Abstract
Localization of three P2X and six P2Y receptors in sinus endothelial cells of the rat spleen was examined by immunofluorescent microscopy, and ultrastructural localization of the detected receptors was examined by immunogold electron microscopy. In immunofluorescent microscopy, labeling for anti-P2Y1, P2Y6, and P2Y12 receptors was detected in endothelial cells, but P2X1, P2X2, P2X4, P2Y2, P2Y4, and P2Y13 receptors was not detected. P2Y1 and P2Y12 receptors were prominently localized in the basal parts of endothelial cells. P2Y6 receptor was not only predominantly localized in the basal parts of endothelial cells, but also in the superficial layer. Triple immunofluorescent staining for a combination of two P2Y receptors and actin filaments showed that P2Y1, P2Y6, and P2Y12 receptors were individually localized in endothelial cells. Phospholipase C-β3, phospholipase C- γ2, and inositol-1,4,5-trisphosphate receptors, related to the release of the intracellular Ca2+ from the endoplasmic reticulum, were also predominantly localized in the basal parts of endothelial cells. In immunogold electron microscopy, labeling for P2Y1, P2Y6, and P2Y12 receptors were predominantly localized in the basal part of endothelial cells and, in addition, in the junctional membrane, basal plasma membrane, and caveolae in the basal part of endothelial cells. Labeling for phospholipase C-β3 and phospholipase C-γ2 was dominantly localized in the basal parts and in close proximity to the plasma membranes of endothelial cells. The possible functional roles of these P2Y receptors in splenic sinus endothelial cells are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endothelial cells lining the splenic sinuses are crucial sites for controlling blood cell passage through the splenic cord and are ultrastructurally quite different from other vascular endothelial cells. In particular, their shape and arrangement are prominent, resembling the staves of a barrel, with a highly ordered network of contractile stress fibers running longitudinally in their basal part, and fenestrated basal lamina barrel-like hoops called “ring fibers” (Drenckhahn and Wagner 1986; Uehara and Miyoshi 1999a). Since the endothelium is devoid of smooth muscles and the endothelial cells are only attached to the ring fibers by focal adhesion, their basal membranes except focal adhesions are exposed to the splenic cord, receiving direct mechanical stimuli from the environment at abluminal sites as well as the luminal surface. Although these structures are believed to be formed for the passage of blood cells in splenic cords surrounding the sinus endothelium, how the passage of blood cells is controlled has not yet been clarified, because sinus endothelial cells adhere predominantly by adherens junctions and poorly developed tight junctions (Uehara and Miyoshi 1999b; Uehara 2006; Uehara and Uehara 2008).
Vascular endothelial cells release ATP, which raises [Ca2+](i) in endothelial cells (Ando and Yamamoto 2009). ATP released from endothelial cells is closely involved in the mechanisms underling the local control of vessel tone and remodeling, as well as the disassembly of vascular endothelial cadherin, migration, proliferation, differentiation, and death during angiogenesis (Burnstock 2008). ATP, as well as ADP, UDP and UTP, acts on P2 receptors on various cells, which have been divided into P2X ligand-gated ion channel and P2Y G protein-coupled receptor families. Seven subtypes of P2X receptors have been cloned and characterized: P2X1, 2, 3, 4, 5, 6, and 7; and 8 subtypes of P2Y receptors: P2Y1, 2, 4, 6, 11, 12 13, and 14 (Ralevic and Burnstock 1998). Vascular endothelial cells have been reported to express P2X1, P2X2, and P2X4 receptors (Loesch and Burnstock 2000; Harrington and Mitchell 2004; Yamamoto et al. 2006) as well as P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, and P2Y12 receptors (Henderson et al. 1995; Motte et al. 1995; Guns et al. 2005; Abbracchio et al. 2006; da Silva et al. 2009). An orhtolog gene of P2Y11 receptor is not detected in the murine genome. In addition, strong expression of P2Y13 receptor has been demonstrated by RT-PCR in the rat spleen where blood capillaries are abundantly distributed (Fumagalli et al. 2004; Abbracchio et al. 2006); however, there is little information on the ultrastructural localization of these subtypes and on comparing the positions of these subtypes in endothelial cells. P2Y1, 2, 4, and 6 receptors signal through a G protein-dependent pathway, activating phospholipase C (PLC)-β and generating inositol-1,4,5-trisphosphate (IP3), which stimulates IP3 receptor to release Ca2+ in the endoplasmic reticulum. Thirteen mammal PLC isozymes have been identified and are divided into six families: PLC-β, -γ, -δ, -ε, -ξ, and -η. PLC-β has four distinct isoforms, β1–4; PLC-γ, which is integrated into the response pathway involving PLC-β, has two isoforms, γ1 and 2 (Rebecchi and Pentyala 2000).
