Abstract
The immunolocalization of protease-activated receptors (PARs) and related proteins in splenic sinus endothelial cells was examined using immunofluorescence and electron microscopy. Immunofluorescence microscopy showed that PAR1 colocalized with PAR2, PAR3, and PAR4. PAR4 colocalized with PAR3 and P2Y12. Myosin heavy chain IIA localized to the outer shape and at the base of cells, but did not colocalize with α-catenin. The localization of di-phosphorylated myosin regulatory light chains (ppMLC) was partially detected on the outer circumference and conspicuously at the base of cells. Macrophage migration inhibitory factor (MIF) also localized in cells. Immunogold electron microscopy revealed the localization of PAR1 on the caveolar membrane, plasma membrane, and junctional membrane of cells. PAR2 and PAR3 localized to the plasma membrane of cells. PAR4 localized to the plasma membrane, depressions in the plasma membrane, and cytoplasmic vesicles. PpMLC was detected in stress fibers, but rarely near the adherens junctions of neighboring cells. MIF localized in vesicles on the apical and basal sides of the Golgi apparatus. Electron microscopy of endothelial cells with saponin extraction showed the depression of many coated pits formed by clathrin from the plasma membrane. Stress fibers developed at the base of cells; however, few actin filaments were observed near adherens junctions. These results indicate that PARs play important roles in splenic sinus endothelial cells, such as in endothelial barrier protection and the maintenance of firm adhesion to ring fibers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The spleen is a lymphoid organ that intervenes between blood vessels and is structurally divided into two components: white pulp and red pulp. White pulp is responsible for the immune system and produces B lymphocytes. Red pulp removes aged red blood cells and stores platelets, blood, and monocytes. The histological structure of the spleen differs between humans, mice, and rats (Steiniger 2015). Mice and rats have marginal zones surrounding lymph nodules, whereas humans do not. Sheathed capillaries encircled by pericytes are distributed in the red pulp of humans, but not in that of mice or rats. Furthermore, red pulp in embryonic humans has hematopoietic tissue that disappears in the middle of development, but initiates hematopoiesis again under specific conditions, such as anemia after birth. On the other hand, hematopoiesis is performed from the embryonic to postnatal stages in mice and rats. However, the structure and functions of red pulp in humans, mice, and rats share some similarities. Red pulp consists of splenic cords of reticular tissue and splenic sinuses of capillaries with wide vascular cavities, blood vessels open in splenic cords, and blood cells in splenic cords pass between splenic sinus endothelial cells and return to capillaries. Macrophages reside in splenic cords and take in aged erythrocytes by phagocytosis, platelets, and blood, and monocytes that transform into macrophages are stored under the capsule (Taylor et al. 2005; Swirski et al. 2009). We have used rats to histochemically and microstructurally examine sinus endothelial cells and elucidate the mechanisms contributing to the passage of blood cells. Our findings revealed concurrent systems in endothelial cells for protecting and dissociating adherens junctions as well as a constant cycle of dissociation and association for these junctions (Uehara and Uehara 2021). Protease-activated receptor (PAR) 1, which belongs to a family comprising four members that are activated by thrombin, a serine protease, plays a significant role in this protection system. Thrombin is presumed to be produced in red pulp, through which blood flow is slow and in which some events induced by PARs occur.
