Abstract
Dendritic arborization and axon outgrowth are critical steps in the establishment of neural connectivity in the developing brain. Changes in the connectivity underlie cognitive dysfunction in neurodevelopmental disorders. However, molecules and associated mechanisms that play important roles in dendritic and axon outgrowth in the brain are only partially understood. Here, we show that microtubule-actin crosslinking factor 1 (MACF1) regulates dendritic arborization and axon outgrowth of developing pyramidal neurons by arranging cytoskeleton components and mediating GSK-3 signaling. MACF1 deletion using conditional mutant mice and in utero gene transfer in the developing brain markedly decreased dendritic branching of cortical and hippocampal pyramidal neurons. MACF1-deficient neurons showed reduced density and aberrant morphology of dendritic spines. Also, loss of MACF1 impaired the elongation of callosal axons in the brain. Actin and microtubule arrangement appeared abnormal in MACF1-deficient neurites. Finally, we found that GSK-3 is associated with MACF1-controlled dendritic differentiation. Our findings demonstrate a novel role for MACF1 in neurite differentiation that is critical to the creation of neuronal connectivity in the developing brain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurons are highly specialized and polarized cells that have two functionally and structurally different processes, axon and dendrites. Neurons receive input signals from neurons and glia through dendrites and transmit output signals to other neurons at axon terminals [1–3]. Thus, normal development of axons and dendrites is important for neural transmission. Abnormalities in these structures can lead to functional brain defects and are implicated in neurodevelopmental disorders such as autism and schizophrenia [4–7]. Formation of axon and dendrites is a dynamic process which requires remodeling of the cytoskeleton controlled by actin and microtubule regulatory proteins [8–10]. However, the key regulators and molecular mechanisms of axon and dendrite formation and further differentiation during brain development are not fully understood.
Microtubule-actin crosslinking factor (MACF1), also called actin cross-linking factor 7, is a member of the plakin family that expressed in numerous tissues including the brain [11–14]. MACF1 is the cytoskeletal crosslinking protein that interacts with F-actin and microtubules to regulate polarization of cells and coordination of cellular movements [15–17]. This protein is essential for embryonic development because the null mice show embryonic lethality with developmental delay [13, 18]. Mutations in the Drosophila homolog of MACF1, Short stop (Shot), lead to abnormal cytoskeleton organization [19–23]. Interestingly, MACF1 is highly expressed in the developing brain and regulates migration of pyramidal neurons in mice [24]. These findings suggest a potential role for MACF1 in neuronal differentiation in the mammalian brain. However, the role of MACF1 and the associated mechanism in dendritic differentiation and axon outgrowth in vivo is not known.
MACF1 is physically associated with GSK-3. For example, MACF1 interacts with GSK-3 in rat tumor cells and human skin stem cells, and the interaction controls microtubule organization [18, 25]. Furthermore, the protein binds to GSK-3 in mouse brain cells. The GSK-3 signaling pathway plays a critical role in neuronal morphogenesis including axon and dendritic outgrowth [26–29]. These findings suggest interplay between MACF1 and GSK-3 signaling in cytoskeleton arrangement and axon/dendrite development.
Here, using a conditional gene targeting strategy combined with in utero electroporation, we investigated the function and the mechanism of MACF1 in neurite differentiation in vivo. We show that MACF1 is required for dendrite arborization and axon outgrowth in the developing brain.
Result
Cell-Autonomous Role of MACF1 in Dendritic Arborization of Pyramidal Neurons
We investigated if MACF1 is required for dendritic differentiation in cortical pyramidal neurons. We first deleted endogenous MACF1 in developing pyramidal neurons by electroporating E14.5 control (MACF1loxP/+) and MACF1loxP/loxP mice in utero with the Dcx-cre-iGFP construct. This in utero electroporation targets the construct onto the ventricular surface of the cerebral cortex where radial neural progenitors and neurons reside. Use of the Dcx-cre-iGFP construct is particularly useful for neuronal targeting. This construct expresses Cre recombinase only in neuronal populations under the Dcx promoter, not in radial neural progenitors [30]. Thus, MACF1 is knocked out selectively in neurons transfected with the Dcx-cre-iGFP. After electroporation, we collected brain tissues at P10. Most control neurons (MACF1 loxP/+; Dcx-cre-iGFP) in layer 2/3 of the cerebral cortex showed a thick apical dendrite extending toward the pial-surface and multiple basal dendrites (Fig. 1a, b). MACF1 deletion led to a loss of primary apical dendrites as the dendrites that grew toward the pia appeared to lose the characteristics of primary apical dendrites. MACF1-deleted neurons (MACF1loxP/loxP; Dcx-cre-iGFP) exhibited more apical dendrites (Fig. 1a, b). The number of dendrites was significantly increased in MACF1-deleted neurons by 64 % compared with control neurons (Fig. 1c). However, the length of total apical dendrites was markedly decreased in MACF1-deleted neurons by 43 % compared with controls. Similarly, the length and the thickness of apical dendrites were decreased by 49 and 48 %, respectively, in MACF1-deleted neurons. MACF1 deletion also led to abnormal formation of basal dendrites. The length of basal dendrites was decreased by 53 % in MACF1 mutant brains compared with controls (Fig. 1c). Furthermore, MACF1-deleted neurons showed a decrease in dendritic lengths in cultures compared with controls (Supplemental Fig. 1), indicating the MACF1 effect on dendritic differentiation is cell-autonomous. Additionally, we assessed orientation of apical dendrites in control and MACF1loxP/loxP; Dcx-cre-iGFP neurons. Apical dendrites of MACF1loxP/loxP; Dcx-cre-iGFP neurons were more horizontally oriented than control dendrites that were mostly aligned verticality toward the pia surface (Fig. 1d). MACF1-deleted dendrites showed wider angles to the vertical line and developed multiple dendrites lacking specific orientation.