We examined the immunohistochemical localization of P2X1, P2X2, P2X4, P2Y1, P2Y2, P2Y4, P2Y6, P2Y12, and P2Y13 receptors, PLC-β1–4, and PLC-γ1–2 in sinus endothelial cells of the rat spleen by confocal laser-scanning microscopy. The ultrastructural localization of detected proteins of P2Y1, P2Y6, and P2Y12 receptors, PLC-β3, and PLC-γ2 was examined by immunogold electron microscopy to elucidate the regional roles of these proteins. Western blotting was carried out to examine the specificity of antibodies and to investigate the molecular weight of the examined P2X and P2Y receptors in the rat spleen.
Materials and methods
Eight-week-old male Wistar rats were anesthetized prior to thoracic aorta cannulation for perfusion with Ringer’s solution, followed by 3% paraformaldehyde in 0.1 M phosphate buffer at pH 7.4. The spleen was removed and the red pulp was cut into small blocks, and then immersed in the same fixative for 1 h. The specimens were rinsed in buffer, infused with 20% polyvinyl pyrrolidone and 2.3 M of sucrose in buffer, and rapidly frozen in liquid nitrogen.
Immunohistochemistry for confocal laser-scanning microscopy
Semi-thin cryosections (about 0.5 μm in thickness) were mounted on glass slides and treated with 3% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) for 10 min. The slides were subsequently incubated with the primary antibody (Table 1) PBS containing 1% BSA at 4°C overnight. After rinsing with PBS, the sections were incubated with secondary antibody conjugated with Alexa 488-conjugated IgG (Molecular Probes, Eugene, USA) containing Alexa-Fluor-546-phalloidin (Molecular Probes) in PBS to demonstrate the superficial layer of endothelial cells by a cortical layer of F-actin, and the basal part of the cell by distinctive stress fibers in sinus endothelial cells to examine the more detailed localization of P2Y receptors in endothelial cells. To examine differences between the distributions of P2Y1 and P2Y6, P2Y1 and P2Y12, and P2Y6 and P2Y12 receptors, some specimens were observed using triple fluorescence immunostaining. After the first reaction of the primary antibody against P2Y1 or P2Y6 receptors and donkey anti-rabbit antibody conjugated with Alexa 488, they were thoroughly washed with PBS containing 0.1% BSA and then incubated for 1 h with 10% goat anti-rabbit antibody in PBS at room temperature. After rinsing with PBS, they were then incubated with the second primary antibody against P2Y1, P2Y6, or P2Y12 receptors at 4°C overnight, rinsed with PBS, and incubated with goat anti-rabbit antibody conjugated with Alexa 633 (Molecular Probes) containing Alexa-Fluor-546-phalloidin. Semi-thin cryosections of the aorta with the vasa vasorum, small intestine, and cardiac muscle of the rat were prepared by the same method as positive controls. Negative controls were also performed. All samples were examined using a laser-scanning confocal microscope (LSM710, Zeiss).
Immunogold labeling for electron microscopy
Ultrathin cryosections were collected on grids and pretreated with 3% BSA. Samples were incubated for 1 h at room temperature with anti-P2Y1, anti-P2Y6, and anti-P2Y12 receptors, and anti-PLC-β3 and anti-PLC-γ2 antibodies (dilution in Table 1) in PBS containing 1% BSA, and were then incubated with 15-nm colloidal gold-conjugated secondary antibody. The specimens were then fixed in 2% glutaraldehyde in 0.1 M phosphate buffer and then incubated in 0.5% uranyl acetate and 1.8% methylcellulose in distilled water. Excess fluid was removed and then the grids were air dried.
Appropriate controls included omission of the first or second primary antibody. They were observed under a Hitachi 7000 electron microscope.
