Abstract
SLC39 proteins are members of the broader ZIP family of metal ion transporters found in organisms at all phylogenetic levels. Most ZIP transporters have eight predicted transmembrane domains and a similar predicted topology. Their biochemical mechanism(s) of substrate transport are not yet known. Where characterized, these proteins have been found to transport metal ions from the cell exterior or lumen of intracellular organelles into the cytoplasm. Furthermore, members of the ZIP family have been implicated in the transport of zinc, iron, and/or manganese indicating that these proteins have diverse functions. There are 14 SLC39-related proteins encoded by the human genome. Studies of SLC39A1, SLC39A2, and SLC39A4, encoding the proteins hZip1, hZip2, and hZip4, have indicated roles in zinc uptake across the plasma membrane of various cell types. Genetic studies have specifically implicated SLC39A4 in the uptake of dietary zinc into intestinal enterocytes. Mutations in SLC39A4 have been identified in patients with acrodermatitis enteropathica, a genetic disease of zinc deficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
History of Slc39-related genes
The SLC39 transporters are members of the ZIP family of metal ion transporters [9, 11, 18]. This designation stands for Zrt-, Irt-like protein, and reflects the first members of this transporter family to be identified. Zrt1 and Zrt2 are the primary zinc uptake transporters in the yeast Saccharomyces cerevisiae and Irt1 is the major iron uptake transporter in roots of Arabidopsis thaliana [6, 20, 35, 37, 41, 42]. Since the initial identification of these proteins, the ZIP family has grown to over 90 members including proteins in bacteria, nematodes, insects, and mammals [11]. Among these, ZupT is a zinc uptake transporter in Escherichia coli [16] and several ZIPs have been implicated in zinc, iron, and/or manganese transport in plants [17, 24, 31, 36]. Database analyses have indicated that there are 14 SLC39-related ZIP proteins encoded by the human genome (Table 1). A dendrogram describing the sequence relationships of human SLC39 proteins is shown in Fig. 1.
The amino acid sequence relationships of SLC39-related proteins. The dendrogram was generated from the amino acid sequences using CLUSTALW. Orphan transporters are designated by their sequence accession number as listed in Table 1. Some example sequence identities are hZip1 versus hZip2 (36%), hZip1 versus NM139177 (27%), and hZip1 versus NM152725 (23%)
Functional characteristics
With no known exceptions, ZIPs transport metal ion substrates across cellular membranes into the cytoplasm. While many members are involved in the uptake of metal ions across the plasma membrane, some efflux metals from intracellular compartments. For example, the yeast Zrt3 protein is responsible for transporting stored zinc out of the lysosome-like vacuole into the cytoplasm for subsequent utilization [27]. Also in yeast, the Atx2 protein appears to transport manganese out of the Golgi [25].
The biochemical mechanisms involved in this transport have not been extensively investigated. In yeast, Zrt1 and Zrt2 are known to be energy dependent [41, 42]. The mechanism of transport used by SLC39 proteins is unclear. Zinc uptake by SLC39A1 and SLC39A2 was found to be energy independent [10, 12]. Zinc uptake by these proteins was not dependent on K+ or Na+ gradients but SLC39A2 activity was stimulated by HCO3 − suggesting a Zn2+/HCO3 − symport mechanism [10]. Alternatively, zinc uptake by these proteins could be driven simply by the concentration gradient of labile zinc likely to exist across the plasma membrane [30].
Description of human SLC39 genes
SLC39A1
SLC39A1, encoding hZip1/ZIRTL, is expressed in a wide variety of tissues and cell types [12, 26]. In transfected K562 cells, hZip1 is localized to the plasma membrane where it confers zinc uptake activity [12]. In other cell types (e.g., COS-7, PC3), hZip1 is localized predominantly to the endoplasmic reticulum [29]. Antisense RNA studies have indicated that hZip1 is responsible for most of the zinc uptake activity detectable in K562 cells suggesting a role in zinc uptake in other cells as well [12]. SLC39A1 expression appears to be regulated by zinc status and by hormone treatment; i.e., mRNA levels are repressed by zinc treatment and induced by prolactin treatment of PC-3 and LNCaP prostate-derived cancer cells [5].