Elimination of MACF1 suppresses dendritic branching in developing cortical neurons. a Neuron-specific deletion of MACF1 leads to abnormal dendritic branching in the developing cortical neurons. Control (MACF1 loxP/+) or MACF1 loxP/loxP embryos were electroporated in utero with Dcx-cre-iGFP at E14.5 to delete MACF1 in cortical pyramidal neurons. The electroporated mice were then sacrificed at P10 and the brain samples were collected. GFP-positive cells were visualized in the lateral cerebral cortex. Scale bar, 25 μm. b Representative single cell traces of soma and dendrites shown in a. c The numbers and lengths of dendrites were quantified. The lengths and thickness of apical and basal dendrites were decreased in MACF1 loxP/loxP neurons compared with controls while the number of primary dendrites was increased. n = 75 cells from five mice for each condition. Statistical significance was determined by two-tailed Student’s t test. *p < 0.05, **p < 0.01, ***p < 0.001. d MACF1 loxP/loxP neurons showed abnormal orientation of apical dendrites in the cerebral cortex
Next, we investigated whether MACF1 regulates initiation, branching, or both of dendritic regrowth. We isolated cortical neurons from control and MACF1loxP/loxP mice and immediately transfected with the Dcx-cre-iGFP construct. After 3 days, we fixed cells and assessed the number and the length of dendrites by immunostaining with anti-MAP2 antibody. MACF1 deletion increased the number of initially regrowing dendrites by 60 % while it decreased primary and total dendrite lengths by 64 % (Fig. 2a, b). Likewise, total length of dendrites was markedly decreased by 69 % in MACF1-deleted neurons compared to controls, indicating that MACF1 deletion impairs the initiation of dendritic regrowth. To assess the effect of MACF1 on dendritic branching, we first cultured control and MACF1-deleted neurons for 6 days, allowing them to generate initial dendrites. Then, we transfected the Dcx-cre-iGFP construct and examined dendritic branching after 6 days. We observed that the number of dendritic branches and the length of total dendrites were markedly decreased in MACF1-deleted neurons by 58 and 71 %, respectively (Fig. 2c, d). These results show that MACF1 regulates both initial dendrite regrowth and later-stage branching.
MACF1 deletion effects in early and later stages of dendritic growth. a MACF1 regulates initial dendritic growth. Cortical neurons from control (MACF1 loxP/+) or MACF1 loxP/loxP brains at E14.5 were cultured and transfected with Dcx-cre-iGFP at 0 day in vitro (DIV). Dendritic morphology was examined in MAP2/GFP-positive neurons at 3 DIV. Bar, 25 μm. b The numbers and lengths of primary and total dendrites were quantified. c Effect of MACF1 in later-stage dendritic branching. E14.5 control and MACF1 loxP/loxP cortical neurons were cultured and transfected with Dcx-cre-iGFP at 6 DIV. MAP2/GFP-positive neurons were assessed at 12 DIV. Bar, 25 μm. d The numbers of dendritic branches were quantified. n = 60 cells from three independent cultures using three mice for each condition. Statistical significance was determined by two-tailed Student’s t test. ***p < 0.001
Like cortical pyramidal cells, neurons in the hippocampus migrate into appropriate layers and establish circuitry networks through dendritic and axonal formation during brain development. Thus, we examined a role of MACF1 in dendrite formation of hippocampal neurons. We deleted MACF1 in the developing hippocampus using an in utero electroporation with a modification of electrode orientation as previously reported [24]. We targeted Dcx-cre-iGFP into the lower part of the lower medial cortex at E14.5, which forms the hippocampus at later developmental stages [31–34]. After electroporation, we collected brain tissues at P10 and examined dendrite formation. MACF1-deletion decreased the length of total dendrites of hippocampal neurons by 62 % compared with controls (Fig. 3). In contrast, the number of primary dendrites was increased by 275 % in MACF1-deleted hippocampal neurons. These results indicate that MACF1 deletion causes a general defect in process outgrowth in the hippocampal neurons. Taken together, our data demonstrate that MACF1 is essential for normal dendrite differentiation of pyramidal neurons during brain development.
MACF1 is required for dendritic arborization of developing hippocampal neurons. a Neuron-specific deletion of MACF1 leads to abnormal dendritic development in the hippocampus. Control (MACF1 loxP/+) or MACF1 loxP/loxP embryos at E14.5 were electroporated in utero with Dcx-cre-iGFP to target hippocampal pyramidal neurons. The electroporated mice were sacrificed at P10 and the brain samples were collected. GFP-positive neurons were assessed in the hippocampus. MACF1 deletion decreased the lengths of apical dendrites of hippocampal neurons. b Representative single cell traces of soma and dendrites shown in a. c The length and the number of dendrites were quantified. n = 70 cells from five mice for each condition. Statistical significance was determined by two-tailed Student’s t test. ***p < 0.001
Dendritic Spine Formation in MACF1-Deleted Neurons
Dendritic spines are the major sites of synaptic input [35–38]. Abnormal formation of dendrites in MACF1-deleted neurons led us to examine a role of MACF1 in spine formation. We cultured cortical neurons from control and MACF1loxP/loxP mice for 5 days and transfected Dcx-cre-iGFP. After 9 days, we assessed dendritic spines. MACF1 deletion decreased the number of dendritic spines by 61 % compared with controls (Fig. 4a, c). Interestingly, MACF1-deleted spines were noticeably long and thin (Fig. 4b). The length of spines was increased by 201 % in MACF1-deleted neurons compared with control neurons (Fig. 4c). Furthermore, the diameters of spine head and neck were decreased by 67 and 39 %, respectively, in MACF1-deleted neurons. These results show that MACF1 regulates dendritic spine formation of cortical pyramidal neurons.
Role of MACF1 in dendritic spine formation. a MACF1-deleted neurons show aberrant dendritic spines. Cortical neurons from E14.5 control (MACF1 loxP/+) and MACF1 loxP/loxP brains were cultured for 5 days, and then transfected with a plasmid encoding Dcx-cre-iGFP. Spines were assessed in GFP-positive neurons at 14 DIV. Bar, 10 μm. b Higher magnification images shown in a. c The numbers, lengths, and sizes of spines were quantified. n = 45 cells from three independent cultures using three mice for each condition. Statistical significance was determined by two-tailed Student’s t test. **p < 0.01, ***p < 0.001
MACF1 Is Required for Axonal Growth and Branching
Callosal axons of projection neurons cross the cortical midline and arrive at the contralateral cortex during development and then they start extensive branching [39–41]. We investigated if MACF1 plays a role in callosal axon development. We labeled layer 2/3 projection neurons in the developing brain by performing in utero electroporation of E14.5 control and MACF1loxP/loxP mice with Dcx-cre-iGFP construct. We collected brain tissues at P14 and assessed callosal axon projections and branches by chasing GFP-labeled neurons and axons along the callosal axon route (Fig. 5a, b). Control neurons expressing GFP (Fig. 5b, left panels) projected callosal axons normally toward the ventricular zone (Fig. 5c, left panel). The axons traversed the midline in the corpus callosum (Fig. 5d, left panel) and reached at the contralateral side (Fig. 5e, left panel). However, MACF1-deleted neurons (Fig. 5b, right panels) showed reduced numbers of callosal axons within the corpus callosum and the contralateral cortex (Fig. 5d, e, right panels). As a result, mutant axon bundles in the corpus callosum appeared to be much thinner (Fig. 5d). The axon numbers were decreased by 45 and 73 % in the mutant corpus callosum and the contralateral side, respectively, compared with control samples (Fig. 5f). A similar pattern of reduced axon bundles were observed in MACF1 loxP/loxP; Nex-cre brains (Supplemental Fig. 2). Axon branching is induced by local signals [42]. We wondered if the correctly innervated axons in the mutant brains normally generate branches. We found that MACF1-deleted axons had a reduction in branching compare with control axons (Fig. 5g, h). In the target area, the number of axon branches was decreased by 74 %. This result suggests that MACF1 deletion suppresses the process of receiving local inputs or disrupts intrinsic signals for axon branching. Our data demonstrate that MACF1 is required for elongation and branching of callosal axons in the developing cortex.