Western blots
Spleens were taken from three 8-week-old male Wistar rats and homogenized in an extraction reagent (Sigma-Aldrich, MO, USA). Protein concentration was measured by Bio-Rad protein assay (Bio-Rad, CA, USA). The sample (20 μg/lane) was loaded onto 10% SDS–polyacrylamide gels using a Bio-Rad Mini-Pprotean 3 cell (Bio-Rad), and then transferred to polyvinyldifluoride membranes (Millipore, MA, USA). The membranes were blocked with 5% nonfat milk powder and 0.05% Tween 20 in Tris-buffered saline (TBS) and incubated overnight at 4°C with the primary antibody (Table 1) in blocking solution. Preabsorption of the antibodies was carried out according to the manufacturer’s instructions. The membranes were washed with TBS containing 0.05% Tween 20, incubated with goat anti-rabbit horseradish peroxidase-conjugated goat anti-rabbit IgG (1:2,000; Invitrogen, CA, USA) at room temperature for 60 min, and washed with TBA-Tween 20. Western blotting luminal reagent ECL and Hyperfilm (Amersham, UK) were used to visualize peroxidase activity. Control for nonspecific binding was determined by omission of the primary antibody.
Results
Western blots
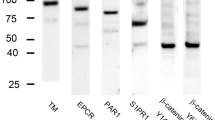
Western blotting analysis was performed using crude splenic tissue extracts. Strong signals for each P2X and P2Y receptor were detected. Two bands for P2X1 and P2X2 receptors were detected at about 48 and 115 kDa, and at about 55 and 64 kDa, respectively. P2X4 receptor was detected at about 65 kDa. P2Y1, P2Y4, and P2Y12 receptors were detected at about 30, 98, and 85 kDa, respectively. Two bands for P2Y2, and P2Y6, and P2Y13 receptors were detected at about 36 and 110, 24 and 94, and 30 and 32 kDa, respectively. When the antibody was preincubated with its specific control peptide antigen for 1 h, no bands were found (Fig. 1).
Western blotting analysis of P2X and P2Y receptors in crude extract from the rat spleen; a antibodies for P2X and P2Y receptors, b preabsorption experiments P2X1 and P2X2 receptors were detected as two bands, and P2X4 receptor was detected as a single band. P2Y1, P2Y4, and P2Y12 receptors were detected as single bands, and P2Y2, P2Y6, and P2Y13 receptors as two bands. In membranes treated with a preabsorption experiment, no bands were found. Molecular weight markers are given in kiloDaltons on the left
Immunohistochemistry for confocal laser-scanning microscopy
Labeling with anti-P2X1, P2X2, and P2X4 receptors was not detected in sinus endothelial cells, although P2X1 receptor was localized in megakaryocytes and P2X2 and P2X4 receptors were localized in smooth muscle cells in splenic trabeculae in the red pulp of the spleen (Fig. 2). Labeling with anti-P2Y2, P2Y4, and P2Y13 receptors was not detected in any cells in the red pulp.
Immunofluorescent localization of P2X1 and P2X2 receptors in semi-thin sections of the red pulp of the spleen by double immunofluorescent staining with phalloidin-conjugated fluorescence; a, b P2X1 receptor is localized in a megakaryocyte (arrow), but not detected in endothelial cells surrounding the sinus lumen (S). c, d P2X2 receptor is localized in smooth muscle cells in a splenic trabecula, but not detected in endothelial cells surrounding the sinus lumen (S). S sinus lumen Bars 5 μm
Labeling for P2Y1, P2Y6, and P2Y12 receptors was detected in sinus endothelial cells. These receptors were prominently localized in the basal parts of sinus endothelial cells. P2Y1 receptor was in the area adjacent to stress fibers and in the regions between stress fibers in the basal part of endothelial cells. P2Y6 receptor was not only localized in the area adjacent to stress fibers and in the regions between stress fibers, but also in the superficial layer of endothelial cells on all sides, and labeling showed a spotted appearance in places. The P2Y12 receptor predominantly lays over stress fibers with a dotted appearance and was sporadically localized between stress fibers and scattered in the apical superficial layer (Fig. 3). Triple immunofluorescent staining for a combination of two P2Y receptors and actin filaments showed that P2Y1, P2Y6, and P2Y12 receptors were individually localized in sinus endothelial cells. These receptors were also distinctively localized in leucocytes, platelets, and megakaryocytes in sinus lumens and splenic cords (Fig. 4).
