Abstract
In this review we consider the multiple functions of developmentally regulated brain protein (drebrin), an actin-binding protein, in the formation of cellular polarity in different cell types. Drebrin has a well-established role in the morphogenesis, patterning and maintenance of dendritic spines in neurons. We have recently shown that drebrin also stabilizes Connexin-43 containing gap junctions at the plasma membrane. The latest literature and our own data suggest that drebrin may be broadly involved in shaping cell processes and in the formation of stabilized plasma membrane domains, an effect that is likely to be of crucial significance for formation of cell polarity in both neuronal and non-neuronal types.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Drebrin: identification, domain structure, and functions in neurons
Developmentally regulated brain protein (drebrin) was first cloned more than a decade ago (Shirao et al. 1988) and found to interact with actin (Shirao 1995). The expression level of drebrin is specifically high in the cerebral cortex, hippocampus, amygdala, thalamus, and striatum (Hayashi et al. 1996; Shirao and Obata 1986). Drebrins are classified into two forms in mammals and three forms in chicken, which arise by alternative splicing from a single gene. On SDS-PAGE gels, the adult-expressed mammalian drebrin A is a protein of apparent molecular weight of ∼125 kDa and the embryonal form drebrin E is ∼115 kDa). Drebrin A mRNA differs from drebrin E mRNA only by the presence of an internal 138-nucleotide sequence insert which is absent from drebrin E mRNA (Jin et al. 2002; see Fig. 1). The expression pattern of each isoform is regulated spatially and temporally in the developing brain. Drebrins contain two main binding domains: an N-terminal ADF homology domain to interact with actin (Lappalainen et al. 1998) and a proline-rich region and C-terminal Homer-binding domain. A schematic of the putative domain structure of drebrins, based on sequence analysis is shown in Fig. 1a. A central 85-amino acid sequence (residues 233–317) is necessary and sufficient for binding to and remodelling of F-actin (Hayashi et al. 1999; see Fig. 1a). Drebrin binding to actin filaments inhibits the binding of tropomyosin and alpha-actinin in vitro and in vivo, resulting in the appearance of thick, curving bundles of actin filaments (Ishikawa et al. 1994; Shirao et al. 1994; Shirao 1995). Clearly, based on the domain structure, there is a potentially large number of interactors for drebrin encoded in the human genome, some of which are now starting to be identified.
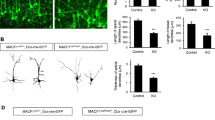
Domain structure of drebrin and presence of drebrin in gap junction plaques. a Drebrin E (embryonal) and A (adult) refer to the different splice isoforms of drebrin; left, proposed comparative models for the function of Cx43/drebrin/actin and E-cadherin/catenin/actin complexes at the interface between the submembrane cytoskeleton and transmembrane proteins. The E-cadherin–catenin–actin signalling system has been described by Jamora and Fuchs (2002); panel reproduced from Butkevich et al. (2004) with permission. b, c Colocalization of drebrin (b) and Cx43 (c) at contacting zones of the cell–cell interface. d–f Both drebrin and Cx43 accumulate in mature outgrowths of transfected COS-7 cells (see arrowhead). Note the absence of both proteins from “less established” outgrowths (arrow). g–h Low level expression of drebrin is not able to shape plasma membrane, i–j increased expression level of drebrin leads to the initiation of lamellipodia-like structures that also contain Cx43
Drebrin was shown to specifically accumulate in dendritic spines where it regulates their shapes and densities, via the rearrangement of cytoskeletal actin filaments (Shirao et al. 1994; Hayashi et al. 1996; Hayashi and Shirao 1999). It has been recently demonstrated that drebrin A (i.e. the neuron-specific, adult isoform) clusters at synapses and governs targeting of the post-synaptic density 95 protein to synapses during normal neuronal development (Takahashi et al. 2006) or upon drebrin overexpression (Mizui et al. 2005). Application of drebrin antisense oligonucleotides strongly decreased the density and width of filopodia-spines in cultured hippocampal neurons (Takahashi et al. 2006). The ability of drebrin to quickly re-shape cellular membranes explains why drebrin is involved in the activity-dependent change of the morphology and density of dendritic spines (Keller 2002; Marrs et al. 2001; Okabe et al. 2001; Shirao and Sekino 2001; Mizui et al. 2005).