MACF1 deletion disrupts callosal axon growth and innervation. a A schematic illustration of callosal axons that traverse the cortical midline and arrive at the contralateral cortex during the first postnatal week. Red rectangles indicate the different regions shown in b–e. b Control (MACF1 loxP/+) or MACF1 loxP/loxP; embryos were electroporated in utero with Dcx-cre-iGFP at E14.5 to target cortical pyramidal neurons. The electroporated mice were then sacrificed at P14 and GFP-positive pyramidal neurons in the lateral cerebral cortex (region 1) were visualized (upper panels). Lower panels showed low magnification images of in utero electroporated brain samples. c–e Neuron-specific deletion of MACF1 leads to abnormal callosal axon growth in the developing cerebral cortex. GFP-positive callosal axons project toward the lower layers of the cerebral cortex (c, region 2), traverse in the cortical midline (d, region 3), arrive the contralateral cortex (e, region 4). f The numbers of callosal axons in each region shown in b–e were assessed. n = 21 sections from five mice for each condition. Statistical significance was determined by two-tailed Student’s t test. ***p < 0.001. g Representative traces of axon branches in region 4. h The numbers of axonal branches shown in g were quantified. n = 128 axons for control and 46 axons using five mice for each condition. Statistical significance was determined by two-tailed Student’s t test. ***p < 0.001
Elimination of MACF1 Disrupts Actin and Microtubule Arrangement
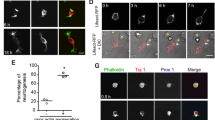
Neurite growth and branching involve the coordinated actions of cytoskeletal rearrangements [43, 44]. We investigated actin and microtubule arrangement and stability in control and MACF1-deleted neurons. We deleted MACF1 by crossing Nex-Cre mouse line that expresses Cre recombinase exclusively in neurons in the developing cerebral cortex [45]. We first examined localization of MACF1 in cortical neurons. MACF1 was found throughout the soma and dendrites with an accumulation at dendrite tips (Fig. 6a). Then, we assessed stabilized actins by labeling with phalloidin conjugated with a fluorescence dye. Polymerized actins labeled by phalloidin-conjugates were mostly bundled and accumulated at the terminal in control cells (Fig. 6b, left panels). However, MACF1-deleted cells did not show clear accumulation patterns at neurite tips (Fig. 6b, right panels). Polymerized actins appeared to be scattered and debundled at neurite tips in MACF1-deleted cells. Additionally, MACF1-deleted cells showed more accumulation of polymerized actins in the cell body than control cells (Fig. 6b, bottom panels). Thus, localization of stable actins at the soma and the neurtie tip was disrupted in MACF1-deleted cells (Fig. 6c), suggesting that MACF1 determines actin stabilization and positioning in the neuron. Finally, we examined microtubule structures at the neurite tip. Microtubules immunostained with a β-tubulin antibody were bundled together in control cells, but they were more debundled in MACF1-deleted cells (Fig. 6d), suggesting unstable microtubules at the neurite tip. Indeed, compared with control cells, MACF1-deleted cells showed a decrease in the level of acetylated tubulin that is a marker for stabilized microtubules (Fig. 6e). Together, these results suggest that MACF1 plays an important role in neurite growth and branching by regulating actin and microtubule arrangement and stabilization.
Actin and microtubule localization and arrangement in MACF1 mutant neurons. a MACF1 expression in cortical neurons. Cortical neurons from E14.5 control (MACF1 loxP/+; Nex-cre) and MACF1 loxP/loxP; Nex-cre brains were cultured for 5 days, and MACF1 localization was examined using immunostaining. Arrows indicate an accumulation of MACF1 in dendritic tips. b MACF1 deletion causes aberrant actin arrangement and localization. Polymerized actins were visualized by phalloidin staining. Top panels show the patterns of polymerized actins at neurite tips. Middle and bottom panels show polymerized actins accumulated in the cytosol. c The levels of polymerized actin (F-actin) intensity in the cell body and at the neurite tip were quantified using ImageJ (NIH) software. n = 21 cells from three independent cultures using three mice for each condition. Statistical significance was determined by two-tailed Student’s t test. ***p < 0.001. d MACF1-deleted neurons show abnormal microtubule arrangement at the neurite tip. e Immunoblotting was performed to measure the levels of α-tubulin or acetylated-tubulin using E14.5 control and MACF1 loxP/loxP; Nex-cre brain lysates (top panel). The levels of each tubulin were quantified in the bottom panel. n = 3 blots using three different lysates from three mice for each condition. Statistical significance was determined by two-tailed Student’s t test. ***p < 0.001
Association of MACF1 with GSK-3 Signaling in Dendrite Differentiation
To identify associated cellular signaling, we investigated an interaction of MACF1 with glycogen synthase kinase-3 (GSK-3) signaling in dendritic arborization. GSK-3 phosphorylates MACF1 in brain and skin cells [24, 25], and the phosphorylation by GSK-3 regulates MACF1 binding to microtubules [25]. Using wild-type control and GSK-3 knockout brain samples, we assessed the level of phosphorylated MACF1 by Western blotting with a MACF1 antibody that detects GSK-3-mediated phosphorylation [25]. The level of phospho-MACF1 was decreased by 51 % in GSK-3 knockout brain lysates compared with control samples (Fig. 7a, b). When phosphorylated by GSK-3, MACF1 is inactivated in skin cells (Wu et al., 2011), suggesting a potential interplay between GSK-3 and MACF1 in neurons. Thus, we examined the role of GSK-3 phosphorylation of MACF1 in dendrite outgrowth by in utero electroporating a control GFP, a constitutively-active GSK-3β (ca-GSK-3β), WT MACF1, MACF1 S:A (phosphorylation-refractile form) [25], or ca-GSK-3β and MACF1 S:A into the E14.5 developing brain. A previous study showed that GSK-3 phosphorylates MACF1 at the C-terminal phosphorylation cluster [25]. MACF1 S:A point mutations convert GSK-3 phosphorylation sites at this cluster to a kinase-refractile version harboring serine to alanine mutations. The S:A mutant was shown to be resistant to GSK-3 phosphorylation. We examined dendrites at P10. Overexpression of ca-GSK-3β suppressed dendrite outgrowth and branching compared with control (Fig. 7c, d). We measured the lengths of total and primary apical dendrites. Overexpression of ca-GSK-3β construct reduced total and primary apical dendrites by 57 and 51 %, respectively (Fig. 7e). Overexpression of WT MACF1 or MACF1 S:A alone showed no significant changes in the number or the length of dendrites (Fig. 7c–e). However, co-expression of MACF1 S:A partially rescued the inhibitory effect of ca-GSK-3β (Fig. 7c, d). Similarly, the numbers of secondary and tertiary apical dendrites that are indicators of dendritic branching were decreased in neurons expressing ca-GSK-3β, and the decreased branches were significantly reversed by coexpression with MACF1 S:A (Fig. 7e). These findings show that GSK-3-mediated phosphorylation of MACF1 is an important mechanism for the MACF1 function in dendritic outgrowth and branching.