PARs are a subfamily of G protein-coupled receptors (GCPRs) that signal through heterotrimeric G proteins, and are activated by the cleavage of a specific site in the extracellular region by protease. PAR1, PAR2, PAR3, and PAR4 have been cloned. PAR1, PAR3, and PAR4 are activated by thrombin, while PAR2 is activated by serine proteases, such as trypsin, activated coagulation factor VII, and activated coagulation factor X (FXa). The four PARs transmit signals alone or by interacting with other members and other GCPRs (Rezaie 2014; Nieman 2016). The thrombin signal by PAR1 in endothelial cells results in two opposite responses to cell adhesion, namely, barrier protection and disruption, in the presence or absence of thrombomodulin. During barrier protection, PAR1 localizes to caveolae and signals via thrombomodulin, activated protein C (APC), and the endothelial protein C receptor (EPCR). At the time of barrier disruption, PAR1 localizes to the plasma membrane and induces the contraction of actin filaments bound to adherens junctions by activating Rho and myosin II via heterotrimeric G proteins, thereby disrupting the endothelial barrier (Duluc and Wojciak-Stothard 2014; Rezaie 2014). Myosin II has three isoforms, A (MHC IIA), B (MHC IIB), and C (MHC IIC). The distribution of MHC IIA and IIB differs, even in each cell, and they also have different functions. Although limited information is currently available on MHC IIC, it is known to be absent in the spleen (Golomb et al. 2004). The ATPase motor domain of myosin II is regulated by the phosphorylation of the myosin regulatory light chain at Thr 18 and/or Ser 19, and there is a division of functions between mono-phosphorylation and di-phosphorylation (ppMLC), with ppMLC functioning in the early stages when the barrier function of endothelial cells is impaired (Hirano and Hirano 2016). The activation of PAR2 in endothelial cells, such as in the aorta, induces blood vessel relaxation (Kawabata et al. 2001). Furthermore, PAR2 is transactivated by cleaved PAR1 and promotes the production of macrophage migratory factor (MIF), a multifunctional molecule that is closely involved in inflammation, immune responses, and cell proliferation, in endothelial cells together with PAR1 during the inflammatory phase induced by thrombin and FXa (O'Brien et al. 2000; Shimizu et al. 2004). PAR3 functions as a cofactor to activate PAR4, regulates PAR1 signaling by receptor dimerization, and is also activated by APC in the presence of EPCR to protect the endothelial barrier (Nakanishi-Matsui et al. 2000; McLaughlin et al. 2007; Burnier and Mosnier 2013). PAR4 has lower affinity for thrombin than PAR1 and, thus, the activation of PAR4 requires high concentrations of thrombin. A previous study reported that PAR4 formed a heterodimer with PAR1 and was activated even at low concentrations of thrombin (Arachiche et al. 2013). Furthermore, PAR4 was shown to form a heterodimer with the purinergic receptor P2Y12, was activated by thrombin and then internalized, and regulated β -arrestin-mediated Akt signals for the activation of integrin (Li et al. 2011; Smith et al. 2017).
To elucidate the function of PARs in splenic sinus endothelial cells, the present study investigated the immunolocalization of PARs, P2Y12, MHC IIA, MHC IIB, ppMLC at T18 and S19, MIF, α-catenin, and clathrin in the endothelial cells of rat splenic sinuses using confocal laser scanning and transmission electron microscopy.
Materials and methods
Western blotting
Spleens removed from 8-week-old male Wistar rats were treated with extraction reagent (Sigma-Aldrich, MO, USA). Extracts were loaded onto 4–15% SDS–polyacrylamide gels for separation using a Bio-Rad Mini-protean 3 cell (Bio-Rad, CA, USA), followed by the transferal of proteins to polyvinyldifluoride membranes (Millipore, MA, USA). Membranes were placed in blocking buffer comprising 5% nonfat milk in Tris-buffered saline with 0.05% Tween 20 (TBS) and then incubated with primary antibodies for PAR2, PAR3, and PAR4. Membranes were washed with TBS, followed by an incubation with goat anti-rabbit horseradish peroxidase-conjugated IgG and then with ECL Prime reagent (GE-Amersham). Hyperfilm ECL (GE-Amersham) was used for chemiluminescence detection.
Confocal immunofluorescence microscopy
Adult male rats were anesthetized using a previously described method (Uehara and Uehara 2014). The red pulp of spleens was cut into pieces and fixed in 3% paraformaldehyde in 0.1 M phosphate buffer at pH 7.4, followed by immersion in 20% polyvinylpyrrolidone and 2.3 M sucrose in the same buffer and rapid freezing in liquid nitrogen. Semi-thin sections were prepared from frozen samples for confocal immunofluorescence microscopy and ultra-thin sections for immunogold electron microscopy.
Semi-thin frozen sections were mounted on glass slides. Triple immunostaining was conducted using a previously described method (Uehara and Uehara 2014, 2016) to investigate the localization of the primary antibody (Table 1) in splenic sinus endothelial cells and elucidate the relationship between PARs. Positive and negative controls for staining were included. Sections were examined using the Zeiss LSM710 confocal microscope (Carl Zeiss Microscopy, Tokyo, Japan).
Electron microscopy
Immunogold labeling
Ultrathin frozen sections were placed on grids and treated with 5% BSA. They reacted the primary antibodies for PAR1, PAR2, PAR3, PAR4, P2Y12, ppMLC, MIF, and clathrin for 1 h and then incubated with the compounded 15-nm colloidal gold secondary antibody. Sections were fixed in 2% glutaraldehyde in 0.1 M phosphate buffer and rinsed with distilled water, followed by infiltration with 0.5% uranyl acetate and 1.8% methylcellulose in distilled water. After the removal of excess liquid, sections were air-dried.