Well in accord with this, antisense oligonucleotide mediated knock-down of drebrin A in whole rat brain resulted in defects in memory formation, sensorimotor gating and cognitive function (Kobayashi et al. 2004). These data also correlate well with the marked decrease of drebrin A that has been found in the brains of Alzheimer’s patients and in patients with Down’s syndrome (Harigaya et al. 1996; Hatanpaa et al. 1999; Shim and Lubec 2002). This observed strong decrease in drebrin levels in the brains of patients with Alzheimer disease and Down syndrome implies a vital requirement for drebrin in the higher brain functions such as: memory, learning and cognition. In conclusion of this part, drebrins are well-established major players in the establishment and maintenance of dendritic spines in neurons.
A novel role for drebrin in the stabilization of gap junctions
In a proteomics search for Connexin-43 [gap junction protein (Cx43)] interactors in brain extracts we have recently identified drebrin as an interactor of the Cx43 cytoplasmic tail (Butkevich et al. 2004). Gap junctions are transmembrane channels between contacting cells that mediate intercellular communication and signalling by permitting the passage of ions, metabolites, and second messengers (Kumar and Gilula 1996; Goldberg et al. 1999; Bukauskas et al. 2000). Drebrin and Cx43 colocalize in contacting zones of the plasma membrane at gap junction plaques (Fig. 1b–f). Depletion of drebrin in cells with siRNA results in impaired cell–cell coupling, internalisation of gap junctions, and targeting of Cx43 to a degradative pathway, whereas drebrin overexpression resulted in stabilization of Cx43-containing gap junctions (Butkevich et al. 2004). We concluded that drebrin is required to maintain Cx43-containing gap junctions in their functional state at the plasma membrane (Butkevich et al. 2004), a novel function for drebrin that has been rapidly adopted by the cell biology community (see Stout et al. 2004).
Interestingly, in contrast to actin, treatment with latrunculin B, a drug that inhibits actin polymerization, did not effect submembrane localization of drebrin, suggesting that other anchoring interactions of drebrin may link this protein to the plasma membrane. If drebrin indeed functions to stabilize transmembrane molecules in microdomains of defined sites at the plasma membrane, microtubule-dependent transport of newly synthesized molecules may be preferentially directed to such sites (especially if microtubule plus-ends get stabilized there), and thus promote their further growth. In accordance with this model, drebrin/Cx43-containing gap junctions turned out to be more stable in “mature” (upper) cell–cell contacts of transfected COS cells (Fig. 1d–f), but both proteins were lacking from “less stable” (lower) contacts.
Drebrin may utilize both the N-terminal ADF and the prolin-rich binding domains to interact simultaneously with cytosolic tails of transmembrane proteins like Cx43 and actin (Fig. 1, upper right; see Butkevich et al. 2004). This puts the Cx43/drebrin complex at the interface between extracellular signals and possible cellular responses that are mediated via the rearrangement of the actin-containing submembrane cytoskeleton. A similar function has been proposed for an E-cadherin/β-catenin/actin complex that acts in signal transduction (Jamora and Fuchs 2002).
Among many different functions of drebrin, submembrane stabilization of plasma membrane-localized proteins (e.g. Cx43 in gap junctions) is likely to have very significant consequences for the cell. If drebrin can stabilize transmembrane channels, e.g. gap junctions, and likely NMDA receptors in neurons (Takahashi et al. 2006), by preventing their internalization/degradation that will generate polarity in selected regions of the plasma membrane. On the other hand, a decreased drebrin expression achieved using siRNA interference triggers removal of Cx43-containing gap junctions from the cell surface and their subsequent degradation, and thus leads to disappearance of cell polarity spots. At least in respect to the Cx43/drebrin interaction we could demonstrate that clearly (Butkevich et al. 2004).
Morphogenic effects of drebrin overexpression in non-neuronal and neuronal cells
Expression of Drebrin E2 has been detected in a wide range of non-neuronal cell types, where it localizes mostly in and near actin-rich lamellipodia and filopodia (see e.g. Peitsch et al. 2001, 2005, 2006). A triggering role of non-neuronal drebrin in the re-shaping of cell morphology has been observed by several groups (Inoue and Shirao 1997; Keon et al. 2000; Peitsch et al. 2006). For example, in non-neuronal cells drebrin may be involved in induction of structures known as podosomes which are e.g. found in primary endothelial cell lines, fibroblast-like cell cultures and HUVEC cells (Linder and Aepfelbacher 2003; Buccione et al. 2004; Spinardi et al. 2004) and in cultured astrocytes, as we have observed (see Fig. 2, upper panel). The presence of drebrin in lamellipodia and filopodia has been described by Peitsch et al. (2001, 2006).