MACF1 mediates GSK-3 signaling in dendritic branching (a) GSK-3 deletion inhibited phosphorylation of MACF1in the developing brain. Phosphorylation of MACF1 was measured by Western blotting using brain lysates from control and GSK-3 knockout mice (GSK-3α−/−; GSK-3βloxP/loxP; Nestin-cre). Control: GSK-3α+/−; GSK-3βloxP/+; Nestin-cre. b Quantification of a. c Suppression of GSK-3 phosphorylation of MACF1 partially restores the inhibitory effect of GSK-3 in dendrite outgrowth. E14.5 mice were electroporated in utero with a GFP, ca-GSK-3β-GFP, WT MACF1, MACF1 S:A-GFP, or ca-GSK-3β-GFP and MACF1 S:A-GFP constructs. Brain sections were prepared at P14 to assess dendrite outgrowth and branching. The overexpression of ca-GSK-3β-GFP inhibited dendrite outgrowth. However, the defective dendrite outgrowth was partially rescued by co-overexpression of ca-GSK-3β-GFP with MACF1 S:A-GFP construct. Scale bar, 25 μm. d Representative single cell traces soma and dendrites shown in c. e The lengths and numbers of total and primary/secondary apical dendrites were quantified. n = 75 cells from five mice for each condition. Statistical significance was determined by one-way ANOVA with Bonferonni correction test. *p < 0.05, **p < 0.01, ***p < 0.001
Discussion
We identified the role of MACF1 in dendritic branching, spine formation, and axon outgrowth in the developing mammalian brain. MACF1 deletion inhibits dendrite/axon length growth, but increases the number of short primary dendrites. Dendritic spines are long and thin, and the density is decreased in MACF1-deficient neurons. MACF1 functions as a downstream effector of GSK-3 to regulate neurite differentiation in developing pyramidal neurons.
Requirement of MACF1 in Neurite Outgrowth and Spine Formation in Developing Neurons
MACF1 is highly expressed in the brain during development [18, 24]. Studies have shown that the Drosophila homolog of MACF1, shot, is involved in neurite outgrowth and branching [19, 20, 46–48]. In mouse brain, MACF1 is required for the migration and positioning of pyramidal neurons in the developing brain [24, 49]. For example, MACF1 deletion using the Dcx-cre-iGFP construct caused abnormal neuron migration leading to scattered positioning of upper layer neurons throughout the cerebral cortex instead of packaging within the layer II/III [24]. Consistently, we observed abnormal neuronal positioning in MACF1loxP/loxP; Dcx-cre-iGFP brains in this study. In addition to the abnormal neuron positioning, thalamocortical and hippocampal commissure projections are abnormal in MACF1; Nestin-Cre mouse brains [49]. In our study, we assessed the dendritic and axonal phenotype from the neurons that are positioned normally within the layer II/III after Dcx-cre-iGFP electroporation. Our results showed that MACF1 deficiency results in aberrant dendrite and axonal differentiation including immature spines and insufficient dendritic branching and callosal axon innervation. The Dcx-cre-iGFP construct uses the mouse doublecortin (Dcx) promoter to drive expression of Cre recombinase in immature and young neurons [30, 50] . Dcx-positive immature neurons are primarily found in the intermediate zone where undifferentiated neurons start migrating toward the pia. Furthermore, Franco et al. (2011) showed that Dcx-cre-iGFP-mediated deletion of Dab1 gene disrupted somal translocation and migration of newly born neurons before neuronal differentiation. Generally, dendrites and axons form after the completion of neuronal migration. Thus, MACF1 deletion mediated by the Dcx-cre-iGFP disrupts early neurite growth of targeted neurons. These data strongly suggest that MACF1 is an essential component of brain wiring by contributing to the establishment of neuronal connections during development. Correct growth and differentiation of axons and dendrites such as branching and innervation are critical prerequisites for proper local neuronal connectivity and eventually long-range circuit formation. Abnormal neural circuit formation is associated with many neurodevelopmental and psychiatric diseases including autism, intellectual disability, and schizophrenia. In this regard, it is interesting to note that MACF1 is an interactome of DISC1 that is a susceptibility factor for schizophrenia [51].
Our data also show that MACF1 controls dendritic spine formation during brain development. MACF1-deficient neurons show a decrease in the number of dendritic spines and an alteration in their shapes, i.e., long and thin spines compared to controls. Dendritic spine is a major site of excitatory synaptic input and thereby important for synaptic plasticity [52, 53]. Our results suggest that MACF1 contributes to synaptic formation, maturation and maintenance in the brain. Aberrant spine morphology and density caused by many neurodevelopmental and psychiatric diseases including autism spectrum disorders and schizophrenia [54, 55]. Synapses undergo dynamic changes responding to a variety of inputs. Whether MACF1 is involved in synaptic plasticity remains to be elucidated.