Immunofluorescent localization of P2Y1, P2Y6, and P2Y12 receptors (green) in semi-thin sections of the sinus endothelium in the red pulp by double immunofluorescent staining with phalloidin-conjugated fluorescence (red) Labeling for phalloidin shows the superficial layer of sinus endothelial cells and a characteristic structure, stress fibers, in the basal parts of sinus endothelial cells; a, b P2Y1 receptor is precisely localized in the basal parts of sinus endothelial cells. P2Y1 receptor is in the area adjacent to stress fibers and in the regions between stress fibers in the basal part of endothelial cells (arrowheads). c, d P2Y6 receptor is localized in the superficial layer of endothelial cells and predominantly in the area adjacent to stress fibers in the basal parts of the endothelial cells (arrows). Labeling shows a spotted appearance in places (arrowhead). e, f P2Y12 receptor overlies stress fibers with a dotted appearance and is sporadically localized between stress fibers (arrows) and scattered in the apical superficial layer (arrowheads). S sinus lumen. Bars 5 μm
Triple immunofluorescent staining for the combination of two P2Y receptors and F-actin in semi-thin sections of the sinus endothelium in the red pulp. a–d P2Y1 (blue), P2Y6 (green) receptors, and F-actin (red). The splenic cord and two sectioned sinusoidal capillaries are observed. One capillary (S1) is transversely sectioned and the other (S2) is longitudinally sectioned. P2Y1 (a arrowheads) and P2Y6 (b arrowheads) receptors are distinctively localized in sinus endothelial cells. The P2Y1 receptor is localized in stress fibers, but the P2Y6 receptor is localized in the regions between stress fibers. The P2Y6 receptor is also localized in the superficial layer of endothelial cells. They are also distinctively localized in leucocytes (L), platelets (P), and megakaryocytes (M) in sinus lumens and splenic cords. The P2Y1 receptor is localized in the cytoplasm of leucocytes and platelets, but P2Y6 receptor is localized in the superficial layer. In megakaryocytes, they are distinctively scattered. e–h P2Y1 (blue), P2Y12 (green) receptor, and F-actin (red) P2Y1 (e arrowheads) and P2Y12 (f arrowhead) receptors are specifically localized in the sinus endothelial cells surrounding the sinus lumen (S). Their localization is subtly different in leucocytes (L) in the splenic cord. i–l P2Y12 (blue), P2Y6 (green) receptors, and F-actin (red). P2Y12 (e arrowheads) and P2Y6 (f arrowheads) receptors are individually localized in sinus endothelial cells surrounding the sinus lumen. Bars 5 μm
Labeling for PLC-β3, PLC-γ2, and IP3 receptors was precisely localized in the basal parts of sinus endothelial cells (Fig. 5). PLC-β3 was prominently localized in the vicinity of stress fibers. PLC-γ2 was predominantly localized over stress fibers and also in the apical superficial layer of endothelial cells. IP3 receptor was markedly and widely localized in the basal part of endothelial cells. Labeling for PLC-β1, PLC-β2, PLC-β4, and PLC-γ1 was not detected in sinus endothelial cells.
Immunofluorescent localization of PLC-β3, PLC-γ2, and IP3 receptor (green) in semi-thin sections of the sinus endothelium in the red pulp by double immunofluorescent staining with phalloidin-conjugated fluorescence (red). a, b PLC-β3 is distinctly localized in the vicinity of stress fibers. c, d PLC-γ2 is clearly localized in the basal parts of the endothelial cells and also in the apical superficial layer of endothelial cells. e, f IP3 receptor is markedly and widely localized in the basal part of endothelial cells. S sinus lumen. Bars 5 μm
Immunogold labeling for electron microscopy
The ultrastructural localization of P2Y1, P2Y6, and P2Y12 receptors, PLC-β3, and PLC-γ2 was examined by immunogold electron microscopy of ultrathin cryosections of the red pulp of the spleen. Labeling for each P2Y1, P2Y6, and P2Y12 receptor was predominantly present in the basal parts of sinus endothelial cells; however, labeling was also present in the lateral junctional membrane, basal plasma membrane, and caveolae and endoplasmic reticulum in the basal part of sinus endothelial cells. In addition, labeling for P2Y6 receptor was localized in caveolae and endoplasmic reticulum in the apical part of sinus endothelial cells and labeling for P2Y12 receptor was collectively present in vesicles in the apical part of sinus endothelial cells (Figs. 6, 7, 8). Labeling for PLC-β3 and PLC-γ2 was predominantly present in the cytoplasm of the basal part of sinus endothelial cells and also localized in the vicinity of the junctional membrane and the basal plasma membrane of endothelial cells and caveolae in the basal part of endothelial cells (Fig. 9).