Drebrin is present in podosomal structures. a–c Cultured rat astrocytes were fixed and stained with a monoclonal antibody against drebrin and rabbit anti-Cx43 antibodies. Laser scan images were obtained. The relative positions along the z-axis of the optical sections are indicated in the upper left corners. Cx43 staining is present only at the lower level of cell–cell contacts but not in the higher level optical sections of the cell. d–f Formation of drebrin/Cx43 complexes may be initiated already upon exit of Cx43 from the Golgi from where both proteins move to the plasma membrane (astrocytes are co-stained with antibodies against drebrin and Cx43 as depicted)
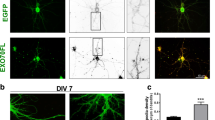
Overexpression of drebrin in different cell lines results in dramatic changes in cell morphology (Peitsch et al. 2001, 2006; see Figs. 3, 4). Raising drebrin levels in non-neuronal cells upto 10–50-fold above its native level (as determined by immunoblotting) lead to the formation of neuronal-like protrusions in Vero cells (Figs. 3, 4), membrane ruffling and recapturing of other proteins in the newly formed protrusions, and/or formation of lamellipodia (Peitsch et al. 2006). Some processes produced in Vero fibroblasts are strongly reminiscent of the initial stages of neuronal differentiation, resembling stages 1, 2 (branching) or stage 3. One of the protrusions may undergo a sharp transition and rapid growth to form a long process that resembles formation of axons (Fig. 3, upper panel—compare behaviour of three transfected cells in a time course of expression; 3D reconstrution of the drebrin-mediated effect). To analyze this in more detail, we transformed Vero cells with a plasmid expressing drebrin-GFP and performed long-term imaging over 14 h of the cell shape changes (see Fig. 4 and accompanying movie). The cell shape changes correlated with the degree of drebrin overexpression (judged by drebrin-GFP signal intensities). A cell with very low expression of drebrin-GFP did not change shape within 14 h, whereas the higher expressing cells in the center and lower left corner show a dramatic response early on. The dynamics of drebrin-induced cell processes was vivid in the first several hours and then slowed down. Longer, neurite-like processes started to form only later (∼8 h after transfection), when drebrin-GFP levels had even further increased. During the entire period of observation, cells were kept in a controlled environment (5% CO2; 37°C; 80% relative humidity) and under these conditions showed no evidence for cell death even at the 14 h time point. Clearly, drebrin can dramatically drive formation of neurite-like cell processes in Vero cells (Fig. 4; movie 1).
Drebrin overexpression drives extension of neurite-like processes in Vero fibroblasts. Upper panel, single cell analyses of drebrin overexpression over a time course revealed the formation of multiple extensions and protrusions from the cell body. One, or several, of the protrusions may undergo a sharp transition to rapid growth forming a long process, resembling an axon in neurons (reconstruction in 3D, right panels). Remodelling of the cell shape by drebrin strongly is strongly reminiscent of early stages of neuronal differentiation (depicted in red). Middle panel, drebrin is highly expressed in dendritic spines. Fixed primary cultured hippocampal neurons were co-stained with anti-drebrin (green) and MAP2 antibody (blue)
Long-term live cell imaging of cell shape changes induced by overexpression of drebrin-GFP. Vero cells transfected with a plasmid expressing drebrin-GFP were imaged with an EM-CCD based sensitive spinning disk confocal system with complete environmental control on the microscope stage (37°C, 5% CO2 and 80% relative humidity), starting 4 h after transfection and for a period of 14 h. Images were taken every 5 min. The frames shown (and the accompanying movie as supplemental material: movie1) demonstrate dramatic shape changes induced by drebrin expression, with the time points indicated. The four frames to the right (shown in inverted contrast, in b/w) show the shape change from a fibroblast-like cell shape to a rounded cell with neurite-like processes at a late stage of the movie. At the 14 h time point there was no evidence for loss of cell viability
What relevance do these findings have for neuronal cells? The development of a neuron is characterized by the transition from a non-polarized state to a state of extreme cell polarity. Long before the differentiation of cell processes into axons and dendrites takes place the cells already undergo a major, critical change in shape (stage 1) to acquire a branched form (stage 2) (Craig and Banker 1994; Bradke and Dotti 2000), followed by further processing to allow the appearance of two structurally and molecularly distinct parts—the axon and dendrites (Dotti and Banker 1987; Dotti et al. 1988; Bass et al. 1989; Goslin and Banker 1989; see Fig. 3, middle panel). Synapses and highly branched dendritic extensions finally decorate maturing neurons, allowing the precise signaling networks of neuronal connectivity to develop. Molecular ensembles regulating these processes have started to be identified. For the specification of an axon, the PAR complex has been shown to be involved and to accumulate at the tip of the axon (Shi et al. 2003; Nishimura et al. 2004; Macara 2004). Unexpectedly, the origin recognition complex (ORC) identified and described recently by Huang et al. (2005) appears to be also responsible for the development of dendrites and spines. It remains to be determined whether ORC subunits are engaged directly in association with the actin cytoskeleton, controlling dendrite and spine growth, or whether the ORC effect is of a more general origin inducing the downstream changes in gene expression that influence cellular metabolism. Clearly, molecular pathways that control the early transition are so far less well defined than the later stages of polarization that specify axons and dendrites, respectively. Given the well known role for drebrin and changes in actin dynamics in spine morphogenesis and dendrite patterning, and in the cell shape changes discussed here, we confidently speculate that drebrin is one of the direct players responsible for the dramatic early transition towards polarity in neurons.
