Cano J, Jiang W, Bello-Laborn H, Hacquoil B, Strobert E, Gangappa S, Weber CJ, Pearson TC, Rajotte RV, Larsen CP (2006) Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med 12:304–306
Cardona K, Milas Z, Strobert E, Cano J, Jiang W, Safley SA, Gangappa S, Hering BJ, Weber CJ, Pearson TC, Larsen CP (2007) Engraftment of adult porcine islet xenografts in diabetic nonhuman primates through targeting of costimulation pathways. Am J Transplant 7:2260–2268
Chimienti F, Devergnas S, Favier A, Seve M (2004) Identification and cloning of a beta-cell-specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes 53:2330–2337
Chimienti F, Favier A, Seve M (2005) ZnT-8, a pancreatic beta-cell-specific zinc transporter. Biometals 18:313–317
Coneyworth LJ, Jackson KA, Tyson J, Bosomworth HJ, van der Hagen E, Hann GM, Ogo OA, Swann DC, Mathers JC, Valentine RA, Ford D (2012) Identification of the human zinc transcriptional regulatory element (ZTRE): a palindromic protein-binding DNA sequence responsible for zinc-induced transcriptional repression. J Biol Chem 287:36567–36581
Cousins RJ, Liuzzi JP, Lichten LA (2006) Mammalian zinc transport, trafficking, and signals. J Biol Chem 281:24085–24089
Dodson G, Steiner D (1998) The role of assembly in insulin’s biosynthesis. Curr Opin Struct Biol 8:189–194
Eide DJ (2004) The SLC39 family of metal ion transporters. Pflugers Arch 447:796–800
Eide DJ (2006) Zinc transporters and the cellular trafficking of zinc. Biochim Biophys Acta 1763:711–722
Gaither LA, Eide DJ (2001) Eukaryotic zinc transporters and their regulation. Biometals 14:251–270
Gyulkhandanyan AV, Lu H, Lee SC, Bhattacharjee A, Wijesekara N, Fox JE, MacDonald PE, Chimienti F, Dai FF, Wheeler MB (2008) Investigation of transport mechanisms and regulation of intracellular Zn2+ in pancreatic alpha-cells. J Biol Chem 283:10184–10197
Hecht G, Eventov-Friedman S, Rosen C, Shezen E, Tchorsh D, Aronovich A, Freud E, Golan H, El-Hasid R, Katchman H, Hering BJ, Zung A, Kra-Oz Z, Shaked-Mishan P, Yusim A, Shtabsky A, Idelevitch P, Tobar A, Harmelin A, Bachar-Lustig E, Reisner Y (2009) Embryonic pig pancreatic tissue for the treatment of diabetes in a nonhuman primate model. Proc Natl Acad Sci USA 106:8659–8664
Hediger MA, Romero MF, Peng JB, Rolfs A, Takanaga H, Bruford EA (2004) The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteins Introduction. Pflugers Arch 447:465–468
Heuchel R, Radtke F, Georgiev O, Stark G, Aguet M, Schaffner W (1994) The transcription factor MTF-1 is essential for basal and heavy metal-induced metallothionein gene expression. EMBO J 13:2870–2875
Jackson KA, Helston RM, McKay JA, O’Neill ED, Mathers JC, Ford D (2007) Splice variants of the human zinc transporter ZnT5 (SLC30A5) are differentially localized and regulated by zinc through transcription and mRNA stability. J Biol Chem 282:10423–10431
Jackson KA, Valentine RA, Coneyworth LJ, Mathers JC, Ford D (2008) Mechanisms of mammalian zinc-regulated gene expression. Biochem Soc Trans 36:1262–1266
Kambe T (2011) An overview of a wide range of functions of ZnT and Zip zinc transporters in the secretory pathway. Biosci Biotechnol Biochem 75:1036–1043
Kambe T, Yamaguchi-Iwai Y, Sasaki R, Nagao M (2004) Overview of mammalian zinc transporters. Cell Mol Life Sci 61:49–68
Langmade SJ, Ravindra R, Daniels PJ, Andrews GK (2000) The transcription factor MTF-1 mediates metal regulation of the mouse ZnT1 gene. J Biol Chem 275:34803–34809
Lemaire K, Ravier MA, Schraenen A, Creemers JW, Van de Plas R, Granvik M, Van LL, Waelkens E, Chimienti F, Rutter GA, Gilon P, in’t Veld PA, Schuit FC (2009) Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc Natl Acad Sci USA 106:14872–14877
Maret W (2000) The function of zinc metallothionein: a link between cellular zinc and redox state. J Nutr 130:1455S–1458S
