Abstract
Techniques involving fluorescein-5-isothiocyanate-conjugated gelatin injection, immunohistochemistry, and in situ reverse transcription/polymerase chain reaction (RT-PCR) revealed a close relationship between vascular endothelial growth factor (VEGF)-A-expressing cells and microvessels in the hypothalamic-pituitary axis of the rat. In situ RT-PCR clearly indicated the presence of VEGF-A mRNA-expressing cells in the pars tuberalis and in the pars distalis both at embryonic day 15.5 (E15.5) and in later developmental stages. The primary capillaries extended along the developing pars tuberalis, whereas the portal vessels penetrated into the pars distalis at E15.5 and subsequently expanded into the lobe to connect with the secondary capillary plexus, emerging in the pars distalis. At the same time, several VEGF-A-positive cells appeared in the pars distalis. These VEGF-A-positive cells were found to correspond to a portion of adrenocorticotropin (ACTH) cells by dual-staining for in situ RT-PCR and immunohistochemistry, suggesting that some ACTH cells have the potential to produce VEGF-A. Thus, the present study suggests that VEGF-A is involved in the development of the primary capillaries and in the vascularization of the pars distalis, but not in the portal vessels since the formation of portal vessels begins at E13.5, before the appearance of VEGF-A in the rostral region of the pars distalis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The hypothalamic-pituitary axis plays a pivotal role in vertebrate endocrine systems with the hypothalamus regulating pituitary function by integrating signals from the environment and the brain. The pituitary gland is composed of an adenohypophysis (the pars distalis, pars intermedia, and pars tuberalis) and a neurohypophysis. The former is derived from an invagination of the oral ectoderm of the stomodeum, known as Rathke’s pouch, whereas the latter is an infundibulum of the diencephalon. Neurohypophyseal hormones are directly secreted from the hypothalamic neurons, whereas hormones in the pars distalis are regulated by hypothalamic neurohormones that are produced in the hypothalamic area and transported to the pars distalis via portal vessels. The pars distalis is vascularized by hypophysial portal vessels that arise from the capillary beds in the median eminence of the hypothalamus (Murakami et al. 1987), and this hypophyseal portal system provides an important link for carrying hormonal information from the central nervous system to the pituitary. The capillaries of the pituitary gland are characterized by richly fenestrated endothelia. Vascular endothelial growth factor (VEGF-A) is known to have a mitogenic effect on the endothelial cells (Ferrara and Henzel 1989). These cells possess high-affinity receptors for VEGF-A (de Vries et al. 1992; Terman et al. 1992), and the binding of this growth factor to receptors renders the endothelium more permeable (Senger et al. 1983; Collins et al. 1993). Cells expressing VEGF-A have been identified and studied in the adult sheep (Jabbour et al. 1997) and rat (Fan and Iseki 1998) by using in situ hybridization or immunohistochemical techniques. However, these studies have not addressed the ontogenic expression of VEGF during the formation of the hypophyseal vessel system.
In a series of experiments aimed at elucidating the molecular and cellular mechanisms underlying the formation of the vascularization in the hypothalamic-pituitary axis, we initially visualized the direct relationship between the distribution of capillaries and that of hormone-producing cells in the rat fetus by using confocal laser-scanning microscopy in combination with the fluorescein 5-isothiocyanate (FITC)-conjugated gelatin injection method and the isolectin staining method. We subsequently identified VEGF-A-expressing cells during cytogenesis of the hypothalamic-pituitary axis by using a dual-staining method together with in situ reverse transcription/polymerase chain reaction (RT-PCR) and immunohistochemistry. Our results indicate that there is a close correlation between VEGF-A expression and vascular formation in the hypothalamic-pituitary axis.
Materials and methods
Animals
All animal experiments were conducted in compliance with the Guide for Care and Use of Laboratory Animals of Shizuoka University. Normal adult rats of the Wistar strain were housed in a temperature-controlled room (22±2°C) with automatically regulated artificial lighting (0700–1900 h daily). Food and water were supplied ad libitum. Mature rats were mated at night. If spermatozoa were found in vaginal smears taken the next morning, that day was designated as day 0.5 of gestation. Pregnant rats were decapitated on day 13.5, 14.5, 15.5, 16.5, 17.5, and 18.5 of gestation, and the fetuses were prepared for histological examination. Normal male adult rats were decapitated at 8 weeks of age and prepared for histological examination in the same manner as the fetuses.