Soluble protein extraction with saponin
To visualize actin filaments in sinus endothelial cells, soluble proteins in the cytoplasm were washed out with saponin (Uehara and Miyoshi 1999; Uehara and Uehara 2010, 2014). Spleens were cut into pieces, infiltrated with 0.5% saponin in HEPES buffer at pH 7.3 for 20 min, and then rinsed with the same buffer. The fixation of samples was performed in 2.5% glutaraldehyde containing 0.2% tannic acid for 1 h, followed by post-fixation in 1% osmium tetroxide in the same buffer for 1 h, dehydration in an ethanol series, and embedding in Epon. Uranyl acetate and lead citrate were used to stain ultrathin sections.
All sections were observed using the Hitachi 7100 electron microscope.
Results
Western blotting
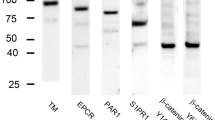
High signal intensities for PAR2, PAR3, and PAR4 were detected at approximately 33, 42, and 55 kDa, respectively (Fig. 1). A previous study conducted a Western blot analysis of PAR1 (Uehara and Uehara 2021).
Confocal immunofluorescence microscopy
Triple immunostaining for the combination of PARs, TM, and actin filaments was performed to examine the localization of PARs in splenic sinus endothelial cells. Since TM specifically localizes around splenic sinus endothelial cells and actin filaments are the main constituents of stress fibers that characteristically localize at the base of these cells, endothelial cells may be identified by the localization of TM and stress fibers. PAR1, PAR2, PAR3, and PAR4 localized around endothelial cells and a merged image of the four subtypes of PARs, TM, and actin filaments revealed that four subtypes of PARs and TM colocalized around endothelial cells. PAR1, PAR3, and PAR4 localized to adjacent cell boundaries (Figs. 2 and 3).
Laser-scanning microscopy of triple immunostaining for the combination of PARs, TM, and actin filaments in semi-thin frozen sections of sinus endothelial cells in red pulp. TM fluorescence (blue) was observed around endothelial cells (b, f, i, and n). Phalloidin (red) visualized stress fibers containing actin filaments, which were conspicuously localized at the base of endothelial cells (c, g, k, and o). a–d Immunolocalization of PAR1 (green), TM, and actin filaments. a PAR1 (arrow) was detected around endothelial cells. Strong fluorescence was observed at adjacent cell boundaries (arrowhead). c, d A merged image showing that PAR1 and TM colocalized around endothelial cells with characteristic stress fibers. e–h Immunolocalization of PAR2 (green), TM, and actin filaments. b’ PAR2 (arrow) localized around endothelial cells. g, h A merged image showing the colocalization of PAR2 and TM around endothelial cells with distinctive stress fibers. i–l Immunolocalization of PAR3 (green), TM, and actin filaments. c’ PAR3 (arrow) was detected around endothelial cells. k, lA merged image showing that PAR3 and TM colocalized around endothelial cells with stress fibers. m–p Immunolocalization of PAR4 (green), TM, and actin filaments. m PAR4 (arrow) was detected around endothelial cells. Strong fluorescence was observed at adjacent cell boundaries (arrowhead). o, p A merged image showing the colocalization of PAR1 and TM around endothelial cells with stress fibers. L sinus lumen, Bars 5 μm
Laser-scanning microscopy of triple immunostaining for the combination of two PARs and actin filaments and for PAR4, P2Y12, and actin filaments in semi-thin frozen sections of sinus endothelial cells in red pulp. Characteristic stress fibers (red) were visualized by phalloidin to identify the splenic sinus endothelium (d, h, i, p, and t). a–d Immunolocalization of PAR1 (green), PAR2 (blue), and actin filaments. PAR1 localized around endothelial cells (arrow) and in the junctional area (arrowhead) of adjacent cells. PAR2 (arrow) was observed around endothelial cells. Merged images (c) showing the colocalization of PAR1 and PAR2. e–h Immunolocalization of PAR1 (green), PAR3 (blue), and actin filaments. PAR1 (e) and PAR3 (f) localized around endothelial cells (arrow) and in the junctional area (arrowhead) of adjacent cells. Merged images (g) showing the colocalization of PAR1 and PAR3. i–l Immunolocalization of PAR1 (green), PAR4 (blue), and actin filaments. PAR1 (i) and PAR4 (j) colocalized around endothelial cells (arrow) and in the junctional area (arrowhead) of adjacent cells. Merged images (k) showing the colocalization of PAR1 and PAR4. m–p The immunolocalization of PAR3 (green), PAR4 (blue), and actin filaments. PAR3 (m) and PAR4 (n) around endothelial cells (arrow). Merged images (o) showing the colocalization of PAR3 and PAR4. q–t Immunolocalization of PAR4 (green), P2Y12 (blue), and actin filaments. PAR4 (q) and P2Y12 (r) localized around endothelial cells (arrow) and in the junctional area (arrowhead) of adjacent cells. L sinus lumen, Bars 5 μm
Triple immunostaining for the combination of two PARs, PAR4 and P2Y12, and actin filaments was performed to examine the relationship between each PAR and PAR4 and P2Y12 in endothelial cells. The following combinations of PARs were used: PAR1 and PAR2, PAR1 and PAR3, PAR1 and PAR4, and PAR3 and PAR4. Immunofluorescence microscopy of these combinations revealed their colocalization around endothelial cells. Immunofluorescence microscopy of the combination of PAR4 and P2Y12 with actin filaments showed the colocalization of PAR4 and P2Y12 around endothelial cells as well as near the junctions of neighboring cells (Fig. 3).