SLC39A2
SLC39A2, encoding hZip2, is expressed at low levels and in only a few tissues [2, 10, 38]. Like hZip1, hZip2 also appears to be involved in zinc uptake. hZip2 localizes to the plasma membrane of transfected K562 cells and confers zinc uptake activity on those cells [10]. As was found for SLC39A1, SLC39A2 expression may also be regulated by zinc; treatment of the THP-1 monocytic cell line, or human peripheral blood mononuclear cells with TPEN, a cell-permeable zinc chelator, resulted in approximately fourfold increases in SLC39A2 mRNA levels [2]. Furthermore, Yamaguchi (1995) found that SLC39A2 expression was induced by contact inhibition in cultured cervical epithelial cells [38].
SLC39A4
hZip4, the product of the SLC39A4 gene, is an important zinc uptake transporter in the human intestine and responsible for absorption of dietary zinc. This conclusion is based on the work of two groups linking SLC39A4 to acrodermatitis enteropathica (AE), a recessive genetic disorder of zinc absorption [23, 39, 40]. Homozygosity mapping using consanguineous families with affected individuals indicated the gene responsible for AE mapped to 8q24.3 [39]. Located in this region was a likely candidate gene, SLC39A4. Sequencing of exons from affected individuals indicated the presence of mutations that were likely to disrupt function of the protein [23, 40]. These mutations were not found in unaffected samples. Moreover, the SLC39A4 gene is expressed in tissues involved in zinc absorption, the small intestine, colon, and kidney, and the hZip4 protein was localized to the apical plasma membrane of mouse enterocytes [40]. These results strongly suggest an important role of hZip4 in dietary zinc uptake and reabsorption of zinc in the kidney. Approximately 10% of the endogenous zinc lost per day in humans is in urine and this loss is reduced under conditions of zinc deficiency suggesting homeostatic regulation of kidney zinc reabsorption [3]. Based on the EST database, two forms of hZip4 may be expressed that have different amino-termini. The more commonly expressed form has a 329 amino acid segment upstream of TM1 including a signal sequence. The alternative form appears to be expressed at lower levels and has an N-terminal tail of 305 amino acids with no signal sequence. This latter form arises from transcription initiation within intron 1 of the longer transcript [40]. So far, no experiments characterizing the regulation of SLC39A4 expression have been published.
Studies of other SLC39 genes
Additional SCL39-related genes are described in Table 1. hZip3, the product of SLC39A3, has not yet been characterized beyond the sequence level [10]. The "LIV-1" gene was cloned because its expression is induced by estrogen in breast cancer cells [28]. LIV-1 expression is also increased by treatment with epidermal growth factor, TGFα, insulin, or IGF-1 [7, 8]. The gene referred to as "BIGM103" also encodes an SLC39-related protein and its expression is induced in monocytes treated with Mycobacterium cell wall and lipopolysaccharide preparations [1]. Moreover, expression of BIGM103 in CHO cells increased zinc accumulation consistent with a potential role in zinc uptake. The "Ke4" gene was identified during the characterization of genes near the major histocompatibility complex on chromosome 6 [19, 21]. Ke4 may share common promoter elements with the retinoid X receptor-β (RXR β), the adjacent divergently transcribed gene, and be repressed by tumor necrosis factor (TNF)-α [33]. Expression of mouse Ke4 in Arabidopsis indicated that this protein could functionally substitute for IAR1, a potential Mn transporter [24]. Finally, mouse Ke4 was localized to ER membranes when expressed in transfected cells [34]. Thus, Ke4 may be an intracellular Mn transporter in mammals.