Injection of the FITC-gelatin conjugate
FITC-gelatin conjugation was carried out according to the method of Hashimoto et al. (1998). A 20 mg aliquot of FITC (Dojin Laboratory, Kumamoto, Japan) was first dissolved in 0.5 ml dimethylsulfoxide (Nacalai tesque, Kyoto, Japan) and then mixed for conjugation in a 20% gelatin (Sigma-Aldrich, St. Louis, Mo.)-water solution (pH 11) at 37°C overnight. The FITC-conjugated gelatin was subsequently dialyzed in phosphate-buffered saline (PBS: 0.01 M sodium phosphate, 0.14 M NaCl, pH 7.4) at 37°C in the dark for 18 days, after which it was stored at 4°C in the dark until use. Following the removal of the uterus from the impregnated rats under ether anesthesia, the FITC-conjugated gelatin, diluted with PBS (1:2), was gently perfused into the umbilical vein, and blood was drained from the umbilical artery. After complete perfusion (1 – 2 ml), the fetuses were fixed in cold 0.5% paraformaldehyde (PFA) solution containing 15% picric acid for approximately 30 min. The fetal heads were then removed and immediately fixed in the same fixative solution at 4°C overnight in the dark.
For the simultaneous visualization of capillary networks and immunohistochemical localization of hormone-producing cells, we used frozen sections. These were obtained from tissues that had been immersed in graded concentrations of sucrose in PBS and embedded in OCT compound (Tissue-Tek, Miles Laboratories, Elkhard, Ind.). The heads were cut into 50-μm-thick sections with a Cryostat (MICROM HM500S-OMV, Walldorf, Germany), and the sections were mounted on silane-coated slides and stored until use.
For the immunohistochemical localiztion of hormone-producing cells, the sections were first dried, then incubated with 1.5% deoxycholic sodium (Sigma-Aldrich) for 4 h in the dark, washed with water, and fixed with 4% PFA in 0.1 M phosphate buffer, pH 7.4, for 30 min. The sections were then washed three times with PBS and blocked with 1% bovine serum albumin (BSA)-PBS overnight. To identify ACTH cells and thyroid-stimulating hormone (TSH) cells, respectively, the sections were incubated with guinea pig anti-rat amidated joining peptide (JP; ST-3; 1:2000; Tanaka and Kurosumi 1992) and rabbit anti-rat TSH (1:2000; Wakabayashi and Tanaka 1988) for 12 h, followed by incubation with Cy3 (indocarbocyanine)-labeled donkey anti-guinea pig IgG (1:400; Jackson, Immunoresearch, West Grove, Pa.) or Cy3-labeled donkey anti-rabbit IgG (1:400; Jackson) overnight. The sections were washed with PBS and mounted in PermaFluor (Immmunon, Pittsburgh, Pa.). Images were obtained with a confocal laser scanning microscope TCS SL (Leica, Wetzlar, Germany) on the laser line at 488 or 543 nm. Optical sections in the Z-axis were obtained at 0.7–2.5 μm increments and projected by maximum pixels.
Staining with isolectin B4
Fetuses were examined from embryonic day 13.5 (E13.5) to E16.5. They were fixed by perfusion via the umbilical vein with a mixture of 4% PFA and 0.1% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4, followed by subsequent fixation with microwaves for 20 s. Following fixation, the fetuses were dehydrated in a graded ethanol series and embedded in Paraplast (Oxford Labware, St. Louis, Mo.). The embedded fetal tissue was then sectioned (4 μm) and mounted on silane-coated slides. Prior to staining, the sections were heated in a retrieval solution (1 mM EDTA in Milli-Q) at 120°C for 3 min in an autoclave (Sanyo, Osaka, Japan), rinsed three times with PBS, and then blocked for non-specific staining by being reacted with 1% BSA-PBS for 1 h. Isolectin B4 staining was performed to investigate the microstructures of endothelial cells according to the method of Gerhardt et al. (2003). The sections were incubated with biotinylated isolectin B4 (Sigma-Aldrich) at a concentration of 20 μg/ml in PBlec (PBS, pH 6.8, 1% Triton X-100, 0.1 mM CaCl2, 0.1 mM MgCl2, 0.1 mM MnCl2) overnight, rinsed three rinses with PBS and incubated for 2 h with Alexa 546-conjugated streptavidin (Molecular Probes, Eugene, Ore.) diluted at 1:200 with 1% BSA-PBS. After they had been rinsed three times with PBS and mounted in PermaFluor (Immunon), the specimens were examined under an Olympus BX-50 microscope equipped with a BX-fluorescence attachment (Olympus Optical, Tokyo, Japan).