The immunofluorescence of MHC IIA, α-catenin, and actin filaments revealed the localization of MHC IIA around endothelial cells, particularly at the basal part. It partially colocalized with stress fibers, but separately from α-catenin (Fig. 4a–d). MHC IIB was not detected in endothelial cells, but localized in the megakaryocytes and leukocytes of splenic cords. Immunofluorescence of ppMLC, MHC IIA, and actin filaments showed the localization of ppMLC to the outer shape of endothelial cells, conspicuous localization at the base of these cells, and partial colocalization with MHC IIA and stress fibers at the base of these cells (Fig. 4e–h).
Laser-scanning microscopy of triple immunostaining for MHC IIA, α-catenin, and actin filaments, and for ppMLC, MHC IIA, and actin filaments in semi-thin frozen sections of sinus endothelial cells in red pulp. a–d Immunolocalization of MHC IIA (green), α-catenin (blue), and actin filaments (red). a, f MHC IIA localized to the outer shape (arrow) of endothelial cells, particularly the basal part. b α-catenin localized to the junctional area of adjacent cells (arrowhead). c A merged image of MHC IIA and α-catenin showing their separate localization. d A merged image of MHC IIA, α-catenin, and actin filaments showing the partial colocalization of MHC IIA and stress fibers at the base of endothelial cells. e–h Immunolocalization of ppMLC (green), MHC IIA (blue), and actin filaments (red). e ppMLC partially localized to the outer shape (arrow) of endothelial cells and conspicuously localized to the base of endothelial cells (arrowhead). g A merged image of ppMLC and MHC IIA showing the partial colocalization of ppMLC in MHC IIA (arrow). h A merged image of ppMLC, MHC IIA, and actin filaments showing that ppMLC, MHC IIA, and stress fibers partially colocalized to the base of endothelial cells (arrow). L sinus lumen, Bars 5 μm
The immunofluorescence of MIF, TM, and actin filaments revealed the localization of MIF to the entire surface of cells identified as splenic sinus endothelial cells by TM and actin filaments (Fig. 5a). The immunofluorescence of ManR, the endosomes and lysosomes of macrophages, and actin filaments demonstrated the distribution of macrophages in splenic cords. Macrophages with well-developed endosomes and lysosomes were identified by labeling the extracellular circumference with an anti-ManR antibody and organelles with an anti-macrophage antibody that responds to endosomes and lysosomes. ManR also localized around endothelial cells with stress fibers at their base. A large number of macrophages were distributed in splenic cords (Fig. 5e).