Already in non-neuronal cells drebrin was shown to act as a polarity-inducing factor that leads to a specific microfilament anchoring at junctional plaques in epithelial cells (Peitsch et al. 1999, 2005). In Vero cells a limited amount of drebrin is present at the plasma membrane but upon establishment of cell–cell contacts the endogenous drebrin redistributes from the Golgi pool to the plasma membrane (Fig. 2). Drebrin has been suggested to move from the Golgi to the plasma membrane in already pre-assembled form of drebrin particles or “drebrosomes” that are transported along cytoskeletal elements (Peitsch et al. 1999, 2001). Alternatively, we consider it possible that drebrin may move from the ER/Golgi to the plasma membrane by being associated with transmembrane proteins, e.g. connexins or transmembrane receptors contained within transport vesicles (see Fig. 2). At the plasma membrane specific regions harboring transmembrane molecules may be stabilized by drebrin and thus become sites for future outgrowths, based on directing microtubule-based transport. Other proteins, e.g. the kinesin KIF3 in axons, appear to do that as well (Takeda et al. 2000; Nishimura et al. 2004).
Here, analysis of cell biological data available on drebrins allowed us to sketch out an important role of drebrin at the initial steps of establishment of cellular polarity, including in neurons and thus by implication in the formation, maintenance and functioning of neuronal networks. Obviously, many data still need to be added to this picture to clarify the relationship of drebrin to other proteins involved in formation of plasma membrane domains and polarity complexes such as ORC and Par-3/Par-6/aPKC operating in conjunction with the small GTPases Cdc42, Rac and/or Rap1 (Makara 2004; Schwamborn and Puschel 2004; Nishimura et al. 2004; Wiggin et al. 2005). It will be fascinating to see the detailed, varied functions of drebrins being unravelled further in the next years to come.
References
Bass PW, Black MM, Banker GA (1989) Changes in microtubule plarity orientation during the development of hippocampal neurons in culture. J Cell Biol 109:3085–3094
Bradke F, Dotti CG (2000) Establishment of neuronal polarity: lessons from cultured hippocampal neurons. Curr Opin Neurobiol 10:574–581
Buccione R, Orth JD, McNiven MA (2004) Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol 5:647–657
Bukauskas FF, Jordan K, Bukauskiene A, Bennett MV, Lampe PD, Laird DW, Verselis VK (2000) Clustering of connexin 43-enhanced green fluorescent protein gap junction channels and functional coupling in living cells. Proc Natl Acad Sci USA 97:2556–2561
Butkevich E, Hulsmann S, Wenzel D, Shirao T, Duden R, Majoul I (2004) Drebrin stabilizes connexin-43 and links gap junctions to the sub-membrane cytoskeleton. Curr Biol 14:650–658
Craig AM, Banker G (1994) Neuronal polarity. Annu Rev Neurosci 17:267–310
Dotti CG, Banker G (1987). Experimentally induced alteration in the polarity of developing neurons. Nature 330:254–256
Dotti CG, Sullivan CA, Banker GA (1988) The establishment of polarity by hippocampal neurons in culture. J Neurosci 8:1454–1468
Goldberg GS, Lampe PD, Nicholson BJ (1999) Selective transfer of endogenous metabolites through gap junctions composed of different connexins. Nat Cell Biol 1:457–459
Goslin K, Banker G (1989) Experimental observations on the development of polarity by hippocampal neurons in culture. J Cell Biol 108:1507–1516
Harigaya Y, Shoji M, Shirao T, Hirai S (1996) Disappearance of actin-binding protein, drebrin, from hippocampal synapses in Alzheimer’s disease. J Neurosci Res 43:87–92