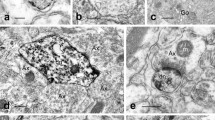
Immunogold electron microscopy of vertical sections of sinus endothelial cells labeled with ant-P2Y1 receptor. a In the basal part of three adjacent sinus endothelial cells, labeling with anti-P2Y1 receptor is predominantly present in the basal parts of sinus endothelial cells (asterisk). Labeling is also present in the lateral junctional membrane (arrows) and the basal plasma membrane (arrowheads). b Higher magnification of the junction of two adjacent cells. Labeling is present in the junctional membrane (arrow). c Higher magnification of the basal part of endothelial cells. Labeling is present in the basal plasma membrane (arrowhead) and caveolae (double arrowheads). RF ring fiber, WP Weibel–Palade body. Bars 100 nm
Immunogold electron microscopy of vertical sections of sinus endothelial cells labeled with ant-P2Y6 receptor. a In three adjacent sinus endothelial cells, labeling with anti-P2Y6 receptor is prominently present in the basal parts of sinus endothelial cells (asterisk). Labeling is also present in the basal plasma membrane (arrowhead). b Higher magnification of the junctions of four adjacent cells. Labeling is present in the junctional membranes (arrows). c The apical part of an endothelial cell. Labeling is present in the caveolae (double arrowheads) and endoplasmic reticulum (arrows) beneath the apical plasma membrane. d The basal part of an endothelial cell. Labeling is present in single (arrowhead) and clustered caveolae (double arrowheads). E1 erythrocyte in the sinus lumen, E2 erythrocyte in the splenic cord, MT mitochondria, RF ring fiber, WP Weibel–Palade body. Bars 100 nm
Immunogold electron microscopy of vertical sections of sinus endothelial cells labeled with anti-P2Y12 receptor. a In adjacent endothelial cells, labeling is present in the junctional plasma membranes (arrows) and scattered in small groups in the cytoplasm of endothelial cells (arrowheads). b Higher magnification of the junction of two adjacent cells. Labeling is present in the junctional membrane (arrows). c The apical part of an endothelial cell. Labeling gathers in a spherical vesicle (arrow). d The basal part of an endothelial cell. Labeling is present in the caveolae (arrowheads) and the basal plasma membrane. E erythrocyte in the sinus lumen, MT mitochondria, RF ring fiber, WP Weibel–Palade body. Bars 100 nm
Immunogold electron microscopy of a vertical section of sinus endothelial cells labeled with anti-PLC-β3 and anti-PLC-γ2. a In adjacent sinus endothelial cells, labeling with anti-PLC-β3 is present in the cytoplasm of sinus endothelial cells. Labeling is also present in the immediate vicinity of the lateral junctional membrane (arrow), the basal plasma membrane (arrowheads), and caveolae (arrowheads). b In adjacent sinus endothelial cells, labeling with anti-PLC-γ2 is present in the basal parts of sinus endothelial cells. Labeling is in close proximity to the plasma membranes of sinus endothelial cells, to which the extended projections of erythrocytes in the splenic cord attach (arrowheads). E erythrocyte in the sinus lumen, E′ erythrocytes protruding their process into the junctions of sinus endothelial cells, MT mitochondria, P platelet in the sinus lumen, RF ring fiber, WP Weibel–Palade body. Bars 100 nm
Discussion
In this study, we demonstrated using immunofluorescence microscopy and immunogold electron microscopy that P2Y1, P2Y6, and P2Y12 receptors were predominantly present in the basal parts of sinus endothelial cells and also in the junctional membrane, basal plasma membrane, and caveolae in the basal part of endothelial cells; however, they were differently distributed in sinus endothelial cells by immunofluorescence microscopy. The distinctive localization of P2Y1, P2Y6, and P2Y12 receptors in splenic sinus endothelial cells imply their particular functions. In addition, the proteins of PLC-β3 and PLC-γ2 related with these receptors were shown to be prominent in the basal part of endothelial cells and in the vicinity of the junctional membrane and the basal plasma membrane of endothelial cells and caveolae in the basal part of endothelial cells. It is well established that P2 receptors on endothelial cells receive ATP and UTP released from themselves and [Ca2+](i) is increased, leading to the release of nitric oxide, reorganization of the cytoskeleton, disassembly of vascular endothelial cadherin, and so on. P2Y1 and P2Y12 receptors mainly respond to ADP and ATP, but do not respond to UDP and UTP. P2Y6 receptor reacts with UDP and UTP. P2Y1 and P2Y6 receptors are associated with the [Ca2+](i) signaling pathway, whereas P2Y12 receptor is associated with cAMP signaling, related to increase in microvascular permeability (Ralevic and Burnstock 1998). In view of the key role of sinus endothelial cells in the filtering out of damaged or senescent cells from the blood in splenic cords, a role for P2Y1, P2Y6, and P2Y12 receptors in this process is suggested and will need to be evaluated.