Laser-scanning microscopy of triple immunostaining for MIF, TM, and actin filaments and for ManR, endosomes and lysosomes of macrophages (macroΦ), and actin filaments in frozen semi-thin sections of sinus endothelial cells in red pulp. a–d Immunolocalization of MIF (green), TM (blue), and actin filaments (red). a Two sinuses (L) were transversely sectioned. MIF (arrow) localized around endothelial cells surrounding sinuses. b TM (arrow) organized around endothelial cells surrounding sinuses. c Stress fibers (arrow) were characteristically located at the base of endothelial cells. The actin filaments of blood cells in splenic cords were observed. d A merged image showing the localization of MIF around endothelial cells. e A merged image of the immunolocalization of ManR (green), macroΦ (blue), and actin filaments (red). ManR localized around macrophages (asterisk) and endothelial cells (arrow) with stress fibers at their base. An anti-macrophage antibody labeled endosomes and lysosomes, which were well developed in macrophages. Many macrophages identified by ManR and macroΦ were detected in splenic cords. L sinus lumen, Bars 5 μm
Electron microcopy
Immunogold labeling
PAR1 labeling was detected in the caveolar membrane, the plasma membrane around endothelial cells, and in the junctional membrane and cytoplasm of neighboring cells (Fig. 6a). PAR2, PAR3, and PAR4 labeling was also observed in the plasma membrane of endothelial cells and in their cytoplasm (Fig. 6b, c, d). PAR4 labeling was frequently located in coated pits, and aggregated labels were located in the cytoplasm (Fig. 6d, e). P2Y12 labeling was detected in the plasma membrane of endothelial cells and the junctional membrane of neighboring cells as well as in the coated pits, but less frequently than that of PAR4 (Fig. 6f, g). ppMLC labeling was observed in the filamentous structures of stress fibers, but rarely near the adherens junction of endothelial cells. Comparisons of the junctions of endothelial cells and those of reticular cells beneath them in the same section revealed fewer gold particles in the former (Fig. 6h, i). MIF labeling was detected in the Golgi apparatus, in vesicles surrounding the Golgi apparatus on both the apical and basal sides, and vesicles near the surface of endothelial cells (Fig. 6j). Clathrin labeling was detected on depressions of the plasma membrane and the membrane of vesicles near the surface (Fig. 7).
Immunogold electron microscopy detected PARs, P2Y12, ppMLC, and MIF labeling in frozen ultra-thin sections of adjacent endothelial cells surrounding the splenic sinus. a PAR1 labeling. Two adjacent endothelial cells are shown. Labeling was observed on the plasma membrane (arrow) and the junctional membrane of adjacent cells (arrowhead). Inset Enlarged image of the apical surface. Labeling was detected on the caveolar membrane (arrowhead). b PAR2 labeling. Two adjacent endothelial cells are shown. Labeling was observed on the plasma membrane (arrow). c PAR3 labeling. Labeling was present on the plasma membrane (arrow). d, e PAR4 labeling. d Three adjacent endothelial cells are shown. Labeling was located on the plasma membrane (arrow). It aggregated in a cluster near the cell surface and at the junction of adjacent cells (arrowhead). e Enlarged image of the cell surface. Labeling was observed on a depression of the plasma membrane (arrow). Clustered labels were present in the cytoplasm (arrowhead). f, g P2Y12 labeling. f Three adjacent cells are shown. Labeling was detected on the plasma membrane (arrow) and the junctional membrane (arrowhead) of adjacent endothelial cells. g Enlarged image of the cell surface. Labeling was observed in a depression of the plasma membrane (arrow). h, i ppMLC labeling. h Two adjacent endothelial cells underneath ring fibers and reticulocytes (RC) are shown. Labeling localized near stress fibers (arrow) at the base of endothelial cells, but was rarely detected at the junctional membrane of adjacent cells (arrowhead). Labeling was present at the junctional membrane of adjacent RC (double arrowheads) and more gold particles were present than in endothelial cells. i The basal part at which adjacent cells join. Endothelial cells adhered by adherens junctions undercoated by an electron dense material at some locations. Labeling was present in the filamentous structures (arrow) of stress fibers, but rarely near adherens junctions (arrowhead). j MIF labeling. Labeling localized on a vesicle near the surface of endothelial cells (arrowhead), in the Golgi apparatus (double arrows), and in vesicles (arrow) surrounding the Golgi apparatus on both the apical and basal sides of the apparatus. L sinus lumen, RF ring fiber, WP Weibel-Palade body. Bars 100 nm
Immunogold electron microscopy detected clathrin labeling in frozen ultra-thin sections of adjacent endothelial cells surrounding the splenic sinus. The apical part at which adjacent cells join. Labeling was present on a depression of the plasma membrane (arrow) and the membrane of a vesicle near the surface (arrowhead). L sinus lumen. Bar 100 nm
Soluble protein extraction with saponin
The saponin treatment removed soluble proteins in endothelial cells, allowing for the clear visualization of actin filaments and organelles. A large number of coated pits with spines formed depressions around the entire surface of endothelial cells. Coated vesicles with spines were detected in the cytoplasm of endothelial cells (Fig. 8a). Stress fibers comprising actin filaments were observed at the base of endothelial cells and ran tangentially parallel to the boundaries of these cells. These fibers contained a central part with high electron density and a surrounding fibrous part. Adjacent endothelial cells were joined by adherens junctions at some locations, but with fewer surrounding actin filaments (Fig. 8b).