Leading process extension of migrating neurons is not just a requirement for neuronal migration but is an important phenomenon for dendritic differentiation. Leading processes of migrating pyramidal neurons differentiate into apical dendrites after completing migration [56]. Then, dendrites undergo arborization by establishing complex branches. We previously showed that MACF1 regulates leading processes of radially migrating pyramidal neurons [24]. For example, elimination of MACF1 disrupts the extension and orientation of leading processes of migrating neurons during brain development, suggesting the role of MACF1 in initial dendritic differentiation. Indeed, we found that MACF1-deficient neurons have aberrant dendrites. Thus, initial formation of dendrites rather than maintenance and shedding appear to cause the defective dendritic phenotypes in MACF1-deficient neurons.
Regulation of Cytoskeleton Components by MACF1
Rearrangements of the actin cytoskeleton and microtubules are crucial for the initial stage of neuronal polarity [57]. Actins and microtubules are enriched in neurite tips and regulate growth and branching of neurites [1, 44, 58]. MACF1 is able to interact with both actins and microtubules and bridges these cytoskeleton components [12, 15, 59]. Mutations in MACF1 gene cause a loss of stable microtubule localization to the periphery of the zebrafish oocyte [60]. Consistently, we found that MACF1-deleted neurons show defective localization of polymerized actins at neurite tips and somas. Our results also show that microtubules are debundled and unstable in MACF1-deficient neurons. Thus, inactivation of MACF1 may cause actin and microtubule instability, and subsequent disruption of cytoskeleton arrangement leading to aberrant neurite outgrowth and differentiation. A schematic model of neurite outgrowth in the absence or presence of MACF1 is presented in Fig. 8. Proper positioning of microtubules and actins is a prerequisite for neurite growth [57]. MACF1 accumulated at neurite tips appears to regulate localization and stability of microtubules and actins during neurite outgrowth.
A schematic model illustrating a role of MACF1 in neurite outgrowth. a MACF1 regulates neurite outgrowth. In control neurons, MACF1 localized at the neurite tip stabilizes microtubules by bundling them together and contributes to accumulate polymerized actins at the neurite tip. In contrast, MACF1 deficiency leads to microtubule debundling at the neurite tip. Also, polymerized actins are reduced and scattered around the neurite tip in the absence of MACF1, but accumulated in the cytosol. b GSK-3 regulates MACF1 activity on dendritic arborization. Unphosphorylated MACF1 is required for primary dendritic polarity. GSK-3 phosphorylation of MACF1 induces short multiple primary dendrites
MACF1 and GSK-3 Signaling in Neurite Growth
MACF1 is associated with a Wnt complex containing GSK-3, APC, Axin, and β-catenin, and knockdown of the protein inhibits the Wnt signaling pathway in cells [18]. GSK-3 regulates the activity of microtubule-associated proteins including APC, CRMP2, CRMP4, MAP1b, MAP2, and Tau via phosphorylation mechanisms [61]. Furthermore, GSK-3 signaling regulates neurite specification, polarity, plasticity, and growth [26–29, 62]. Importantly, GSK-3 controls the activity of MACF1 via physical interaction and phosphorylation in skin stem cells [25]. We have also previously shown that GSK-3 binds to and phosphorylates MACF1 in brain tissues [24]. This phosphorylation is important for MACF1 to bind and control microtubules [25]. Inhibition of GSK-3β induces dendrite outgrowth in cultured cortical and hippocampal neurons [63]. Our results are consistent with the previous finding because overexpression of active GSK-3 inhibits dendritic branching and the active GSK-3-mediated inhibition is partially suppressed by co-overexpression with MACF1 S:A. These data suggest that MACF1 is a downstream target of GSK-3 signaling in dendritic differentiation. We noted that overexpression of ca-GSK-3 did not completely phenocopy the dendritic morphology in MACF1-deleted neurons. GSK-3 has multiple targets that are associated with neurite development [62, 64]. While active GSK-3 suppresses MACF1 activity, it may also modify other microtubule and actin binding proteins. Thus, we think that the outcome of active GSK-3 overexpression may result from a combination of each substrate phenotype. However, the decreased dendritic lengths and branch numbers in neurons expressing active GSK-3 are similar to the MACF1 phenotypes.
In summary, we show a novel role of MACF1 in dendrite arborization and axon extension in the developing mammalian brain. GSK-3 as an upstream kinase is associated with the function of MACF1. Our findings may help develop a better understanding of neural connectivity during brain development.
Materials and Methods
Plasmids
A constitutively-active GSK-3β (S9A) plasmid was generously provided by Dr. James Woodgett (Samuel Lunenfeld Research Institute). Dcx-cre-iGFP was described previously [30]. MACF1-GFP S:A plasmid was a generous gift from Dr. Elaine Fuchs (Howard Hughes Medical Institute, The Rockefeller University).
Mice
Mice were handled according to our animal protocol approved by the University of Nebraska Medical Center. MACF1 floxed mouse [25] was described previously. Nex-cre mouse [45] was used to generate conditional MACF1 knockout mice (MACF1loxP/loxP; Nex-cre).
Immunohistochemistry
Immunohistochemical labeling of embryonic brain sections or dissociated neural cells was performed as described previously [65, 66]. The following primary antibodies were used: chicken anti-GFP (Invitrogen), rabbit anti-GFP (Invitrogen), mouse anti-β-III Tubulin (Phosphosolutions), rabbit anti-acetyl-α-tubulin (Cell Signaling), and mouse anti-β-tubulin (Upstate). Appropriate secondary antibodies conjugated with Alexa Fluor dyes (Invitrogen) were used to detect primary antibodies. Polymerized F-actins were detected by labeling with phalloidin-Alexa 568 (Invitrogen).
In Utero Electroporation
In utero electroporation was performed as described previously [24, 66]. Briefly, timed pregnant female mice from E14.5 day of gestation were deeply anesthetized and the lateral ventricles of an embryonic brain were injected with plasmid DNA (2 μg/μl) and 0.001 % fast green using a Picospritzer II (Parker Inc.). Electroporation was achieved by using BTX ECM830 elecroporator (5 pulses with 100-ms length separated by 900-ms intervals were applied at 45 V). Embryos were allowed to develop in utero for the indicated time. For hippocampal gene delivery, the electrodes were placed at an angle to the opposite way of cortical targeting as described previously [37].