Mohanasundaram D, Drogemuller C, Brealey J, Jessup CF, Milner C, Murgia C, Lang CJ, Milton A, Zalewski PD, Russ GR, Coates PT (2011) Ultrastructural analysis, zinc transporters, glucose transporters and hormones expression in new world primate (Callithrix jacchus) and human pancreatic islets. Gen Comp Endocrinol 174:71–79
Murgia C, Devirgiliis C, Mancini E, Donadel G, Zalewski P, Perozzi G (2009) Diabetes-linked zinc transporter ZnT8 is a homodimeric protein expressed by distinct rodent endocrine cell types in the pancreas and other glands. Nutr Metab Cardiovasc Dis 19:431–439
Nicolson TJ, Bellomo EA, Wijesekara N, Loder MK, Baldwin JM, Gyulkhandanyan AV, Koshkin V, Tarasov AI, Carzaniga R, Kronenberger K, Taneja TK, Libert S, Froguel P, Scharfmann R, Stetsyuk V, Ravassard P, Parker H, Gribble FM, Reimann F, Sladek R, Hughes SJ, Johnson PR, Masseboeuf M, Burcelin R, Baldwin SA, Liu M, Lara-Lemus R, Arvan P, Schuit FC, Wheeler MB, Chimienti F, Rutter GA (2009) Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes 58:2070–2083
Overbeck S, Uciechowski P, Ackland ML, Ford D, Rink L (2008) Intracellular zinc homeostasis in leukocyte subsets is regulated by different expression of zinc exporters ZnT-1 to ZnT-9. J Leukoc Biol 83:368–380
Palmiter RD, Huang L (2004) Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. Pflugers Arch 447:744–751
Pound LD, Sarkar SA, Benninger RK, Wang Y, Suwanichkul A, Shadoan MK, Printz RL, Oeser JK, Lee CE, Piston DW, McGuinness OP, Hutton JC, Powell DR, O’Brien RM (2009) Deletion of the mouse Slc30a8 gene encoding zinc transporter-8 results in impaired insulin secretion. Biochem J 421:371–376
Renner S, Braun-Reichhart C, Blutke A, Herbach N, Emrich D, Streckel E, Wunsch A, Kessler B, Kurome M, Bahr A, Klymiuk N, Krebs S, Puk O, Nagashima H, Graw J, Blum H, Wanke R, Wolf E (2013) Permanent neonatal diabetes in INSC94Y transgenic pigs. Diabetes 62:1505–1511
Rutter GA (2010) Think zinc: new roles for zinc in the control of insulin secretion. Islets 2:49–50
Schweiger M, Steffl M, Amselgruber WM (2005) Determination of target cells for cholecystokinin in the porcine pancreas. Ann Anat 187:209–214
Smidt K, Pedersen SB, Brock B, Schmitz O, Fisker S, Bendix J, Wogensen L, Rungby J (2007) Zinc-transporter genes in human visceral and subcutaneous adipocytes: lean versus obese. Mol Cell Endocrinol 264:68–73
Sun Y, Ma X, Zhou D, Vacek I, Sun AM (1996) Normalization of diabetes in spontaneously diabetic cynomolgus monkeys by xenografts of microencapsulated porcine islets without immunosuppression. J Clin Invest 98:1417–1422
Tomita T, Matsubara O (2000) Immunocytochemical localization of metallothionein in human pancreatic islets. Pancreas 20:21–24
Wang X, Zhou B (2010) Dietary zinc absorption: a play of Zips and ZnTs in the gut. IUBMB Life 62:176–182
Wenzlau JM, Moua O, Sarkar SA, Yu L, Rewers M, Eisenbarth GS, Davidson HW, Hutton JC (2008) SlC30A8 is a major target of humoral autoimmunity in type 1 diabetes and a predictive marker in prediabetes. Ann N Y Acad Sci 1150:256–259
Wijesekara N, Dai FF, Hardy AB, Giglou PR, Bhattacharjee A, Koshkin V, Chimienti F, Gaisano HY, Rutter GA, Wheeler MB (2010) Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia 53:1656–1668
Zhong ML, Chi ZH, Shan ZY, Teng WP, Wang ZY (2012) Widespread expression of zinc transporter ZnT (SLC30) family members in mouse endocrine cells. Histochem Cell Biol 138:605–616
Acknowledgments
The authors express their gratitude to Mrs. Müller and Mrs. Kallenberger for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schweiger, M., Steffl, M. & Amselgruber, W.M. The zinc transporter ZnT8 (slc30A8) is expressed exclusively in beta cells in porcine islets. Histochem Cell Biol 140, 677–684 (2013). https://doi.org/10.1007/s00418-013-1137-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-013-1137-2