Biochemical and structural information
Most ZIP proteins have eight transmembrane (TM) domains and similar predicted topologies (Fig. 2). Aspects of this topology model have been confirmed for yeast [13, 14] and some mammalian ZIP transporters [10, 12]. While most loops between TM domains are quite short, a longer loop region is frequently found between TM domains III and IV. This region often contains a histidine-rich domain with the sequence (HX) n where n generally ranges from 3 to 5. The function of this domain is not yet clear. Determinants of substrate specificity have been mapped to the extracellular loop between TM II and III in Irt1 [32]. For some SCL39 proteins (e.g., Liv-1), this regions is also rich in histidine residues. Finally, some SLC39 proteins (e.g., SLC39A4) have long N-terminal domains. Transmembrane domains IV and V are particularly amphipathic and contain conserved histidine residues frequently with adjacent polar or charged amino acids. Given their sequence conservation and amphipathic nature, TM IV and V are predicted to line a cavity in the transporter through which the substrate passes. Consistent with this hypothesis, conserved residues in these regions are essential for function [32].
Topological model of SLC39 proteins. Transmembrane (TM) domains are indicated in red. The histidine-rich region in the cytoplasmic loop between TM III and IV, conserved histidines and charged/polar residues in TM IV and V, and the location of substrate specificity determinants mapped in Irt1 are also shown
Physiological and pathological implications
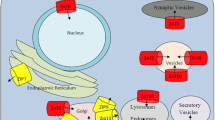
Like other ZIP transporters, SLC39-related proteins are likely to play diverse roles in cell and organismal physiology (Fig. 3). The products of some of these genes, e.g., SLC39A1, SLC39A2, and SLC39A4, are or appear to be involved in zinc uptake across the plasma membrane. Other members may function in the uptake of other metal ions (e.g., Fe and Mn) or may transport metal ion substrates across organellar membranes. In this latter role, SLC39-related proteins may be involved in metal ion homeostasis within the compartment or, perhaps, play a role in mobilizing stored metal ions for use under deficiency conditions. Finally, some ZIP transporters (e.g., Zrt1 and Irt1) have been shown to be capable of cadmium transport [4, 15, 22]. Therefore, SLC39 proteins may play a role in cadmium uptake into the intestine and into other tissues of the body. Consistent with this hypothesis, zinc uptake by SLC39A1 and SLC39A2 is strongly inhibited by cadmium suggesting that this toxic metal may also be a substrate for these transporters [10, 12].
Roles of SLC39 proteins in metal metabolism. Available evidence indicates that hZip1, hZip2, and hZip4 are responsible for zinc uptake in cells where they are expressed. Based on their similarity to yeast ZIP proteins, it is possible that human SLC39 proteins transport metal ions out of intracellular compartments. Furthermore, some SLC39 proteins may transport substrates other than zinc (e.g., Fe or Mn)
Given the importance of zinc and other metal ions, it is likely that SLC39-related proteins will have far-reaching pathological implications. The only case where some of these implications are known is SLC39A4, the gene responsible for AE. If left untreated, AE patients can suffer severe zinc deficiency that results in growth impairment, immune system dysfunction, alopecia, severe dermatitis, and mental disorders. Successful treatment of AE can be achieved by supplementing the diet of these patients with zinc. This suggests that additional zinc transporters are present in the intestine for dietary zinc absorption.
The SLC39 transporters are not the only proteins responsible for zinc transport in humans. DMT1/SLC11 may also play a role in dietary zinc uptake in the intestine and into various tissues. Moreover, the activity of SLC39 proteins is closely integrated with the activity of members of the SLC30 family. While SLC39 proteins transport metal ions into the cytosol, SLC30 proteins efflux that zinc either out of the cell or into organelles. For example, dietary zinc taken up into enterocytes of the intestine via SLC39A4 may be exported across the basolateral membrane into the bloodstream by Znt-1/SLC30A1 and/or ZnT-4/SLC30A4. Similarly, zinc taken up into cells of other tissues by SLC39 transporters (e.g., SLC39A1) may be transported into the Golgi by Znt-5/SLC30A5. Thus, zinc homeostasis and utilization in humans involves the coordinated activity of members of several zinc transporter families.