In situ RT-PCR protocol
Animals were perfused through the umbilical vein with a mixture of 4% PFA and 0.1% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4, by using the method described in the previous section. Adult rats were perfused through the heart, and their pituitary glands were excised and further fixed by immersion in the same fixative for 2 days. After fixation, the tissues were dehydrated through a graded ethanol series, cleated in methyl benzoate/celloidin and embedded in Paraplast. Sections (4 μm) were cut, mounted on silane-coated slides, and pretreated with 1 U/μl DNase I (Takara, Kyoto, Japan) in 1× DNase buffer containing 2 U/μl RNase inhibitor (Toyobo, Osaka, Japan) at 37°C overnight. Following the DNase treatment, the sections were washed (2×1 min) with RNase-free PBS and Mill-Q. One-step in situ RT-PCR was performed with the rTth enzyme, which combines cDNA synthesis and PCR amplification in a single reaction mixture, in a thermal cycler (PCR Express Thermo Hybaid, ASTEC, Fukuoka, Japan). The RT-PCR kit containing rTth DNA polymerase (Toyobo) was used, and the reaction mixture (50 μl in total per section) was as follows: 10 μl 5× reaction buffer, 6 μl 2.5 mM dNTPs, 1 μl RNase inhibitor (stock: 10 U/μl), 23 μl Milli-Q, 5 μl 25 mM Mn(OAc)2, 1.2 μl each primer (stock: 25 pmol/μl), 0.6 μl 1 mM digoxigenin-11-dUTP (Roche Molecular Biochemicals, Meylan, France), and 2 μl rTth DNA polymerase (stock: 2.5 U/μl).
Rat VEGF-A is organized into eight exons, and alternative splicing of the gene produces four isoforms of the gene product. Consequently, the primers, corresponding to sequence in exons 3 and 4 of the VEGF-A gene, were designed to amplify the domain common to the four isoforms (Conn et al. 1990; Ishii et al. 2002). The sequences of the sense (primer 1) and antisense (primer 2) primers were as follows: VEGF-A primer 1, 5'-GAAGTTCATGGACGTCTACC-3'; VEGF-A primer 2, 5'-CATCTCTCCTATGTGCTGGC-3'. The cDNA was synthesized at 60°C for 30 min. PCR amplification consisted of an initial denaturation step of 94°C for 3 min, followed by 20 reaction cycles of extension (72°C, 60 s) and denaturation (94°C, 30 s), and termination with a final extension reaction at 72°C for 7 min. The specimens were fixed with a mixture of 4% PFA and 0.1% glutaraldehyde in Milli-Q for 10 min at 4°C and washed twice in 0.1× SSC (standard saline citrate) for 20 min at 45°C and once in washing buffer for 5 min. After a blocking step, the sections were incubated with alkaline-phosphatase-conjugated sheep anti-digoxigenin Fab antibody (Roche) for 2 h at 37°C. The label was detected with nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolylphosphate (Roche) by incubating the sections for the relevant length of time. To check the specificity of the staining, we carried out in situ RT-PCR by using a reaction solution lacking rTth polymerase.
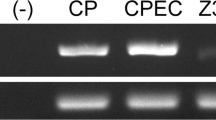
As a control, the plasmid DNA for rat VEGF-A was used. PCR amplification (40 cycles with specific primers) was performed for pGEM-3Z vectors containing a 633-bp fragment (12–644 bp) of rat VEGF-A cDNA (Conn et al. 1990). PCR was also carried out with the cDNA that had been extracted from the tissue sections of Paraplast-embedded or fresh adult rat pituitaries, and the resultant PCR products were electrophoresed through a 2% agarose gel, together with those from the VEGF-A cDNA.
