Abstract
Circulating hemocytes of the silkworm can be classified by fluorescence microscopy following staining with acridine orange and propidium iodide. Based on their fluorescence characteristics, three groups of circulating hemocytes can be distinguished. The first group, granulocytes and spherulocytes, is positive for acridine orange and contain bright green fluorescent granules when observed by fluorescence microscopy. In granulocytes, these green granules are heterogeneous and relatively small. In contrast, in spherulocytes, the green granules appear more homogenous and larger. The second group of hemocytes consists of prohemocytes and plasmatocytes. These cells appear faint green following staining with acridine orange and do not contain any green fluorescent granules in the cytoplasm. Prohemocytes are round, and their nuclei are dark and clear within a background of faint green fluorescence. Inside the nucleus there are one or two small bright green fluorescent bodies. Plasmatocytes are irregularly shaped and their nuclei are invisible. Oenocytoids belong to the third group, and their nuclei are positive for propidium iodide. Therefore, all five types of circulating hemocytes of the silkworm, including many peculiar ones that are difficult to identify by light microscopy, can now be easily classified by fluorescence microscopy following staining with acridine orange and propidium iodide. In addition, we show that hemocytes positive for acridine orange and propidium iodide are in fact living cells based on assays for hemocyte composition, phagocytosis, and mitochondrial enzyme activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Circulating insect hemocytes provide an excellent model system to study cell development and differentiation. Since hemocytes play important roles in the insect immune system, fighting against pathogens and parasites by phagocytosis, encapsulation, and nodule formation, etc. (Lackie 1988; Wago 1991; Hoffmann et al. 1996; Carton and Nappi 1997, 2001; Lavine and Strand 2002), the research on larval hemocytes is now being paid much more attention. However, accurate classification is an important prerequisite for the study of larval hemocytes. Although the identification of larval hemocytes by light microscopy is common, this conventional method of hemocyte classification has been the source of frequent controversy (Arnold 1979). In the silkworm, there are five types of circulating hemocytes: prohemocyte, granulocyte, spherulocyte, plasmatocyte, and oenocytoid (Nittono 1960; Akai and Sato 1973; Beaulaton 1979; Wago 1991; Yamashita and Iwabuchi 2001). Identification of each type by light microscopy has often been perplexing, especially some granulocytes which are difficult to distinguish from prohemocytes even for experienced researchers.
The recent use of monoclonal antibodies (mAbs) to identify insect hemocytes is an exciting development because of the promise of high specificity (Chain et al. 1992; Mullett et al. 1993; Willott et al. 1994; Strand and Johnson 1996; Gardiner and Strand 1999). However, mAbs specific enough for identification of each hemocyte have not been isolated yet. In addition to mAbs, specific enzyme activity, for example, protease, acid phosphatase, phenoloxidase, peroxidase, and esterase, has been utilized to identify insect hemocytes (Chain and Anderson 1983; Glupov et al. 1997; Shirae and Saito 2000; Inoue et al. 2001; Hillyer and Christensen 2002; Hillyer et al. 2003). Unfortunately, these assays are very time consuming and impractical when many hemocytes have to be examined. A disadvantage of the use of mAbs or enzyme activity in the identification of larval hemocytes is the unavoidably repeated washing steps which may result in the loss of hemocytes from glass coverslips or slides. Therefore, the need to develop an exact and easy way to classify insect hemocytes still exists. Here we show that circulating hemocytes of the silkworm can be easily classified by fluorescence microscopy following staining with acridine orange and propidium iodide.
Materials and methods
Experimental animals
The pnd pS strain of the silkworm, Bombyx mori, was used for this experiment. Larvae were reared on an artificial diet and kept at 25°C under a 16-h light and 8-h dark photocycle. Larval age was given in days described by Kiguchi and Agui (1981) for larval molting stage and Kiguchi et al. (1985) for the fifth instar and metamorphic stages. Day 0 indicates the day when larval ecdysis occurs. Most of the fifth instar larvae begin to wander on day 5.