Hatanpaa K, Isaacs KR, Shirao T, Brady DR, Rapoport SI (1999) Loss of proteins regulating synaptic plasticity in normal aging of the human brain and in Alzheimer disease. J Neuropathol Exp Neurol 58:637–643
Hayashi K, Shirao T (1999) Change in the shape of dendritic spines caused by overexpression of drebrin in cultured cortical neurons. J Neurosci 19:3918–3925
Hayashi K, Ishikawa R, Ye LH, Takata K, Kohama K, Shirao T (1996) Modulatory role of drebrin in the cytoskeleton within dendritic spines in the rat cerebral cortex. J Neurosci 16:7161–7170
Hayashi K, Ishikawa R, Kawai-Hirai R, Takagi T, Taketomi A, Shirao T (1999) Domain analysis of the actin-binding and actin-remodeling activities of drebrin. Exp Cell Res 253:673–680
Huang Z, Zang K, Reichardt LF (2005) The origin recognition core complex regulates dendrite and spine development in postmitotic neurons. J Cell Biol 170:527–535
Inoue HK, Shirao T (1997) Neurite formation induced in neuroblastoma cells and genetically altered non-neuronal cells. J Electron Microsc (Tokyo) 46:497–502
Ishikawa R, Hayashi K, Shirao T, Xue Y, Takagi T, Sasaki Y, Kohama K (1994) Drebrin, a development-associated brain protein from rat embryo, causes the dissociation of tropomyosin from actin filaments. J Biol Chem 269:29928–29933
Jamora C, Fuchs E (2002) Intercellular adhesion, signalling and the cytoskeleton. Nat Cell Biol 4:E101–E108
Jin M, Tanaka S, Sekino Y, Ren Y, Yamazaki H, Kawai-Hirai R, Kojima N, Shirao T (2002) A novel, brain-specific mouse drebrin: cDNA cloning, chromosomal mapping, genomic structure, expression, and functional characterization. Genomics 79:686–692
Keller A (2002) Use-dependent inhibition of dendritic spines. Trends Neurosci 25:541–544
Keon BH, Jedrzejewski PT, Paul DL, Goodenough DA (2000) Isoform specific expression of the neuronal F-actin binding protein, drebrin, in specialized cells of stomach and kidney epithelia. J Cell Sci 113:325–336
Kobayashi RY Sekino, Shirao T, Tanaka S, Ogura T, Inada K, Saji M (2004) Antisense knockdown of drebrin A, a dendritic spine protein, causes stronger preference, impaired pre-pulse inhibition, and an increased sensitivity to psychostimulant. Neurosci Res 49:205–217
Kumar NM, Gilula NB (1996) The gap junction communication channel. Cell 84:381–388
Lappalainen P, Kessels MM, Cope MJ, Drubin DG (1998) The ADF homology (ADF-H) domain: a highly exploited actin-binding module. Mol Biol Cell 9:1951–1959
Linder S., Aepfelbacher M (2003) Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol 13:376–385
Macara IG (2004) Par proteins: partners in polarization. Curr Biol 14:R160–R162
Marrs GS, Green SH, Dailey ME (2001) Rapid formation and remodeling of postsynaptic dendrites in developing dendrites. Nat Neurosci 4:1006–1013
Mizui T, Takahashi H, Sekino Y, Shirao T (2005) Overexpression of drebrin A in immature neurons induces the accumulation of F-actin and PSD-95 into dendritic filopodia, and the formation of large abnormal protrusions. Mol Cell Neurosci 30:149–157
Nishimura T, Kato K, Yamaguchi T, Fukata Y, Ohno S, Kaibuchi K (2004) Role of the PAR-3–KIF3 complex in the establishment of neuronal polarity. Nat Cell Biol 6:328–334
Okabe S, Miwa A, Okado H (2001) Spine formation and correlated assembly of presynaptic and postsynaptic molecules. J Neurosci 21:6105–6114
Peitsch WK, Grund C, Kuhn C, Schnolzer M, Spring H, Schmelz M, Franke WW (1999) Drebrin is a widespread actin-associating protein enriched at junctional plaques, defining a specific microfilament anchorage system in polar epithelial cells. Eur J Cell Biol 78:767–778