Dual mRNA and protein staining
Following staining of the mRNA, the sections were washed in 10 mM TRIS-HCl containing 1 mM EDTA, fixed with 4% PFA in Milli-Q for 10 min, washed twice with Milli-Q (5 min each) and three times with PBS (5 min each). The sections were then treated for antigen retrieval by heating in a retrieval solution (1 mM EDTA in Milli-Q) at 120°C for 3 min in an autoclave (Sanyo, Osaka, Japan), washed three times with PBS, blocked with 1% BSA-PBS for 1 h, and immunolabeled by the immunofluorescence method. They were then incubated with guinea pig anti-amidated JP (ST-3; 1:2,000; Tanaka and Kurosumi 1992), rabbit anti-rat TSH (1:2,000; Wakabayashi and Tanaka 1988), rabbit anti-rat pituitary glycoprotein hormone α-subunit (αGSU; ST-7; 1:1,000; Tanaka et al. 1997), rabbit anti-rat prolactin (PRL; 1:1,000; supplied by Prof. K. Wakabayashi), guinea pig anti-human growth hormone (GH; 1:2,000; Kato et al. 2004), mouse monoclonal antibody against ovine luteinizing hormone β (LHβ; 1:1000; Uehara et al. 2001), or mouse monoclonal antibody against S-100 protein (Immuno Biological Laboratories, Fujioka, Japan) for 21 h, followed by FITC-labeled donkey anti-guinea pig IgG (1:400; Jackson), FITC- or Cy3-labeled donkey anti-mouse IgG (1:400; Jackson) or Alexa 488-donkey anti-rabbit IgG (1:200; Molecular probes) for 2 h. The sections were washed with PBS, mounted in PermaFluor (Immunon) and observed under the fluorescence microscope.
Results
Formation of the vascular system in the hypothalamic-pituitary axis
We first examined the functional relationship between the vascular system in the hypothalamic-pituitary axis and ACTH or TSH cells by means of confocal laser-scanning microscopy on specimens infused with FITC-labeled gelatin.
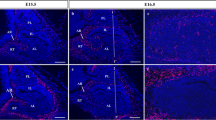
At E13.5, a tongue-like cell cord (Atwell’s process) extended from the antero-ventral part of Rathke’s pouch beneath the median eminence (this eventually gives rise to the pars tuberalis). Portal vessels first appeared in the mesenchymal space of Atwell’s recess, at this stage located dorsal to the developing pars tuberalis (Fig. 1a). The portal vessels did not penetrate into the pars distalis and the pars tuberalis (Fig. 1a–c), but many capillaries had started to penetrate into the pars nervosa (Fig. 1a). At E14.5, no capillaries had penetrated into the pars distalis, but several capillaries had begun to invade the pars tuberalis from the stomatodeal roof (Fig. 1d–f). At E15.5, the portal vessels had begun to penetrate into the pars distalis from its ventral region (Fig. 2a,b), whereas in the pars tuberalis, the number of the capillaries increased from E14.5 to E15.5 (Fig. 2c). At E16.5, the number of primary capillaries had increased still further along the pars tuberalis and Atwell’s recess, and the portal vessels had clearly invaded the pars distalis to connect with the secondary capillary plexus emerging in the pars distalis (Fig. 2d–f). When sections of E14.5 brains, including the pituitary gland, that had been injected with FITC-gelatin were stained with anti-amidated JP or anti-TSH, immunoreactive TSH cells became visible in the pars tuberalis (Fig 3a), but no immunoreactive ACTH and TSH cells were found in the pars ditalis. At E15.5, ACTH cells appeared in the ventro-dorsal region of the pars distalis, but TSH cells had not yet appeared (Fig. 3b). The ACTH cells were located near the site where the capillaries had penetrated. Large polygonal ACTH cells, which were distributed throughout the ventro-dorsal region of the pars distalis, lay on the secondary capillaries (Fig. 3c). However, the pars intermedia lacked blood vessels. A few TSH cells were observed in the rostral region of the pars distalis, but these did not border on the secondary capillaries (Fig. 3d).