Hemocyte classification by light microscopy
An 8 μl volume of larval hemolymph was dropped onto a slide and covered with a coverslip. Following the standard criteria of hemocyte classification of the silkworm (Nittono 1960; Akai and Sato 1973; Beaulaton 1979; Wago 1991; Yamashita and Iwabuchi 2001), circulating hemocytes were first identified using a light microscope (Olympus, BH-2).
Use of acridine orange and propidium iodide to stain circulating hemocytes for classification by fluorescence microscopy
To maintain the physiological environment of hemocytes, larval insect saline (130 mM NaCl, 5.0 mM KCl, and 1.0 mM CaCl2; pH 7.0) was used to dissolve acridine orange and propidium iodide. To observe circulating hemocytes, 10 μl larval hemolymph was added to 2.5 μl acridine orange (10 μg/ml) and 2.5 μl propidium iodide (2 μg/ml) on Parafilm and mixed thoroughly. Next, 8 μl of this mixture was immediately dropped onto a slide, covered, and viewed with the appropriate fluorescence filters using a differential interference fluorescence microscope (Nikon Eclipse E600 System). Each type of hemocyte classified by fluorescence microscopy was classified again by differential interference contrast according to the above. To scan and image hemocytes, the Radiance 2000 confocal scanning laser microscope (BioRad) was used. Confocal fluorescent images were processed with the Lasersharp 2000 software. From the late fourth larval stage to the wandering stage, circulating hemocytes were checked daily.
Phagocytosis of foreign matter and necrotic cells
For better understanding of the phagocytic function, about 9×106 fluorescent microbeads [FluoSpheres Polystyrene Microspheres for Tracer Studies, 1.0 μm φ, Red (580/605); Molecular Probes] per gram larval body weight were injected into each larva (fifth larval stage) with a microliter syringe (25 μl; Hamilton) in order to allot approximately five to ten fluorescent microbeads to each circulating hemocyte. After about 6 h, when all free fluorescent microbeads had disappeared from the hemolymph, circulating hemocytes were classified by acridine orange and propidium iodide staining as described before.
To view necrotic cells inside hemocytes, hemocytes were released from five anterior hemopoietic organs into 100 μl Grace’s Insect Cell Culture Medium (Gibco BRL), opsonized with 10 μl silkworm hemolymph (Kawasaki 1989, 1995) and 5 μl propidium iodide, by mechanical shredding on a glass coverslip, and cultured in sealed dishes at 25°C for 20 min. The purpose of adding propidium iodide is to label naked nuclei before they are phagocytosed. Following incubation, hemocytes were dyed with acridine orange and propidium iodide and viewed as described before.
MTT test
Tetrazolium salt 3-(4,5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT; Dojindo) was diluted in Grace’s Insect Cell Culture Medium to make a 5 mg/ml solution. To test the activity of mitochondrial enzymes in hemocytes, a volume of 10 μl circulating hemolymph was added to 2.5 μl acridine orange (10 μg/ml) and 2.5 μl propidium iodide (2 μg/ml) on Parafilm, mixed, and then incubated for 3 min in order to ensure labeling of all hemocytes. Next, 20 μl MTT solution was added and mixed thoroughly. A sample of this hemolymph mixture (8 μl) was dropped on a slide and immediately observed and imaged using a differential interference fluorescence microscope (Nikon Eclipse E600 System). In the absence of detergent, the insoluble purple formazan crystals are confined inside the cells, making them appear purple. Hemocytes of larvae in the fifth larval stage were tested, but only data from larvae on day 2 (V-2) are presented due to the same results.
In situ apoptosis detection: the TUNEL method
Hemolymph pooled from at least ten individuals on day 0 of wandering stage (W-0), when the circulating hemocyte population is most abundant and easy to collect (Ling et al. 2003b), was centrifuged at 3,000 rpm for 5 min and precipitated cells were fixed in 4% paraformaldehyde for 20 min, embedded in paraffin, sectioned (5 μm in thickness), and fixed on slides coated with Biobond Tissue Section Adhesive (BB International). After deparaffinization and dehydration, hemocytes were stained using the In Situ Cell Death Detection kit, TMR Red (Roche). The procedure suggested by the manufacturer was followed carefully with a slight modification: samples were incubated in 5 μg/ml proteinase K (Wako) at 25°C for 15 min. After TUNEL labeling, slides were washed in PBS three times and counterstained with Hoechst 33342 (2 μg/ml) to stain the nuclei. Hemocytes were observed and imaged using a differential interference fluorescence microscope (Nikon Eclipse E600 System).