Morphometry
For the quantification of lengths, numbers, or thickness of primary dendrites and branches, images of 20 different brain sections containing the corpus callosum from more than five mice were taken with Zeiss LSM510 and LSM710 confocal microscopes and a Nikon Eclipse epifluorescence microscope attached with a QImaging CCD camera. For spine quantification and cytoskeleton analysis in cultured cells, ten mice for each experiment (control mice, n = 5; mutant mice, n = 5) were used. More than 20 fields scanned horizontally and vertically were analyzed in each condition. Cell numbers examined were described in figure legends. The images were analyzed by using ZEN (Zeiss), LSM image browser (Zeiss), QCapture software (QImaging), and ImageJ (NIH). The calculated values were averaged, and some results were recalculated as relative changes versus control.
Primary Neuron Cultures
Primary neuronal culture was described previously [24, 67]. Briefly, cerebral cortices from E13.5–16.5 mice were isolated and dissociated with trituration after trypsin/EDTA treatment. Then, the cells were plated onto poly-d-lysine/ laminin-coated coverslips and cultured in the medium containing neurobasal medium, 5 % serum, B27 and N2 supplements.
Cell Transfection
Mouse cortical neurons were transfected with various plasmids as described in a previous paper [29]. Embryonic cortices were dissociated and suspended in 100 μl of Amaxa electroporation buffer containing 1–10 μg of plasmid DNA. Then, suspended cells were electroporated with an Amaxa Nucleofector apparatus. After electroporation, cells were plated onto coated coverslips and the medium was changed 4 h later to remove the remnant transfection buffer. For transfecting DNA constructs into attached cells, lipofectamine (Invitrogen)-mediated transfection was performed according to the manufacturer’s protocol.
Western Blotting
Western blotting was performed as described previously [68, 69]. Lysates from E14.5 telencephalon were prepared using RIPA buffer, and the protein content was determined by a Bio-Rad Protein Assay system. Proteins were separated on 4–12 % SDS-PAGE gradient gel and transferred onto nitrocellulose membrane. Then, the membrane was incubated with rabbit anti-acetyl-α-tubulin (Cell Signaling), mouse anti-α-tubulin (Sigma), or rabbit anti-GAPDH (Cell Signaling) at 4 °C overnight. Appropriate secondary antibodies conjugated to HRP were used (Cell Signaling) and the ECL reagents (Amersham) were used for immunodetection. For quantification of band intensity, blots from three independent experiments for each molecule of interest were used. Signals were measured using ImageJ software and represented by relative intensity versus control. GAPDH was used as an internal control to normalize band intensity.
Statistical Analysis
Normal distribution was tested using Kolmogorov–Smirnov test and variance was compared. Unless otherwise stated, statistical significance was determined by two-tailed Student’s t test for two-population comparison and one-way analysis of variance followed by Bonferonni correction test for multiple comparisons. Data were analyzed using GraphPad Prism and presented as mean (±) SEM. P values were indicated in figure legends.
References
Arimura N, Kaibuchi K (2007) Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat Rev Neurosci 8(3):194–205. doi:10.1038/nrn2056
Craig AM, Banker G (1994) Neuronal polarity. Annu Rev Neurosci 17:267–310. doi:10.1146/annurev.ne.17.030194.001411
Witte H, Bradke F (2008) The role of the cytoskeleton during neuronal polarization. Curr Opin Neurobiol 18(5):479–487. doi:10.1016/j.conb.2008.09.019
Bellon A (2007) New genes associated with schizophrenia in neurite formation: a review of cell culture experiments. Mol Psychiatry 12(7):620–629. doi:10.1038/sj.mp.4001985
Jan YN, Jan LY (2010) Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci 11(5):316–328. doi:10.1038/nrn2836
Kaufmann WE, Moser HW (2000) Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex 10(10):981–991
Pardo CA, Eberhart CG (2007) The neurobiology of autism. Brain Pathol 17(4):434–447. doi:10.1111/j.1750-3639.2007.00102.x
Hur EM, Saijilafu ZFQ (2012) Growing the growth cone: remodeling the cytoskeleton to promote axon regeneration. Trends Neurosci 35(3):164–174. doi:10.1016/j.tins.2011.11.002
Geraldo S, Gordon-Weeks PR (2009) Cytoskeletal dynamics in growth-cone steering. J Cell Sci 122(Pt 20):3595–3604. doi:10.1242/jcs.042309
Siegrist SE, Doe CQ (2007) Microtubule-induced cortical cell polarity. Genes Dev 21(5):483–496. doi:10.1101/gad.1511207
Fuchs E, Karakesisoglou I (2001) Bridging cytoskeletal intersections. Genes Dev 15(1):1–14
Jefferson JJ, Leung CL, Liem RK (2004) Plakins: goliaths that link cell junctions and the cytoskeleton. Nat Rev Mol Cell Biol 5(7):542–553. doi:10.1038/nrm1425
Kodama A, Karakesisoglou I, Wong E, Vaezi A, Fuchs E (2003) ACF7: an essential integrator of microtubule dynamics. Cell 115(3):343–354
Lin CM, Chen HJ, Leung CL, Parry DA, Liem RK (2005) Microtubule actin crosslinking factor 1b: a novel plakin that localizes to the Golgi complex. J Cell Sci 118(Pt 16):3727–3738. doi:10.1242/jcs.02510
Leung CL, Sun D, Zheng M, Knowles DR, Liem RK (1999) Microtubule actin cross-linking factor (MACF): a hybrid of dystonin and dystrophin that can interact with the actin and microtubule cytoskeletons. J Cell Biol 147(6):1275–1286
Sun D, Leung CL, Liem RK (2001) Characterization of the microtubule binding domain of microtubule actin crosslinking factor (MACF): identification of a novel group of microtubule associated proteins. J Cell Sci 114(Pt 1):161–172
Suozzi KC, Wu X, Fuchs E (2012) Spectraplakins: master orchestrators of cytoskeletal dynamics. J Cell Biol 197(4):465–475. doi:10.1083/jcb.201112034