Formation of the vascular system detectable by the FITC-conjugated gelatin injection method at E13.5 and E14.5 (AR Atwell’s recess, PN pars nervosa, RP Rathke’s pouch). The portal vessels (arrows) appear initially at E13.5 (a, b), extending from the primary capillaries (arrowheads). The capillaries are not visible in the developing pars tuberalis (PT) at E13.5 (c). The portal vessels (arrowheads) expand into the pituitary primordium at E14.5 but do not penetrate into the pars distalis (PD; d, e). A few capillaries begin to penetrate into the PT (f) by E14.5. a, d Sagittal sections. b, e Horizontal sections. c, f Frontal sections. The lines 1 – 1' and 2 – 2' indicate the direction of sections as shown in b–f. Bar 80 μm
Formation of the vascular system detectable by the FITC-conjugated gelatin injection method at E15.5 and E16.5 (AR Atwell’s recess, PN pars nervosa). The portal vessels (arrows) penetrate into the pars distalis (PD) at E15.5. The primary capillary plexus (arrowheads) develops expansively along the developing pars tuberalis (PT), and the secondary capillaries (double arrowheads) are visible at E15.5 in the PD. The capillaries that penetrate into the PT increase in number (c). Development of the primary capillaries (arrowheads) and the secondary capillaries (double arrowheads) are visible at E16.5 (d, e). a, d Sagittal sections. b, e Horizontal sections. c, f Frontal section. The lines 1 – 1' and 2 – 2' indicate the direction of sections as shown in b–f. Bars 80 μm (a, d), 200 μm (b, e), 40 μm (c, f)
Double-staining of the vascular system and hormone-secreting cells (arrowheads ACTH cells close to the capillaries, PI pars intermedia). Immunopositive TSH cells are seen in the pars tuberalis (PT) at E14.5, but capillaries are not observed in the PT (a). ACTH cells appear in the ventral part of the pars distalis (PD) at E15.5, close to the elongating tips of the capillaries of the portal vessel (b). At E16.5, many portal vessels penetrate into the PD, and most of the ACTH cells are located close to the secondary capillaries (c). Most TSH cells are seen in the PD at E17.5, but not close to the secondary capillaries (d). Bars 40 μm (a), 80 μm (b–d)
In a second series, thin sections of fetal tissue from E12.5 to E16.5 were stained with isolectin B4. At E13.5, portal vessels derived from the subtuberal capillary plexus were observed in Atwell’s recess; higher resolution images of the isolectin B4-stained sections also revealed the presence of endothelial cells having several filopodia at the tips of the vascular sprouts (Fig. 4a). These filopodia extended in the direction of vascular expansion. By E15.5, the portal vessels had penetrated into the pars distalis from Atwell’s recess; filopodia were also observed extending from endothelial cells of the blood capillaries of the pars distalis (Fig. 4b).
Staining of endothelial cells by isolectin B4 (RP Rathke’s pouch). Filopodia (arrowheads) at the tip of the portal vessels are found in Atwell’s recess (AR) at E13.5 (a). After the portal vessels penetrate into the pars distalis (PD), filopodia also extend (arrowhead) from the endothelial cells of the secondary capillaries (b). Bars 100 μm (a), 10 μm (b)
Identification and appearance of VEGF-A-expressing cells
To identify cells positive for VEGF-A mRNA, we carried out a double-staining protocol by using in situ RT-PCR for detecting VEGF-A mRNA and immunofluoresence for detecting the pituitary hormones. Reaction product indicating VEGF-A mRNA expression was clearly observed in the pars tuberalis of adult rats (Fig. 5a). The absence of a positive reaction when the rTth enzyme was omitted from the reaction mixture indicated the validity of the in situ RT-PCR methodology used (Fig. 5b). The authenticity of the in situ RT-PCR for VEGF-A was confirmed by solution-phase PCR, which revealed the expected bands with the same primer pairs when tested on the harvested pituitary sections and plasmid DNA of rat VEGF-A (Fig. 6).
Expression of VEGF-A mRNA in the adult pars tuberalis. VEGF-A mRNA is found in most of the cells of the pars tuberalis (arrows in a). No positive reaction for VEGF-A mRNA is visible when the rTth polymerase is omitted in the reaction (b). VEGF-A mRNA-expressing cells (d) correspond to TSH cells (arrowheads) and folliculo-stellate cells (double arrowheads), which were detected with the anti-TSH (c) and anti-S-100 protein (e) antibodies. Bars 50 μm (a, b), 10 μm (c–e)
When sections of the adult pituitary were immunostained with anti-amidated JP, anti-LHβ, anti-TSH, anti-αGSU, anti-PRL, anti-GH, and anti-S-100 protein antibodies after detection with VEGF-A mRNA, the presence of VEGF-A mRNA was visible in most of the TSH cells and folliculo-stellate cells in the pars tuberalis (Fig. 5c–e) and in a small population of hormone-producing cells, in particular, ACTH cells, TSH cells, and GH cells in the pars distalis (Fig. 7).
VEGF-A mRNA-positive cells first appeared throughout the pars tuberalis at E15.5, and this extent of staining was also observed in this area in later stages (Fig. 8). In the pars distalis at E15.5 and E16.5, VEGF-A mRNA-expressing cells corresponded to a portion of ACTH cells, particularly those located in the ventral region into which the secondary capillaries penetrated (Fig. 9).