Results
Hemocyte types and morphology under the fluorescence microscope
After staining with acridine orange and propidium iodide, circulating hemocytes of the silkworm can be categorized into three groups based on their fluorescence characteristics. However, within each group, hemocytes can be further categorized based on more subtle differences.
Granulocytes and spherulocytes are characterized by bright green fluorescent granules in the cytoplasm
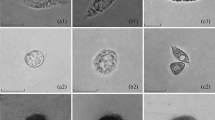
Granulocytes and spherulocytes stained with acridine orange contain many granules that emit bright green fluorescence when observed under a fluorescence microscope. In granulocytes, these green granules are irregularly shaped and vary in size: large and small ones can coexist in the same cell (Fig. 1A, C, E). In the silkworm, many granulocytes are so small that they are not easily distinguished from prohemocytes (Fig. 2B) by light microscopy. However, using fluorescence microscopy, even the very small granulocyte can be easily distinguished from the prohemocyte (compare Fig. 1C and Fig. 2A).
Morphology of granulocytes (A–F) and spherulocytes (G, H) using fluorescence microscopy (A, C, E, G) or differential interference contrast (B, D, F, H). Circulating granulocytes, which appear either a little irregularly shaped (A, B), round (C–F), or small (C, D), contain many green granules of different sizes (A, C, E). In spherulocytes, these green granules are generally larger and similar in size (G). Bar 10 μm
Morphology of prohemocytes (A, B) and plasmatocytes (C, D) using fluorescence microscopy (A, C) or differential interference contrast (B, D). Prohemocytes and plasmatocytes are stained a faint green by acridine orange (A, C). The nuclei of prohemocytes appear dark within the background of faint green fluorescence (A). However, nuclei are invisible in plasmatocytes that are irregularly shaped with extended pseudopodia (D). Within the nuclei of prohemocytes, one or two weak green fluorescent bodies are observed. Bar 10 μm
In circulating spherulocytes, green granules are almost similar in size and generally larger than those in granulocytes (Fig. 1G). By differential interference contrast, those granules in spherulocytes are clearly visible (Fig. 1H). However, in granulocytes they are not so obvious (Fig. 1B, D, F).
Prohemocytes and plasmatocytes stain faintly green and do not contain cytoplasmic green fluorescent granules
Following staining with acridine orange, prohemocytes and plasmatocytes can be easily distinguished from granulocytes and spherulocytes because they generally appear faint green and do not contain bright green fluorescent granules in their cytoplasm when observed by fluorescence microscopy (Fig. 2). Prohemocytes are round, and their nuclei are dark and clear within a background of faint green fluorescence (Fig. 2A). Inside the nucleus there are one or two small bright green fluorescent bodies, which are unlike the cytoplasmic granules observed in granulocytes and spherulocytes because of their locations.
Plasmatocytes are irregularly shaped and stain homogeneously faint green, making their nuclei invisible (Fig. 2C). When using a longer exposure time, we could also see the pseudopodium of plasmatocytes by fluorescence microscopy.
In summary, prohemocytes and plasmatocytes are stained a homogeneous faint green and do not contain bright green cytoplasmic granules. In addition, prohemocytes are characterized by bright green bodies in the nuclei. Thus, prohemocytes and plasmatocytes can be clearly distinguished from granulocytes and spherulocytes by staining with acridine orange.
Oenocytoid nuclei stain red with propidium iodide
Because of their fragile nature, oenocytoids can be stained with propidium iodide as we expected (Fig. 3A, D, G, H). Interestingly, oenocytoid nuclei also stained red even when the membranes appeared intact (Fig. 3A, G, H). A lot of circulating oenocytoids are easily disrupted and nuclei are expelled after bleeding (Fig. 3D–F). Some oenocytoids have double nuclei (Fig. 3H). Generally, these double nuclei are relatively small when compared to those shown in Fig. 3A, D, G.