Electron microscopy of sinus endothelial cells treated with saponin. Soluble proteins in endothelial cells were removed, and the cytoplasm was electron-lucent. Actin filaments and organelles were clearly visible. a The apical surface of an endothelial cell. Coated pits with spines or a polygonal mesh (arrow) formed depressions from the cell surface. A coated vesicle with spines was present in the cytoplasm (double arrows). b The basal part of two tangentially sectioned adjacent endothelial cells. Stress fibers were visible at the base of endothelial cells and ran slightly parallel to the cell boundary. They consisted of a central part with high electron density and a surrounding fibrous part. Adjacent cells were joined by adherens junctions at some locations (arrow); however, there were few actin filaments near adherens junctions. L sinus lumen, RF ring fiber, SF stress fibers. Bar 100 nm
Discussion
Confocal immunofluorescence microscopy and immunogold electron microscopy revealed that PAR1, PAR2, PAR3, PAR4, P2Y12, and their related proteins localized to the sinus endothelial cells of red pulp. Since blood flow in red pulp is slow and its stores blood and platelets, it is regarded as a production site of thrombin. Therefore, each PAR is presumed to plays important roles in red pulp.
The distribution and functions of MHC IIA and MHC IIB have been reported to differ in cultured fibroblasts. In the migrating phase, MHC IIA localizes to the lamella and posterior region, MHC IIB colocalizes with MHC IIA in the posterior region, and ppMLC is present in the restricted region, which is rich in MHC IIA. In the quiescent phase, MHC IIA is enriched in peripheral stress fibers with ppMLC, whereas MHC IIB is not (Saitoh et al. 2001). In the present study, MHC IIA localized around endothelial cells, particularly their base, and partially colocalized with stress fibers; however, MHC IIB was not detected in endothelial cells in the present study. ppMLC also conspicuously localized at the base of these cells. Based on these findings, MHC IIA plays a role in the induction of contractions at the base of endothelial cells.
The thrombin signal by PAR1 in endothelial cells results in two opposite reactions: barrier protection and disruption. The localization of PAR1 differs between the two reactions. In barrier disruption, stimulated PAR1 activates Rho and myosin II and contracts actin filaments bound to adherens junctions, resulting in the disruption of these junctions (Burnier and Mosnier 2013; Duluc and Wojciak-Stothard 2014; Rezaie 2014). Adherens junctions in vascular endothelial cells dynamically and strictly control vascular permeability to maintain homeostasis, and comprise an adhesion molecule complex containing vascular endothelial-cadherin, p120, β-catenin, and α-catenin, which binds to actin filaments. When contractile force is generated by myosin II, α-catenin binds recruited vinculin, which connects actin filaments to the adhesion complex and stabilizes cell adhesion. The contractile force generated by myosin II is indispensable for the stability of adherens junctions and an adhesion complex not connected to actin filaments exhibits high instability and random motion (Hong et al. 2013; Gloushankova et al. 2017). In the present study, electron microscopy on saponin-treated samples revealed that very few actin filaments attached to the adherens junctions adjoining neighboring endothelial cells. In addition, immunogold electron microscopy for ppMLC generating contractile force demonstrated that labeling localized in the filamentous structure of stress fibers, but rarely near adherens junctions in endothelial cells. Contractile force was presumed to be generated around stress fibers, but not adherens junctions. These results indicate that the adherens junctions of splenic sinus endothelial cells are unstable, and are also not disrupted by PAR1 stimulated by thrombin.
PAR1 localizes to caveolae during barrier protection and to the plasma membrane during barrier disruption. We previously reported that PAR1 localized to both the plasma and caveolar membranes of splenic sinus endothelial cells, with its localization to the caveolar membrane being involved in barrier protection with thrombomodulin, APC, and EPCR (Uehara and Uehara 2021). PAR3 was previously shown to be activated by APC in the presence of EPCR and contributed to vascular barrier protection (Burnier and Mosnier 2013), and also regulated PAR1 signaling by receptor dimerization (McLaughlin et al. 2007). In the present study, immunofluorescence microscopy showed that PAR3 localized around endothelial cells and colocalized with PAR1. PAR3 in splenic sinus endothelial cells contribute to barrier protection and may enhance the PAR1 signal.