Chen HJ, Lin CM, Lin CS, Perez-Olle R, Leung CL, Liem RK (2006) The role of microtubule actin cross-linking factor 1 (MACF1) in the Wnt signaling pathway. Genes Dev 20(14):1933–1945. doi:10.1101/gad.1411206
Gao FB, Brenman JE, Jan LY, Jan YN (1999) Genes regulating dendritic outgrowth, branching, and routing in Drosophila. Genes Dev 13(19):2549–2561
Lee S, Harris KL, Whitington PM, Kolodziej PA (2000) Short stop is allelic to kakapo, and encodes rod-like cytoskeletal-associated proteins required for axon extension. J Neurosci 20(3):1096–1108
Prokop A, Uhler J, Roote J, Bate M (1998) The kakapo mutation affects terminal arborization and central dendritic sprouting of drosophila motorneurons. J Cell Biol 143(5):1283–1294
Reuter JE, Nardine TM, Penton A, Billuart P, Scott EK, Usui T, Uemura T, Luo L (2003) A mosaic genetic screen for genes necessary for drosophila mushroom body neuronal morphogenesis. Development 130(6):1203–1213
Sanchez-Soriano N, Travis M, Dajas-Bailador F, Goncalves-Pimentel C, Whitmarsh AJ, Prokop A (2009) Mouse ACF7 and drosophila short stop modulate filopodia formation and microtubule organisation during neuronal growth. J Cell Sci 122(Pt 14):2534–2542. doi:10.1242/jcs.046268
Ka M, Jung EM, Mueller U, Kim WY (2014) MACF1 regulates the migration of pyramidal neurons via microtubule dynamics and GSK-3 signaling. Dev Biol 395(1):4–18. doi:10.1016/j.ydbio.2014.09.009
Wu X, Shen QT, Oristian DS, Lu CP, Zheng Q, Wang HW, Fuchs E (2011) Skin stem cells orchestrate directional migration by regulating microtubule-ACF7 connections through GSK3beta. Cell 144(3):341–352. doi:10.1016/j.cell.2010.12.033
Jiang H, Guo W, Liang X, Rao Y (2005) Both the establishment and the maintenance of neuronal polarity require active mechanisms: critical roles of GSK-3beta and its upstream regulators. Cell 120(1):123–135. doi:10.1016/j.cell.2004.12.033
Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K (2005) GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell 120(1):137–149. doi:10.1016/j.cell.2004.11.012
Zhou FQ, Zhou J, Dedhar S, Wu YH, Snider WD (2004) NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron 42(6):897–912. doi:10.1016/j.neuron.2004.05.011
Kim WY, Zhou FQ, Zhou J, Yokota Y, Wang YM, Yoshimura T, Kaibuchi K, Woodgett JR (2006) Essential roles for GSK-3s and GSK-3-primed substrates in neurotrophin-induced and hippocampal axon growth. Neuron 52(6):981–996. doi:10.1016/j.neuron.2006.10.031
Franco SJ, Martinez-Garay I, Gil-Sanz C, Harkins-Perry SR, Muller U (2011) Reelin regulates cadherin function via Dab1/Rap1 to control neuronal migration and lamination in the neocortex. Neuron 69(3):482–497. doi:10.1016/j.neuron.2011.01.003
Grove EA, Tole S, Limon J, Yip L, Ragsdale CW (1998) The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development 125(12):2315–2325
Grove EA, Tole S (1999) Patterning events and specification signals in the developing hippocampus. Cereb Cortex 9(6):551–561
Mangale VS, Hirokawa KE, Satyaki PR, Gokulchandran N, Chikbire S, Subramanian L, Shetty AS, Martynoga B et al (2008) Lhx2 selector activity specifies cortical identity and suppresses hippocampal organizer fate. Science 319(5861):304–309. doi:10.1126/science.1151695
Monuki ES, Porter FD, Walsh CA (2001) Patterning of the dorsal telencephalon and cerebral cortex by a roof plate-Lhx2 pathway. Neuron 32(4):591–604
Cingolani LA, Goda Y (2008) Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci 9(5):344–356. doi:10.1038/nrn2373
Frost NA, Shroff H, Kong H, Betzig E, Blanpied TA (2010) Single-molecule discrimination of discrete perisynaptic and distributed sites of actin filament assembly within dendritic spines. Neuron 67(1):86–99. doi:10.1016/j.neuron.2010.05.026
Harnett MT, Makara JK, Spruston N, Kath WL, Magee JC (2012) Synaptic amplification by dendritic spines enhances input cooperativity. Nature 491(7425):599–602. doi:10.1038/nature11554
Sanders J, Cowansage K, Baumgartel K, Mayford M (2012) Elimination of dendritic spines with long-term memory is specific to active circuits. J Neurosci 32(36):12570–12578. doi:10.1523/JNEUROSCI.1131-12.2012
Courchet J, Lewis TL Jr, Lee S, Courchet V, Liou DY, Aizawa S, Polleux F (2013) Terminal axon branching is regulated by the LKB1-NUAK1 kinase pathway via presynaptic mitochondrial capture. Cell 153(7):1510–1525. doi:10.1016/j.cell.2013.05.021
Ivy GO, Killackey HP (1981) The ontogeny of the distribution of callosal projection neurons in the rat parietal cortex. J Comp Neurol 195(3):367–389. doi:10.1002/cne.901950302
Wang CL, Zhang L, Zhou Y, Zhou J, Yang XJ, Duan SM, Xiong ZQ, Ding YQ (2007) Activity-dependent development of callosal projections in the somatosensory cortex. J Neurosci 27(42):11334–11342. doi:10.1523/JNEUROSCI.3380-07.2007
Llano I, Tan YP, Caputo C (1997) Spatial heterogeneity of intracellular Ca2+ signals in axons of basket cells from rat cerebellar slices. J Physiol 502(Pt 3):509–519
Jones SB, Lu HY, Lu Q (2004) Abl tyrosine kinase promotes dendrogenesis by inducing actin cytoskeletal rearrangements in cooperation with Rho family small GTPases in hippocampal neurons. J Neurosci 24(39):8510–8521. doi:10.1523/JNEUROSCI.1264-04.2004
Mattila PK, Lappalainen P (2008) Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol 9(6):446–454. doi:10.1038/nrm2406
Goebbels S, Bormuth I, Bode U, Hermanson O, Schwab MH, Nave KA (2006) Genetic targeting of principal neurons in neocortex and hippocampus of NEX-Cre mice. Genesis 44(12):611–621. doi:10.1002/dvg.20256
Gregory SL, Brown NH (1998) kakapo, a gene required for adhesion between and within cell layers in Drosophila, encodes a large cytoskeletal linker protein related to plectin and dystrophin. J Cell Biol 143(5):1271–1282
Kolodziej PA, Jan LY, Jan YN (1995) Mutations that affect the length, fasciculation, or ventral orientation of specific sensory axons in the Drosophila embryo. Neuron 15(2):273–286