Expression of VEGF-A mRNA in the developing pars tuberalis (PT/arrowheads). No positive reaction for VEGF-A mRNA is detectable at E14.5 (a). VEGF-A mRNA is detected at E15.5 (b) and in later stages (c–f) in the pars tuberalis (PD pars distalis, PI pars intermedia, PN pars nervosa). Bars 50 μm (a–d, f), 100 μm (e)
Discussion
The present study demonstrates the close relationship between VEGF-A mRNA expression in the developing pituitary and the formation of the vascular system in the hypothalamic-pituitary axis of the rat. Our first object was to examine the formation of vascularization in the hypothalamic-pituitary axis by using FITC-labeled gelatin. Since this method had the advantage of simultaneously identifying the hormone-secreting cells (Itoh et al. 2000), we used anti-amidated JP and anti-TSH as markers for elucidating the spatial and temporal relationship between appearance of hormone-producing cells and the blood vessel system in the pars distalis. ACTH cells and TSH cells are known to appear at an earlier developmental stage than other pituitary hormones (Watanabe and Daikoku 1979). The FITC-labeled gelatin method revealed that,although the portal vessels had expanded into Atwell’s recess by E13.5, they had not entered the pars tuberalis and the pars distalis; by E14.5, the capillaries from the stomatodeal roof had invaded the pars tuberalis. This finding was consistent with previous reports involving the use of Indian ink (Daikoku et al. 1981; Szabo and Csanyi 1982). Our isolectin B4 staining method provided additional data indicating the appearance of filopodia, from the primary capillaries, at the tip of portal vessels at E13.5. This morphological evidence suggested that the capillaries had spread out in the direction of the pars distalis.
There is some disagreement concerning the precise cellular sites in the pituitary glands at which VEGF-A is secreted. Based on an immunohistochemical technique, Fan and Iseki (1998) have reported that VEGF-A protein is expressed in a portion of the TSH cells located in the rat pituitary gland, whereas Jabbour et al. (1997), using in situ hybridization and immunohistochemistry, have shown that VEGF-A is expressed in the pars tuberalis of the sheep pituitary gland. The primers used in the in situ RT-PCR protocol described here amplify the domain common to the four isoforms of the rat VEGF-A. Therefore, all possible forms of VEGF-A can be detected in this system. We have found that VEGF-A mRNA is expressed in the pars tuberalis and in the pars distalis in adult rats. These results have been confirmed by solution-phase RT-PCR on pituitary sections and with plasmid DNA for rat VEGF-A by using the same primer pairs. Thus, our results support those of Jabbour et al. (1997), although they do not precisely identify VEGF-A-expressing cells.
In adult rats, the pars tuberalis is composed of two main cell types: TSH cells and folliculo-stellate cells (Stoeckel et al. 1973; Gross 1984; Wittkowski et al. 1999). Our in situ RT-PCR method has demonstrated that VEGF-A-expressing cells primarily correspond to TSH cells and folliculo-stellate cells in the pars tuberalis. Conversely, VEGF-A mRNA is detectable in a small proportion of all pituitary hormone-secreting cells in the pars distalis. This finding is in agreement with the results of Ferrara and Henzel (1989) who have demonstrated that folliculo-stellate cells secrete VEGF-A. Many studies have been performed that have resulted in the identification of VEGF-A-expressing cells, but few investigations have aimed at elucidating the exact cellular localization of this growth factor. VEGF-A is known to be expressed in several cell types of human and rat tissues, including those of the brain, lung, heart, liver, kidney, spleen, testis, ovary, uterus, placenta, pancreas, and adrenal gland (Monacci et al. 1993; Shifren et al. 1994). Because VEGF-A is expressed in such a wide range of cell types, VEGF-A not only might affect the induction of active vascular proliferation, but may also be active in the maintenance of the differentiated state of the blood vessels (Alon et al. 1995). Our observation that VEGF-A is expressed in a small population of several hormone-secreting cell types indicates its role in the functional maintenance of blood vessels.