Morphology of oenocytoids using fluorescence microscopy (A, D, G, H) or differential interference contrast (C, F). B is combined from A and C, and E from D and F. Most oenocytoids have intact membranes (A, G, H). Because of their fragile characteristics, the membranes of some oenocytoids rupture and their nuclei are expelled (E, F). Some oenocytoids were shown to have two nuclei (H). Bar 10 μm
Are hemocytes that are positive for acridine orange and propidium iodide living cells?
It is well known that acridine orange binds to the DNA of apoptotic cells and emits green fluorescence, and that propidium iodide binds the nuclei of necrotic cells and emits red fluorescence after being excited (Wyllie et al. 1998; Foglieni et al. 2001). Granulocytes and spherulocytes in question here may appear apoptotic because of the presence of bright green fluorescent granules after being stained with acridine orange (Fig. 1). Since oenocytoids are positive for propidium iodide staining (Fig. 3), these cells may be suggested to be necrotic. However, it is unlikely that all these cells are dead or dying all the time among other living circulating hemocytes. Clearly, the life and death of these cells needs further investigation.
Circulating hemocyte composition
To determine the frequencies of the different types of hemocytes in the fifth larval stage, we used the fluorescence method described above. From the fourth molting stage (IV-M) to the wandering stage (W-1), the relative number of oenocytoids and spherulocytes is small and fluctuates during the time tested (Fig. 4). The percentage of circulating prohemocytes begins to decrease after day 3 of the fifth larval stage (V-3). Throughout the fifth larval stage, the relative number of granulocytes is the highest compared to the other types of hemocytes (at least 60%). However, it would be unusual to sustain over 60% of apoptotic cells in circulating hemolymph every day if judged according to the presence of acridine orange-positive green granules.
Circulating hemocyte composition. Hemocytes of larvae from IV-M (fourth molting stage) to W-1 (day 1 of the wandering stage) were stained with acridine orange and propidium iodide and classified as described before. Over 60% of circulating hemocytes are granulocytes that are positive for acridine orange staining during the fifth larval stage. PL Plasmatocyte, SP spherulocyte, OE oenocytoid, GR granulocyte, PR prohemocyte
Phagocytosis activities in granulocytes
Fluorescent microbeads were introduced into larvae during the fifth larval stage by injection. After 6 h, most granulocytes positive for acridine orange had phagocytosed the microbeads (Fig. 5A–C). This experiment proves that most of the granulocytes positive for acridine orange are not apoptotic at all because it would be impossible for any apoptotic cells to carry out phagocytosis.
Phagocytosis of fluorescent microbeads by circulating granulocytes. The fluorescent microbeads were introduced into larvae by injection. Hemocytes were serially scanned under the Radiance 2000 confocal scanning laser microscope (BioRad) with a step of 1 μm. Only one section was printed. The acridine orange-positive granulocyte (A–C) can phagocytose the fluorescent microbeads. Bar 20 μm
Few TUNEL-positive dead cells in the circulating hemocytes
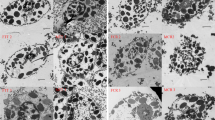
Circulating hemocytes can also become aged and die, as do other cells. To determine the frequency of cell death in hemocytes, we specifically labeled the ends of broken DNA, which is indicative of apoptosis, by TUNEL labeling. According to the result of this assay, TUNEL-positive apoptotic small particles (arrowheads in Fig. 6B, C) were often observed dispersing in the cells with TUNEL-negative nuclei (arrows in Fig. 6A, B). However, few hemocytes with TUNEL-positive nuclei were observed, suggesting that they might be immediately phagocytosed by other hemocytes.