Peitsch WK, Hofmann I, Pratzel S, Grund C, Kuhn C, Moll I, Langbein L, Franke WW (2001) Drebrin particles: components in the ensemble of proteins regulating actin dynamics of lamellipodia and filopodia. Eur J Cell Biol 80:567–579
Peitsch WK, Hofmann I, Bulkescher J, Hergt M, Spring H, Bleyl U, Goerdt S, Franke WW (2005) Drebrin, an actin-binding, cell-type characteristic protein: induction and localization in epithelial skin tumors and cultured keratinocytes. J Invest Dermatol 125:761–774
Peitsch WK, Bulkescher J, Spring H, Hofmann I, Goerdt S, Franke WW (2006) Dynamics of the actin-binding protein drebrin in motile cells and definition of a juxtanuclear drebrin-enriched zone. Exp Cell Res 312:2605–2618
Schwamborn JC, Puschel AW (2004) The sequential activity of the GTPases Rap1B and Cdc42 determines neuronal polarity. Nat Neurosci 7:923–929
Shi SH, Jan LY, Jan YN (2003) Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell 112:63–75
Shim KS, Lubec G (2002) Drebrin, a dendritic spine protein, is manifold decreased in brains of patients with Alzheimer’s disease and Down syndrome. Neurosci Lett 324:209–212
Shirao T (1995) The roles of microfilament-associated proteins, drebrins, in brain morphogenesis: a review. J Biochem (Tokyo) 117:231–236
Shirao T, Obata K (1986) Immunochemical homology of 3 developmentally regulated brain proteins and their developmental change in neuronal distribution. Brain Res 394:233–244
Shirao T, Sekino Y (2001) Clustering and anchoring mechanisms of molecular constituents of postsynaptic scaffolds in dendritic spines. Neurosci Res 40:1–7
Shirao T, Kojima N, Kato Y, Obata K (1988) Molecular cloning of a cDNA for the developmentally regulated brain protein, drebrin. Brain Res 464:71–74
Shirao T, Hayashi K, Ishikawa R, Isa K, Asada H, Ikeda K, Uyemura K (1994) Formation of thick curving bundles of actin by drebrin A expressed in fibroblasts. Exp Cell Res 215:145–153
Spinardi L, Rietdorf J, Nitsch L, Bono M, Tacchetti C, Way M, Marchisio PC (2004) A dynamic podosome-like structure of epithelial cells. Exp Cell Res 295:360–374
Stout C, Goodenough DA, Paul DL (2004) Connexins: functions without junctions. Curr Opin Cell Biol 16:507–512
Takahashi H, Mizui T, Shirao T (2006) Down-regulation of drebrin A expression suppresses synaptic targeting of NMDA receptors in developing hippocampal neurons. J Neurochem 97(Suppl 1):110–115
Takeda S, Yamazaki H, Seog DH, Kanai Y, Terada S, Hirokawa N (2000) Kinesin superfamily protein 3 (KIF3) motor transports fodrin-associating vesicles important for neurite building. J Cell Biol 148:1255–1265
Wiggin GR, Fawcett JP, Pawson T (2005) Polarity proteins in axon specification and synaptogenesis. Dev Cell 8:803–816
Acknowledgments
The authors are grateful to Drs. Eugenia Butkevich and Piotr Bregestovski for critical reading of early versions of this manuscript. We thank Dr. Robin Battye (Quorum Technologies; http://www.quorumtechnologies.com/) for his invaluable help with long-time imaging of cells expressing drebrin, at the “3D Microscopy of Living Cells” course held in 2006 at the University of British Columbia, Vancouver, Canada (http://www.3dcourse.ubc.ca/index.htm). This work was supported by the Wellcome Trust (Senior Fellowship to R.D.; grant number 047578).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Movie 1 (AVI 8.9 mb)
Rights and permissions
About this article
Cite this article
Majoul, I., Shirao, T., Sekino, Y. et al. Many faces of drebrin: from building dendritic spines and stabilizing gap junctions to shaping neurite-like cell processes . Histochem Cell Biol 127, 355–361 (2007). https://doi.org/10.1007/s00418-007-0273-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-007-0273-y