The in situ RT-PCR protocol described here has revealed that VEGF-A mRNA begins to be expressed in the pars distalis from E15.5 onward and continues to be expressed into adulthood. The capillaries start to enter into the pars distalis at E15.5 (these are subsequently referred to as the secondary capillary plexus). We have also been able to demonstrate that, at E15.5, the portal vessels in Atwell’s recess have entered the pars distalis, precisely at the site at which the ACTH cells are located. We have subsequently determined that these ACTH cells express VEGF-A mRNA. Our findings suggest that the induction and growth of the secondary capillary plexus is dependent on VEGF-A secreted from ACTH cells. However, the branching of the portal vessels from the primary capillaries occurs in the mesenchymal space of Atwell’s recess at E13.5. Surprisingly, no signs of VEGF-A expression are visible in Atwell’s recess at this stage. The absence of any detectable VEGF-A here may be attributable to its being expressed below the detection limit of our technique. A tissue-specific angiogenic factor (endocrine-gland-derived VEGF) has recently been identified in steroidogenic glands, i.e., adrenal cortex, testis, ovary, and placenta (LeCouter et al. 2001). This factor does not belong to the VEGF-A family but instead is a member of a structurally related class of peptides that include the secreted proteins from Bombina variegata, designated Bv8. Consequently, another possible explanation may be that unknown angiogenic factors are involved in the initial formation of the portal vessels.
We have also demonstrated that VEGF-A mRNA is intensely expressed in the developing pars tuberalis. The presumptive pars tuberalis extends rostrally as development proceeds, and the adult pars tuberalis develops into two to three cell layers that are located beneath the median eminence. We have observed that, in the upper parts that border the median eminence, a number of the primary capillaries develop along the forming pars tuberalis. This observation suggests that VEGF-A mRNA expression in the developing pars tuberalis is involved in the formation of the primary capillary plexus by delivering VEGF-A protein to the primary capillaries plexus that has expanded into the upper parts of the pars tuberalis. This manner of secretion may well be possible because VEGF-A protein has a signal sequence peptide.
VEGF-A acts primarily as an endothelial cell mitogen via the specific receptors Flk-1 (fetal liver kinase-1) and Flt-1 (fms-like tyrosine kinase). In adult rats, Flk-1 is expressed in the endothelial cells of many capillaries and in all types of pituitary hormone-secreting cells in the pars distalis (Vidal et al. 2002). We propose that VEGF-A acts via the Flk-1 receptor expressed on the endothelial cells of the capillaries in the developing pituitary. However, whether the VEGF-A receptor is expressed on the endothelial cells of the capillaries of the portal vessels remains an interesting problem, because no VEGF-A signal has been detected at any of the sites near the growing portal vessels.
In summary, our results demonstrate that, whereas VEGF-A expression is involved in the induction and maintenance of the primary and secondary capillary plexus in the hypothalamic-pituitary axis, it is not involved in the induction of the portal vessels.
References
Alon T, Hemo I, Itin A, Pe’er J, Stone J, Keshet E (1995) Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med 1:1024–1028
Collins PD, Connolly DT, Williams TJ (1993) Characterization of the increase in vascular permeability induced by vascular permeability factor in vivo. Br J Pharmacol 109:195–199
Conn G, Bayne ML, Soderman DD, Kwok PW, Sullivan KA, Palisi TM, Hope DA, Thomas KA (1990) Amino acid and cDNA sequences of a vascular endothelial cell mitogen that is homologous to platelet-derived growth factor. Proc Natl Acad Sci USA 87:2628–2632
Daikoku S, Kawano H, Abe K, Yoshinaga K (1981) Topographical appearance of adenohypophysial cells with special reference to the development of the portal system. Arch Histol Jpn 44:103–116
Fan L, Iseki S (1998) Immunohistochemical localization of vascular endothelial growth factor in the endocrine glands of the rat. Arch Histol Cytol 61:17–28
Ferrara N, Henzel WJ (1989) Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun 161:851–858
Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C (2003) VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161:1163–1177
Gross DS (1984) The mammalian hypophyseal pars tuberalis: a comparative immunocytochemical study. Gen Comp Endocrinol 56:283–298
Hashimoto H, Ishikawa H, Kusakabe M (1998) Simultaneous observation of capillary nets and tenascin in intestinal villi. Anat Rec 250:488–492