MIF is a multifunctional molecule that plays an important role in inflammation, immune responses, and cell proliferation and is expressed in various types of cells, including endothelial cells and immune cells, under normal physiological conditions. Previous studies reported that MIF was induced by thrombin via PAR2 transactivated by cleaved PAR1 in endothelial cells (O'Brien et al. 2000; Shimizu et al. 2004). In the present study, immunofluorescence microscopy showed the co-localization of PAR1 and PAR2 and the localization of MIF to sinus endothelial cells. Moreover, immunogold electron microscopy revealed that MIF localized to the Golgi apparatus, its surrounding vesicles on both the apical and basal sides, and in vesicles near the surface of endothelial cells. These results indicate that MIF is produced in endothelial cells and secreted on both the apical and basal sides, and that PAR1 and PAR2 may play a role in this process. Furthermore, MIF secreted by endothelial cells has been reported to promote the recruitment of leukocytes by altering the endothelial expression of E-selectin, ICAM-1, VCAM-1, and chemokines (Cheng et al. 2010). In the splenic cord, through which blood flow is slow, MIF may attract leukocytes towards sinus endothelial cells, creating blood flow through the sinus, thereby facilitating the return of blood cells to the sinus.
Macrophages in splenic cords are one subset of tissue macrophages. Tissue macrophages are distinguished from bone marrow-derived macrophages that are mobilized from blood during inflammation. They are normally present in tissues and play a role in immune responses, tissue repair, and the removal of aged cells as well as a tissue-specific role. Macrophages in the splenic cord are derived from the yolk sac, express the glycoprotein F4/80 on their surface, take in aged erythrocytes by phagocytosis, and are essential for iron recycling. It currently remains unclear why many macrophages remain in splenic cords. MIF may be involved in limiting macrophage migration.
Splenic sinus endothelial cells are barrel-shaped, adhere by focal adhesion to ring fibers, which are hoop-like deformations of the basement membrane, and have well-developed stress fibers at their base. Focal adhesion is the site at which cells adhere to the extracellular matrix via integrin and bind stress fibers regulated by focal adhesion kinase (FAK). The heterodimer of PAR4 and P2Y12 was previously shown to be stimulated in platelets and arrestin was recruited, resulting in the internalization of the heterodimer and activation of integrin via Akt signaling (Smith et al. 2017). Previous studies demonstrated the localization of integrin αvβ5 at the base of rat splenic sinus endothelial cells (Uehara and Uehara 2014). Immunofluorescence microscopy in the present study revealed the colocalization of PAR4 and P2Y12 in endothelial cells, while immunogold electron microscopy and electron microscopy showed the internalization of PAR4 by coated pits formed by clathrin. Therefore, PAR4 and P2Y12 are considered to jointly activate integrin αvβ5 and attach endothelial cells to ring fibers; however, they may not be internalized in the form of a heterodimer. Furthermore, the localization of phosphorylated FAK at Y925 (FAK Y925) to the base of sinus endothelial cells was demonstrated (Uehara and Uehara 2016) and thrombin was shown to induce the phosphorylation of FAK at Y925 via PAR1 and sphingosine 1-phosphate receptor 1 (Shikata et al. 2003). Therefore, in sinus endothelial cells, FAK Y925 is considered to play a role in the formation of well-developed stress fibers. Collectively, these findings and the present results indicate that PAR1 and PAR4 are essential for maintaining the firm adhesion of endothelial cells to ring fibers.