The antibodies for P2X and P2Y receptors used in this study were confirmed by Western blotting performed with antibodies raised against these receptor subtypes in crude splenic extraction. Pre-incubation of the examined antibodies with the corresponding immunogenic peptides prevented immunoreactive bands, indicating that the bands were specific to the receptors. In previous studies, P2X1, P2X2, P2X3, P2X4, P2X5, P2X6, and P2X7 receptors in the rat thymus and thyroid were indicated by two bands of 70 and 140 kDa, estimated to form dimers (Glass et al. 2000; Glass and Burnstock 2001). P2Y1 and P2Y2 receptors in the hamster proximal urethra were detected at about two bands of 40 and 45 kDa, and three bands of 30, 50, and 120 kDa, respectively (Pinna et al. 2005). P2Y6 receptor was detected at 36, 40, and 55 kDa in the mouse colon (Grbic et al. 2008) and at 50 kDa in human endothelial cells (Riegel et al. 2011). P2Y12 receptor showed a band of 42–44 kDa in the tissues of the rat brain (Amadio et al. 2006); however, the molecular weights of the detected bands of the examined receptors in this study were different from those in previous studies. The examined receptors in the extraction might form polymers or be fragmented. Furthermore, they might show considerable variation in the size of the detected proteins, which are assumed to be brought out by alternative splicing and the presence of alternative spliced isoforms together with post-translational modifications such as glycosylation (Glass et al. 2000).
Although P2Y1, P2Y6, and P2Y12 receptors were prominently localized in the basal part of the endothelial cells in immunofluorescent microscopy, immunogold electron microscopy revealed that they were localized in the junctional membrane and the basal plasma membrane of endothelial cells and caveolae in the basal part of endothelial cells. In the mouse mammary gland, release of ATP, UTP, and UDP from secretory epithelial cells with the expression of P2Y receptors was induced by contraction of myoepithelial cells stimulated by oxytocin, working as a paracrine to enhance or regulate the contraction of myoepithelial cells by oxytocin. Furthermore, the release of nucleotides induced the increase of [Ca2+](i) in surrounding secretory cells (Enomoto et al. 1992, 1994; Nakano et al. 1997). Sinus endothelial cells are stimulated by nucleotides by P2Y1, P2Y6, and P2Y12 receptors in the junctional and plasma membrane and caveolae, and consequently these receptors in the cytoplasm of the basal part of endothelial cells are considered to be transported to the junctional and plasma membrane to enhance the information. In addition, the release of nucleotides from sinus endothelial cells receiving environmental stimuli might induce an increase of [Ca2+](i) in surrounding sinus endothelial cells.
In bovine aortic endothelial cells, ATP-induced [Ca2+](i) increase is initiated on the rim of centralized caveolin and propagates throughout the entire cell as a Ca2+ wave (Isshiki et al. 2002). ATP-induced [Ca2+](i) increase in sinus endothelial cells is likely to be initiated at the caveolae, the P2Y1 receptor of which is localized in the basal part of sinus endothelial cells. Although numerous caveolae are distributed throughout splenic sinus endothelial cells (Uehara and Miyoshi 1999c), P2Y1 receptor is localized in caveolae in the basal part of endothelial cells. Sinus endothelial cells are a crucial site for controlling blood cell passage through the splenic cord beneath endothelial cells. P2Y1 receptor might play an important role in controlling blood cell passage through phenomena including cytoskeletal reorganization and disassembly of vascular endothelial cadherin, and so on. In addition, P2Y12 and P2Y1 receptors are localized in the basal plasma membrane and caveolae. The P2Y12 receptor is also activated by ATP and reduces the formation of cAMP. cAMP regulates a wide range of cellular processes, including differentiation, secretion, gene transcription, regulation of cell shape, cytoskeleton remodeling, adhesion, migration, and microvascular permeability. There is a possibility that the P2Y12 receptor is involved in controlling blood cell passage between endothelial cells by cAMP, regulating in part the dynamic opening and closing of cell–cell adherens junctions (Dejana et al. 2008).
The P2Y6 receptor was localized in the vicinity of stress fibers and in the superficial layer of endothelial cells on all sides. The P2Y6 receptor contributes to endothelium-dependent relaxation of the aorta by UDP (Bar et al. 2008). The sinus endothelium lacks smooth muscles, but includes well-developed stress fibers in the basal part of endothelial cells, considered to be contractile (Katoh et al. 2011); therefore, the P2Y6 receptor may be involved in the relaxation of stress fibers. Furthermore, intestinal inflammation increases the expression of P2Y6 receptor on epithelial cells and the release of chemokine CXCL8 from them by UDP (Grbic et al. 2008). CXCL8, one of the major mediators for leucocytes to transmigrate through endothelial cells, is also synthesized by endothelial cells and stored in Weibel–Palade bodies (Hol et al. 2009). Weibel–Palade bodies are abundant in sinus endothelial cells. The P2Y6 receptor in sinus endothelial cells is considered to participate in the immune system and/or might be correlated with leucocyte migration through sinus endothelial cells without inflammation.