Strumpf D, Volk T (1998) Kakapo, a novel cytoskeletal-associated protein is essential for the restricted localization of the neuregulin-like factor, vein, at the muscle-tendon junction site. J Cell Biol 143(5):1259–1270
Goryunov D, He CZ, Lin CS, Leung CL, Liem RK (2010) Nervous-tissue-specific elimination of microtubule-actin crosslinking factor 1a results in multiple developmental defects in the mouse brain. Mol Cell Neurosci 44(1):1–14. doi:10.1016/j.mcn.2010.01.010
Wang X, Qiu R, Tsark W, Lu Q (2007) Rapid promoter analysis in developing mouse brain and genetic labeling of young neurons by doublecortin-DsRed-express. J Neurosci Res 85(16):3567–3573. doi:10.1002/jnr.21440
Camargo LM, Collura V, Rain JC, Mizuguchi K, Hermjakob H, Kerrien S, Bonnert TP, Whiting PJ et al (2007) Disrupted in Schizophrenia 1 Interactome: evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Mol Psychiatry 12(1):74–86. doi:10.1038/sj.mp.4001880
Bourne JN, Harris KM (2008) Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci 31:47–67. doi:10.1146/annurev.neuro.31.060407.125646
Harris KM, Kater SB (1994) Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu Rev Neurosci 17:341–371. doi:10.1146/annurev.ne.17.030194.002013
Chapleau CA, Larimore JL, Theibert A, Pozzo-Miller L (2009) Modulation of dendritic spine development and plasticity by BDNF and vesicular trafficking: fundamental roles in neurodevelopmental disorders associated with mental retardation and autism. J Neurodev Disord 1(3):185–196. doi:10.1007/s11689-009-9027-6
Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM (2011) Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci 14(3):285–293. doi:10.1038/nn.2741
Marin O, Valiente M, Ge X, Tsai LH (2010) Guiding neuronal cell migrations. Cold Spring Harb Perspect Biol 2(2):a001834. doi:10.1101/cshperspect.a001834
Li R, Gundersen GG (2008) Beyond polymer polarity: how the cytoskeleton builds a polarized cell. Nat Rev Mol Cell Biol 9(11):860–873. doi:10.1038/nrm2522
Polleux F, Snider W (2010) Initiating and growing an axon. Cold Spring Harb Perspect Biol 2(4):a001925. doi:10.1101/cshperspect.a001925
Roper K, Gregory SL, Brown NH (2002) The ‘spectraplakins’: cytoskeletal giants with characteristics of both spectrin and plakin families. J Cell Sci 115(Pt 22):4215–4225
Gupta T, Marlow FL, Ferriola D, Mackiewicz K, Dapprich J, Monos D, Mullins MC (2010) Microtubule actin crosslinking factor 1 regulates the Balbiani body and animal-vegetal polarity of the zebrafish oocyte. PLoS Genet 6(8):e1001073. doi:10.1371/journal.pgen.1001073
Zhou FQ, Snider WD (2005) Cell biology. GSK-3beta and microtubule assembly in axons. Science 308(5719):211–214
Hur EM, Zhou FQ (2010) GSK3 signalling in neural development. Nat Rev Neurosci 11(8):539–551. doi:10.1038/nrn2870
Lim CS, Walikonis RS (2008) Hepatocyte growth factor and c-Met promote dendritic maturation during hippocampal neuron differentiation via the Akt pathway. Cell Signal 20(5):825–835. doi:10.1016/j.cellsig.2007.12.013
Sutherland C (2011) What are the bona fide GSK3 substrates? Int J Alzheimers Dis 2011:505607. doi:10.4061/2011/505607
Kim WY, Fayazi Z, Bao X, Higgins D, Kazemi-Esfarjani P (2005) Evidence for sequestration of polyglutamine inclusions by Drosophila myeloid leukemia factor. Mol Cell Neurosci 29(4):536–544. doi:10.1016/j.mcn.2005.04.005
Ka M, Condorelli G, Woodgett JR, Kim WY (2014) mTOR regulates brain morphogenesis by mediating GSK3 signaling. Development 141(21):4076–4086. doi:10.1242/dev.108282
Kim WY, Wang X, Wu Y, Doble BW, Patel S, Woodgett JR, Snider WD (2009) GSK-3 is a master regulator of neural progenitor homeostasis. Nat Neurosci 12(11):1390–1397. doi:10.1038/nn.2408
Kim WY, Horbinski C, Sigurdson W, Higgins D (2004) Proteasome inhibitors suppress formation of polyglutamine-induced nuclear inclusions in cultured postmitotic neurons. J Neurochem 91(5):1044–1056. doi:10.1111/j.1471-4159.2004.02788.x
Kim WY, Gonsiorek EA, Barnhart C, Davare MA, Engebose AJ, Lauridsen H, Bruun D, Lesiak A et al (2009) Statins decrease dendritic arborization in rat sympathetic neurons by blocking RhoA activation. J Neurochem 108(4):1057–1071
Acknowledgments
We thank Dr. Robert Norgren for valuable comments on the manuscript. Research reported in this publication was supported by an award from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number R01NS091220, an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under award number P20GM103471, a grant from NE DHHS (Stem Cell 2012-05), and a grant from Alzheimer’s Association (NIRP-12-258440) to WYK.
Author Contribution
M.K. and W.K. conceived, designed, performed and analyzed the study. W.K. supervised the work. M.K. and W.K. wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
MACF1 deletion effect on dendrites in cultured neurons (a) Cortical neurons from control (MACF1 loxP/+; Nex-cre) and MACF1 loxP/loxP; Nex-cre brains at E14.5 were cultured for 3 days. Dendrites were visualized by MAP2 immunostaining. (b) The number and the length of dendrites were quantified. n = 75 cells from 3 independent cultures using 3 mice for each condition. Statistical significance was determined by two-tailed Student’s t-test. ***p < 0.001 (PDF 82 kb).
Supplemental Fig. 2
MACF1 deletion effect on callosal axon projection Callosal axons from P0 control (MACF1 loxP/+; Nex-cre) and MACF1 loxP/loxP; Nex-cre brains were examined. Brains were immunostained with an L1 antibody. Reduced intensity of L1 fluorescence was shown in MACF1 loxP/loxP; Nex-cre brains compared with controls (PDF 212 kb).
Rights and permissions
About this article
Cite this article
Ka, M., Kim, WY. Microtubule-Actin Crosslinking Factor 1 Is Required for Dendritic Arborization and Axon Outgrowth in the Developing Brain. Mol Neurobiol 53, 6018–6032 (2016). https://doi.org/10.1007/s12035-015-9508-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9508-4