References
Begum NA, Kobayashi M, Moriwaki Y, Matsumoto M, Toyoshima K, Seya T (2002) Mycobacterium bovis BCG cell wall and lipopolysaccharide induce a novel gene BIGM103, encoding a 7-TM protein: Identification of a new protein family having Zn-transporter and Zn-metalloprotease signatures. Genomics 80:630–645
Cao J, Bobo JA, Liuzzi JP, Cousins RJ (2001) Effects of intracellular zinc depletion on metallothionein and ZIP2 transporter expression and apoptosis. J Leukoc Biol 70:559–566
Chesters JK (1997) Zinc. In: Handbook of nutritionally essential mineral elements. Dekker, New York
Connolly EL, Fett JP, Guerinot ML (2002) Expression of the IRT1 metal transporter is controlled by metals at the level of transcript and protein accumulation. Plant Cell 14:1347–1357
Costello LC, Liu Y, Zou J, Franklin RB (1999) Evidence for a zinc uptake transporter in human prostate cancer cells which is regulated by prolactin and testosterone. J Biol Chem 274:17499–17504
Eide D, Broderius M, Fett J, Guerinot ML (1996) A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci USA 93:5624–5628
El-Tanani MK, Green CD (1996) Insulin/IGF-1 modulation of the expression of two estrogen-induced genes in MCF-7 cells. Mol Cell Endocrinol 121:29–35
El-Tanani MK, Green CD (1997) Interaction between estradiol and growth factors in the regulation of specific gene expression in MCF-7 human breast cancer cells. J Steroid Biochem Mol Biol 60:269–276
Eng BH, Guerinot ML, Eide D, Saier MH (1998) Sequence analyses and phylogenetic characterization of the ZIP family of metal ion transport proteins. J Membr Biol 166:1–7
Gaither LA, Eide DJ (2000) Functional expression of the human hZIP2 zinc transporter. J Biol Chem 275:5560–5564
Gaither LA, Eide DJ (2001) Eukaryotic zinc transporters and their regulation. Biometals 14:251–270
Gaither LA, Eide DJ (2001) The human ZIP1 transporter mediates zinc uptake in human K562 erythroleukemia cells. J Biol Chem 276:22258–22264
Gitan RS, Eide DJ (2000) Zinc-regulated ubiquitin conjugation signals endocytosis of the yeast ZRT1 zinc transporter. Biochem J 346:329–336
Gitan RS, Luo H, Rodgers J, Broderius M, Eide DJ (1998) Zinc-induced inactivation of the yeast ZRT1 zinc transporter occurs through endocytosis and vacuolar degradation. J Biol Chem 273:28617–28624
GomesDS, Fragoso LC, Riger CJ, Panek AD, Eleutherio EC (2002) Regulation of cadmium uptake by Saccharomyces cerevisiae. Biochim Biophys Acta 1573:21–25
Grass G, Wong MD, Rosen BP, Smith RL, Rensing C (2002) ZupT is a Zn(II) uptake system in Escherichia coli. J Bacteriol 184:864–866
Grotz N, Fox T, Connolly E, Park W, Guerinot ML, Eide DJ (1998) Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proc Natl Acad Sci USA 95:7220–7224
Guerinot ML (2000) The ZIP family of metal transporters. Biochim Biophys Acta 1465:190–198
Hanson IM, Trowsdale J (1991) Colinearity of novel genes in the class II regions of the MHC in mouse and human. Immunogenetics 34:5–11
Henricque R, Jasik J, Klein M, Martinoia E, Feller U, Schell J, Pais MS, Koncz C (2002) Knockout of Arabidopsis metal transporter IRT1 results in iron deficiency accompanied by cell differentiation defects. Plant Mol Biol 50:587–597
Janatipour M, Naumov Y, Ando A, Sugimura K, Okamoto N, Tsuji K, Abe K, Inoko H (1992) Search for MHC-associated genes in human: five new genes centromeric to HLA-DP with yet unknown functions. Immunogenetics 35:272–278
Korshunova YO, Eide D, Clark WG, Guerinot ML, Pakrasi H (1999) The IRT1 protein from Arabidopsis is a metal transporter with a broad substrate range. Plant Mol Biol 40: 37–44