Activated PLC-β by a G-protein produces IP3 to mobilize [Ca2+](i) via IP3 receptor from intracellular calcium storage sites. In our previous studies (Uehara et al. 2004; Uehara 2005), Ca2+-storing tubulovesicular structures within sinus endothelial cells were established using tissue sections treated with osmium ferricyanide, and IP3 type 1 receptors and calreticulin, a Ca2+-binding protein, have been shown to be localized in the endoplasmic reticulum in the junctional membrane and caveolae on immunogold analysis by electron microscopy. It is likely that P2Y1and P2Y6 receptors activate G-protein and PLC-β3 and produce IP3 to mobilize [Ca2+](i) via IP3 receptor from intracellular calcium storage sites.
PLC-γ activation is involved in its association with and phosphorylation by receptor and non-receptor tyrosine kinases, as well as its interaction with specialized adaptor molecules and other second messenger molecules. PLC-γ is implicated in remodeling actin-based cytoskeletons, such as focal adhesions, stress fibers, filopodia, and lamellipodia to change their shape and adherence, and purposeful movement by environmental stimuli (Rebecchi and Pentyala 2000). Furthermore, PLC-γ is integrated into response pathways involving PLC-β, where it operates to prolong the calcium response. In the present study, both PLC-γ2 and PLC-β were localized in the basal parts of endothelial cells and in close proximity to the plasma membrane. PLC-γ might operate to prolong the calcium response in sinus endothelial cells.
References
Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA (2006) International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58:281–341
Amadio S, Tramini G, Martorana A, Viscomi MT, Sancesario G, Bernardi G, Volonte C (2006) Oligodendrocytes express P2Y12 metabotropic receptor in adult rat brain. Neuroscience 141:1171–1180
Ando J, Yamamoto K (2009) Vascular mechanobiology: endothelial cell responses to fluid shear stress. Circ J 73:1983–1992
Bar I, Guns PJ, Metallo J, Cammarata D, Wilkin F, Boeynams JM, Bult H, Robaye B (2008) Knockout mice reveal a role for P2Y6 receptor in macrophages, endothelial cells, and vascular smooth muscle cells. Mol Pharmacol 74:777–784
Burnstock G (2008) Dual control of vascular tone and remodelling by ATP released from nerves and endothelial cells. Pharmacol Rep 60:12–20
da Silva CG, Specht A, Wegiel B, Ferran C, Kaczmarek E (2009) Mechanism of purinergic activation of endothelial nitric oxide synthase in endothelial cells. Circulation 119:871–879
Dejana E, Orsenigo F, Lampugnani MG (2008) The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci 121:2115–2122
Drenckhahn D, Wagner J (1986) Stress fibers in the splenic sinus endothelium in situ: molecular structure, relationship to the extracellular matrix, and contractility. J Cell Biol 102:1738–1747
Enomoto K, Furuya K, Yamagishi S, Maeno T (1992) Mechanically induced electrical and intracellular calcium responses in normal and cancerous mammary cells. Cell Calcium 13:501–511
Enomoto K, Furuya K, Yamagishi S, Oka T, Maeno T (1994) The increase in the intracellular Ca2+ concentration induced by mechanical stimulation is propagated via release of pyrophosphorylated nucleotides in mammary epithelial cells. Pflugers Arch 427:533–542
Fumagalli M, Trincavelli L, Lecca D, Martini C, Ciana P, Abbracchio MP (2004) Cloning, pharmacological characterisation and distribution of the rat G-protein-coupled P2Y(13) receptor. Biochem Pharmacol 68:113–124
Glass R, Burnstock G (2001) Immunohistochemical identification of cells expressing ATP-gated cation channels (P2X receptors) in the adult rat thyroid. J Anat 198:569–579
Glass R, Townsend-Nicholson A, Burnstock G (2000) P2 receptors in the thymus: expression of P2X and P2Y receptors in adult rats, an immunohistochemical and in situ hybridisation study. Cell Tissue Res 300:295–306