References
Arachiche A, Mumaw MM, de la Fuente M, Nieman MT (2013) Protease-activated receptor 1 (PAR1) and PAR4 heterodimers are required for PAR1-enhanced cleavage of PAR4 by α-thrombin. J Biol Chem 288:32553–32562
Burnier L, Mosnier LO (2013) Novel mechanisms for activated protein C cytoprotective activities involving noncanonical activation of protease-activated receptor 3. Blood 122:807–816
Cheng Q, McKeown SJ, Santos L, Santiago FS, Khachigian LM, Morand EF, Hickey MJ (2010) Macrophage migration inhibitory factor increases leukocyte-endothelial interactions in human endothelial cells via promotion of expression of adhesion molecules. J Immunol 185:1238–1247
Duluc L, Wojciak-Stothard B (2014) Rho GTPases in the regulation of pulmonary vascular barrier function. Cell Tissue Res 355:675–685
Gloushankova NA, Rubtsova SN, Zhitnyak IY (2017) Cadherin-mediated cell-cell interactions in normal and cancer cells. Tissue Barr 5:e1356900
Golomb E, Ma X, Jana SS, Preston YA, Kawamoto S, Shoham NG, Goldin E, Conti MA, Sellers JR, Adelstein RS (2004) Identification and characterization of nonmuscle myosin II-C, a new member of the myosin II family. J Biol Chem 279:2800–2808
Hirano M, Hirano K (2016) Myosin di-phosphorylation and peripheral actin bundle formation as initial events during endothelial barrier disruption. Sci Rep 6:20989
Hong S, Troyanovsky RB, Troyanovsky SM (2013) Binding to F-actin guides cadherin cluster assembly, stability, and movement. J Cell Biol 201:131–143
Kawabata A, Kuroda R, Nakaya Y, Kawai K, Nishikawa H, Kawao N (2001) Factor Xa-evoked relaxation in rat aorta: involvement of PAR-2. Biochem Biophys Res Commun 282:432–435
Li D, D’Angelo L, Chavez M, Woulfe DS (2011) Arrestin-2 differentially regulates PAR4 and ADP receptor signaling in platelets. J Biol Chem 286:3805–3814
McLaughlin JN, Patterson MM, Malik AB (2007) Protease-activated receptor-3 (PAR3) regulates PAR1 signaling by receptor dimerization. Proc Natl Acad Sci U S A 104:5662–5667
Nakanishi-Matsui M, Zheng YW, Sulciner DJ, Weiss EJ, Ludeman MJ, Coughlin SR (2000) PAR3 is a cofactor for PAR4 activation by thrombin. Nature 404:609–613
Nieman MT (2016) Protease-activated receptors in hemostasis. Blood 128:169–177
O’Brien PJ, Prevost N, Molino M, Hollinger MK, Woolkalis MJ, Woulfe DS, Brass LF (2000) Thrombin responses in human endothelial cells. Contributions from receptors other than PAR1 include the transactivation of PAR2 by thrombin-cleaved PAR1. J Biol Chem 275:13502–13509
Rezaie AR (2014) Protease-activated receptor signalling by coagulation proteases in endothelial cells. Thromb Haemost 112:876–882
Saitoh T, Takemura S, Ueda K, Hosoya H, Nagayama M, Haga H, Kawabata K, Yamagishi A, Takahashi M (2001) Differential localization of non-muscle myosin II isoforms and phosphorylated regulatory light chains in human MRC-5 fibroblasts. FEBS Lett 509:365–369
Shikata Y, Birukov KG, Birukova AA, Verin A, Garcia JG (2003) Involvement of site-specific FAK phosphorylation in sphingosine-1 phosphate- and thrombin-induced focal adhesion remodeling: role of Src and GIT. Faseb j 17:2240–2249
Shimizu T, Nishihira J, Watanabe H, Abe R, Honda A, Ishibashi T, Shimizu H (2004) Macrophage migration inhibitory factor is induced by thrombin and factor Xa in endothelial cells. J Biol Chem 279:13729–13737
Smith TH, Li JG, Dores MR, Trejo J (2017) Protease-activated receptor-4 and purinergic receptor P2Y12 dimerize, co-internalize, and activate Akt signaling via endosomal recruitment of β-arrestin. J Biol Chem 292:13867–13878
Steiniger BS (2015) Human spleen microanatomy: why mice do not suffice. Immunology 145:334–346
Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ (2009) Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325:612–616
Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S (2005) Macrophage receptors and immune recognition. Annu Rev Immunol 23:901–944
Uehara K, Miyoshi M (1999) Stress fiber networks in sinus endothelial cells in the rat spleen. Anat Rec 254:22–27
Uehara K, Uehara A (2010) Vimentin intermediate filaments: the central base in sinus endothelial cells of the rat spleen. Anat Rec (hoboken) 293:2034–2043
Uehara K, Uehara A (2014) Integrin alphavbeta5 in endothelial cells of rat splenic sinus: an immunohistochemical and ultrastructural study. Cell Tissue Res 356:183–193
Uehara K, Uehara A (2016) Differentiated localizations of phosphorylated focal adhesion kinase in endothelial cells of rat splenic sinus. Cell Tissue Res 364:611–622
Uehara K, Uehara A (2021) Immunohistochemical study of dissociation and association of adherens junctions in splenic sinus endothelial cells. Cell Tissue Res 384:25–33
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All animals used in the present study were processed according to the animal welfare regulations of Japan. All processes were approved by the Committee of Experimental Animals of Fukuoka University.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Uehara, K., Uehara, A. Immunolocalization of protease-activated receptors in endothelial cells of splenic sinuses. Cell Tissue Res 386, 605–615 (2021). https://doi.org/10.1007/s00441-021-03535-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-021-03535-3