Morphology of phagocytosed necrotic cells inside hemocytes
Since oenocytoids are positively stained red by propidium iodide, they are probably necrotic. Necrotic cells are of course able to be eliminated by other living hemocytes. However, it is difficult to identify them visually because there are no standard criteria of morphology for reference. To view necrotic cells inside hemocytes after being phagocytosed, hemocytes released from hemopoietic organs were cultured for 20 min, after adding propidium iodide. Following incubation, hemocytes were stained with acridine orange and propidium iodide and viewed as described before. A propidium iodide-labeled isolated nucleus is shown being phagocytosed by another hemocyte (Fig. 7A–C). The phagocytosed part, contained within the hemocyte, cannot be stained by acridine orange at all and appears dark when using a green fluorescence filter to view them (Fig. 7A). Therefore, hemocytes that have phagocytosed necrotic cells can be easily identified. However, among circulating hemocytes, we never found any hemocytes that have large dark areas, except their nuclei, under the fluorescence microscope in the normal larvae.
Morphology of necrotic cells inside hemocytes. The image in the middle is combined from those imaged with green (left) and red (right) filters. Naked nuclei were produced by mechanically shredding hemopoietic organs followed by immediate staining with propidium iodide (B, C). The naked nucleus (arrowheads) does not stain with acridine orange and appears dark when being viewed with a green fluorescence filter (A). Arrows point to a free oenocytoid that was released from hemopoietic organs. Bar 20 μm
Mitochondrial enzyme activity in oenocytoids
We applied an MTT assay to verify that the propidium iodide-positive cells are indeed living hemocytes instead of necrotic cells. As is well known, MTT is broken down in metabolically active cells to form insoluble purple formazan crystals through the activity of mitochondrial enzymes (Mosmann 1983), providing an assay by which to identify living cells. In this assay, the large oenocytoid with a propidium iodide-positive nucleus turned purple (Fig. 8A, B), indicating that they are not necrotic. Similarly, granulocytes also showed mitochondrial enzyme activity (Fig. 8A, B).
Morphology of oenocytoid (OE) and granulocyte (GR) after being incubated with MTT. Both the propidium iodide-positive oenocytoid (suggested by red nucleus in B) and acridine orange-positive granulocyte (suggested by green fluorescent granules in B) can cleave MTT into insoluble purple formazan crystals, which makes them appear purple (A). Bar 10 μm
Discussion
The combination of acridine orange and propidium iodide is an efficient method to study apoptosis and necrosis (Cañete et al. 2001; Foglieni et al. 2001). However, we found that they can also be used to classify circulating hemocytes of the silkworm, Bombyx mori. When stained with acridine orange, granulocytes and spherulocytes are easily distinguished from prohemocytes and plasmatocytes, based on the presence of bright green fluorescent granules in the cytoplasm. The latter two types of hemocytes do not contain bright green fluorescent granules in their cytoplasm. The dark nuclei of prohemocytes are clearly visible within the background of faint green fluorescence, although the green granules inside the nuclei of prohemocytes are unknown structures. The irregular morphology and the absence of green fluorescent cytoplasmic granules make plasmatocytes easily distinguishable from the other types of hemocytes.
Since acridine orange binds to the DNA of apoptotic cells, one may wonder whether the hemocytes positive for acridine orange are dead or dying cells. If so, the circulating granulocytes stained with acridine orange, which form the majority (almost 60% or more) of all hemocytes in the silkworm (Fig. 4), would be apoptotic. However, it seems unlikely that over half of the circulating hemocyte population is apoptotic. In fact, granulocytes in circulation that have green granules were shown to phagocytose injected fluorescent microbeads (Fig. 5A–C). Obviously, it would be impossible for apoptotic cells to phagocytose foreign particles. Moreover, the TUNEL assay showed that TUNEL-positive small particles (arrowheads in Fig. 6C) can be phagocytosed and accumulated in TUNEL-negative hemocytes (arrows in Fig. 6B).
Oenocytoids are easily classified even under the light microscope because of their relatively large morphology, fragile nature, and opaque appearance when compared to other hemocytes (Wago 1991). We showed that the nuclei of oenocytoids are stained red with propidium iodide so that oenocytoids are easily distinguished from all other types of hemocyte under the fluorescence microscope.