Ishii H, Oota I, Arakawa T, Takuma T (2002) Differential gene expression of vascular endothelial growth factor isoforms and their receptors in the development of the rat masseter muscle. Arch Oral Biol 47:505–510
Itoh J, Kawai K, Serizawa A, Yasumura K, Ogawa K, Osamura R (2000) A new approach to three-dimensional reconstructed imaging of hormone-secreting cells and their microvessel environments in rat pituitary glands by confocal laser scanning microscopy. J Histochem Cytochem 48:569–578
Kato H, Kuwako K, Suzuki M, Tanaka S (2004) Gene expression patterns of pro-opiomelanocortin-processing enzymes PC1 and PC2 during postnatal development of rat corticotrophs. J Histochem Cytochem 52:943–957
Jabbour HN, Boddy SC, Lincoln GA (1997) Pattern and localisation of expression of vascular endothelial growth factor and its receptor flt-1 in the ovine pituitary gland: expression is independent of hypothalamic control. Mol Cell Endocrinol 134:91–100
LeCouter J, Kowalski J, Foster J, Hass P, Zhang Z, Dillard-Telm L, Frantz G, Rangell L, DeGuzman L, Keller GA, Peale F, Gurney A, Hillan KJ, Ferrara N (2001) Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature 412:877–884
Monacci WT, Merrill MJ, Oldfield EH (1993) Expression of vascular permeability factor/vascular endothelial growth factor in normal rat tissues. Am J Physiol 264:C995–C1002
Murakami T, Kikuta A, Taguchi T, Ohtsuka A, Ohtani O (1987) Blood vascular architecture of the rat cerebral hypophysis and hypothalamus. A dissection/scanning electron microscopy of vascular casts. Arch Histol Jap 50:133–176
Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF (1983) Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219:983–985
Shifren JL, Doldi N, Ferrara N, Mesiano S, Jaffe RB (1994) In the human fetus, vascular endothelial growth factor is expressed in epithelial cells and myocytes, but not vascular endothelium: implications for mode of action. J Clin Endocrinol Metab 79:316–322
Stoeckel ME, Porte A, Hindelang-Gertner C, Dellmann HD (1973) A light and electron microscopic study of the pre- and postnatal development and secretory differentiation of the pars tuberalis of the rat hypophysis. Z Zellforsch Mikrosk Anat 142:347–365
Szabo K, Csanyi K (1982) The vascular architecture of the developing pituitary-median eminence complex in the rat. Cell Tissue Res 224:563–577
Tanaka S, Kurosumi K (1992) A certain step of proteolytic processing of proopiomelanocortin occurs during the transition between two distinct stages of secretory granules maturation in rat anterior pituitary corticotrophs. Endocrinology 131:779–786
Tanaka S, Kurabuchi S, Mochida H, Hayashi H, Wakabayashi K (1997) Production and characterization of specific anti-peptide antiserum against α-subunit of rat pituitary glycoprotein hormones. J Histochem Cytochem 45:985–990
Terman BI, Dougher-Vermazen M, Carrion ME, Dimitrov D, Armellino DC, Gospodarowicz D, Bohlen P (1992) Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun 187:1579–1586
Uehara M, Yaoi Y, Suzuki M, Takata K, Tanaka S (2001) Differential localization of prohormone convertase (PC1 and PC2) in two distinct types of secretory granules in rat pituitary gonadotrophs. Cell Tissue Res 304:43–49
Vidal S, Lloyd RV, Moya L, Scheithauer BW, Kovacs K (2002) Expression and distribution of vascular endothelial growth factor receptor Flk-1 in the rat pituitary. J Histochem Cytochem 50:533–540
Vries C de, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT (1992) The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science 255:989–991
Wakabayashi K, Tanaka S (1988) Assessment of specificity of antiserum for immunohistochemistry. Acta Histochem Cytochem 21:221–229
Watanabe YG, Daikoku S (1979) An immunohistochemical study on the cytogenesis of adenohypophysial cells in fetal rats. Dev Biol 68:557–567
Wittkowski W, Bockmann J, Kreutz MR, Bockers TM (1999) Cell and molecular biology of the pars tuberalis of the pituitary. Int Rev Cytol 185:157–194
Author information
Authors and Affiliations
Corresponding author
Additional information
This investigation was supported in part by a grant-in-aid for scientific research from the Ministry of Education, Science, Sports, and Culture of Japan to S.T.
Rights and permissions
About this article
Cite this article
Nakakura, T., Yoshida, M., Dohra, H. et al. Gene expression of vascular endothelial growth factor-A in the pituitary during formation of the vascular system in the hypothalamic-pituitary axis of the rat. Cell Tissue Res 324, 87–95 (2006). https://doi.org/10.1007/s00441-005-0115-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-005-0115-y