Grbic DM, Degagne E, Langlois C, Dupuis AA, Gendron FP (2008) Intestinal inflammation increases the expression of the P2Y6 receptor on epithelial cells and the release of CXC chemokine ligand 8 by UDP. J Immunol 180:2659–2668
Guns PJ, Korda A, Crauwels HM, Van Assche T, Robaye B, Boeynaems JM, Bult H (2005) Pharmacological characterization of nucleotide P2Y receptors on endothelial cells of the mouse aorta. Br J Pharmacol 146:288–295
Harrington LS, Mitchell JA (2004) Novel role for P2X receptor activation in endothelium-dependent vasodilation. Br J Pharmacol 143:611–617
Henderson DJ, Elliot DG, Smith GM, Webb TE, Dainty IA (1995) Cloning and characterisation of a bovine P2Y receptor. Biochem Biophys Res Commun 212:648–656
Hol J, Kuchler AM, Johansen FE, Dalhus B, Haraldsen G, Oynebraten I (2009) Molecular requirements for sorting of the chemokine interleukin-8/CXCL8 to endothelial Weibel–Palade bodies. J Biol Chem 284:23532–23539
Isshiki M, Ando J, Yamamoto K, Fujita T, Ying Y, Anderson RG (2002) Sites of Ca(2+) wave initiation move with caveolae to the trailing edge of migrating cells. J Cell Sci 115:475–484
Katoh K, Kano Y, Noda Y (2011) Rho-associated kinase-dependent contraction of stress fibres and the organization of focal adhesions
Loesch A, Burnstock G (2000) Ultrastructural localisation of ATP-gated P2X2 receptor immunoreactivity in vascular endothelial cells in rat brain. Endothelium 7:93–98
Motte S, Communi D, Pirotton S, Boeynaems JM (1995) Involvement of multiple receptors in the actions of extracellular ATP: the example of vascular endothelial cells. Int J Biochem Cell Biol 27:1–7
Nakano H, Furuya K, Furuya S, Yamagishi S (1997) Involvement of P2-purinergic receptors in intracellular Ca2+ responses and the contraction of mammary myoepithelial cells. Pflugers Arch 435:1–8
Pinna C, Glass R, Knight GE, Bolego C, Puglisi L, Burnstock G (2005) Purine- and pyrimidine-induced responses and P2Y receptor characterization in the hamster proximal urethra. Br J Pharmacol 144:510–518
Ralevic V, Burnstock G (1998) Receptors for purines and pyrimidines. Pharmacol Rev 50:413–492
Rebecchi MJ, Pentyala SN (2000) Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol Rev 80:1291–1335
Riegel AK, Faigle M, Zug S, Rosenberger P, Robaye B, Boeynaems JM, Idzko M, Eltzschig HK (2011) Selective induction of endothelial P2Y6 nucleotide receptor promotes vascular inflammation. Blood 117:2548–2555
Uehara K (2005) Localization of TRPC1 channel in the sinus endothelial cells of rat spleen. Histochem Cell Biol 123:347–356
Uehara K (2006) Distribution of adherens junction mediated by VE-cadherin complex in rat spleen sinus endothelial cells. Cell Tissue Res 323:417–424
Uehara K, Miyoshi M (1999a) Stress fiber networks in sinus endothelial cells in the rat spleen. Anat Rec 254:22–27
Uehara K, Miyoshi M (1999b) Tight junction of sinus endothelial cells of the rat spleen. Tissue Cell 31:555–560
Uehara K, Miyoshi M (1999c) Tubular invaginations with caveolae and coated pits in the sinus endothelial cells of the rat spleen. Histochem Cell Biol 112:351–358
Uehara K, Uehara A (2008) Localization of claudin-5 and ZO-1 in rat spleen sinus endothelial cells. Histochem Cell Biol 129:95–103
Uehara K, Onoue H, Jeyakumar LH, Fleischer S, Uehara A (2004) Localization of ryanodine receptor 3 in the sinus endothelial cells of the rat spleen. Cell Tissue Res 317:137–145
Yamamoto K, Sokabe T, Matsumoto T, Yoshimura K, Shibata M, Ohura N, Fukuda T, Sato T, Sekine K, Kato S, Isshiki M, Fujita T, Kobayashi M, Kawamura K, Masuda H, Kamiya A, Ando J (2006) Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat Med 12:133–137
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uehara, K., Uehara, A. P2Y1, P2Y6, and P2Y12 receptors in rat splenic sinus endothelial cells: an immunohistochemical and ultrastructural study. Histochem Cell Biol 136, 557–567 (2011). https://doi.org/10.1007/s00418-011-0859-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-011-0859-2