Kury S, Dreno B, Bezieau S, Giraudet S, Kharfi M, Kamoun R, Moisan JP (2002) Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet 31:239–240
Lasswell J, Rogg LE, Nelson DC, Rongey C, Bartel B (2000) Cloning and characterization of IAR1, a gene required for auxin conjugate sensitivity in Arabidopsis. Plant Cell 12:2395–2408
Lin S-J, Culotta VC (1996) Suppression of oxidative damage by Saccharomyces cerevisiae ATX2, which encodes a manganese-trafficking protein that localizes to Golgi-like vesicles. Mol Cell Biol 16:6303–6312
Lioumi M, Ferguson CA, Sharpe PT, Freeman T, Marenholz I, Mischke D, Heizmann C, Ragoussis J (1999) Isolation and characterization of human and mouse ZIRTL, a member of the IRT1 family of transporters, mapping within the epidermal differentiation complex. Genomics 62:272–280
MacDiarmid CW, Gaither LA, Eide DJ (2000) Zinc transporters that regulate vacuolar zinc storage in Saccharomyces cerevisiae. EMBO J 19:2845–2855
Manning DL, Robertson JF, Ellis IO, Elston CW, McClelland RA, Gee JM, Jones RJ, Green CD, Cannon P, Blamey RW (1994) Oestrogen-regulated genes in breast cancer: association of pLIV1 with lymph node involvement. Eur J Cancer 30A:675–678
Milon B, Dhermy D, Pountney D, Bourgeois M, Beaumont C (2001) Differential subcellular localization of hZip1 in adherent and non-adherent cells. FEBS Lett 507:241–246
Outten CE, O'Halloran TV (2001) Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 292:2488–2492
Pence NS, Larsen PB, Ebbs SD, Letham DL, Lasat MM, Garvin DF, Eide DJ, Kochian LV (2000) The molecular physiology of heavy metal transport in the Zn/Cd hyperaccumulator Thlaspi caerulescens. Proc Natl Acad Sci USA 97:4956–4960
Rogers EE, Eide DJ, Guerinot ML (1999) Altered cation selectivity in an Arabidopsis metal transporter. Proc Natl Acad Sci USA 97:12356–12360
Sugawara A, Uruno A, Nagata T, Taketo MM, Takeuchi K, Ito S (1998) Characterization of mouse retinoid X receptor (RXR)-beta gene promoter: negative regulation by tumor necrosis factor (TNF)-alpha. Endocrinology 139:3030–3033
Suzuki A, Endo T (2002) Ermelin, an endoplasmic reticulum transmembrane protein, contains the novel HELP domain conserved in eukaryotes. Gene 284:31–40
Varotto C, Maiwald D, Pesaresi P, Jahns P, Salamini F, Leister D (2002) The metal transporter IRT1 is necessary for iron homeostasis and efficient photosynthesis. Plant J 31:589–599
Vert G, Briat JF, Curie C (2001) Arabidopsis IRT2 gene encodes a root-periphery iron transporter. Plant J 26:181–189
Vert G, Grotz N, Dedaldechamp F, Gaymard F, Guerinot ML, Briat JF, Curie C (2002) IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14:1223–1233
Yamaguchi S (1995) Subtraction cloning of growth arrest inducible genes in normal human epithelial cells. Kokubyo Gakkai Zasshi 62:78–93
Wang K, Pugh EW, Griffen S, Doheny KF, Mostafa WZ, al-Aboosi MM, el-Shanti H, Gitschier J (2001) Homozygosity mapping places the acrodermatitis enteropathica gene on chromosomal region 8q24.3. Am J Hum Genet 68:1055–1060
Wang K, Zhou B, Kuo YM, Zemansky J, Gitschier J (2002) A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. Am J Hum Genet 71:66–73
Zhao H, Eide DJ (1996) The yeast ZRT1 gene encodes the zinc transporter of a high affinity uptake system induced by zinc limitation. Proc Natl Acad Sci USA 93:2454–2458
Zhao H, Eide DJ (1996) The ZRT2 gene encodes the low affinity zinc transporter in Saccharomyces cerevisiae. J Biol Chem 271:23203–23210
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eide, D.J. The SLC39 family of metal ion transporters. Pflugers Arch - Eur J Physiol 447, 796–800 (2004). https://doi.org/10.1007/s00424-003-1074-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-003-1074-3