Based on the clear morphological characteristics of oenocytoids under the light microscope, these propidium iodide-positive hemocytes were confirmed to be oenocytoids. Since propidium iodide binds to the nuclei of necrotic cells, we wonder whether these positive nuclei for propidium iodide are necrotic. However, MTT assays showed that the in vitro oenocytoids can still cleave the chemical into formazan crystals, which makes them appear purple shortly after incubation (Fig. 8). Obviously, oenocytoids have a strong mitochondrial enzyme activity, which is typical of living cells (Wyllie et al. 1998). In addition, when checking larvae of different generations, oenocytoids are consistently positive for propidium iodide staining in every generation (data not shown). Thus, oenocytoids with nuclei positive for propidium iodide are living cells in circulation. Now, all five types of circulating hemocytes in the silkworm, B. mori, can be easily classified by fluorescence microscopy following staining with acridine orange and propidium iodide.
In the silkworm, B. mori, classification of hemocytes has historically been based on their morphology at the light microscope level (Iwasaki 1930; Nittono 1960). Although more precise characterization of each type of hemocyte at the electron microscope level was also done (Akai and Sato 1973), classification of a mass of hemocytes still has to depend upon light microscope examination. When observed under the light microscope, distinguishing between some granulocytes and prohemocytes is not always easy, especially when they are almost the same in size. Moreover, oenocytoids become fragile following bleeding (Wago 1991), and often release their nuclei out of the cells. Again it is also confusing to discriminate between prohemocytes and the nuclei released from oenocytoids since they are roughly the same shape and size. Our method described above is extremely useful for such discrimination of peculiar cells that are difficult to identify by ordinary light microscope observation. However, our work also suggests that the use of chemicals for studying hemocyte death and life should be done in a careful manner. Phagocytosed dead cells in granulocytes and nuclei of oenocytoids stained red by propidium iodide may be mistaken for apoptotic or necrotic cells, thereby compromising the judgment.
Our primary interest is to understand how regeneration and differentiation occur in the heavy ion irradiated hemopoietic organs of the silkworm. In a previous paper, we showed that the hemopoietic organs can regenerate even after irradiation with heavy ions at a high dose of more than 100 Gy in the silkworm, B. mori (Ling et al. 2003a). This is an interesting and convenient phenomenon to study not only the possible involvement of hemopoietic stem cells but also their differentiation process. We are currently investigating the process and the regeneration mechanism using the fluorescence method for the classification of hemocytes.
Finally, the fluorescence method we described here can be modified to be used with other kinds of insect because of the similarities in the functioning of the immune system among insects.
References
Akai H, Sato S (1973) Ultrastructure of the larval hemocytes of the silkworm, Bombyx mori L. (Lepidoptera: Bombycidae). Int J Insect Morphol Embryol 2:207–231
Arnold JW (1979) Controversies about hemocyte types in insects. In: Gupta AP (ed) Insect hemocytes. Cambridge University Press, Cambridge, UK, pp 231–258
Beaulaton J (1979) Hemocytes and hemocytopoiesis in silkworms. Biochimie 61:157–164
Cañete M, Juarranz A, López-Nieva P, Alonso-Torcal C, Villanueva A, Stockert JC (2001) Fixation and permanent mounting of fluorescent probes after vital labelling of cultured cells. Acta Histochem 103:117–126
Carton Y, Nappi AJ (1997) Drosophilia cellular immunity against parasitoids. Parasitol Today 13:218–227
Carton Y, Nappi AJ (2001) Immunogenetic aspects of the cellular immune response of Drosophilia against parasitoids. Immunogenetics 52:157–164
Chain BM, Anderson RS (1983) Observations on the cytochemistry of the hemocytes of an insect, Galleria mellonella. J Histochem Cytochem 31:601–607
Chain BM, Leyshon-Sørland K, Siva-Jothy MT (1992) Haemocyte heterogeneity in the cockroach Periplaneta americana analysed using monoclonal antibodies. J Cell Sci 103:1261–1267
Foglieni C, Meoni C, Davalli AM (2001) Fluorescent dyes for cell viability: an application on prefixed conditions. Histochem Cell Biol 115:223–229
Gardiner EMM, Strand MR (1999) Monoclonal antibodies bind distinct classes of hemocytes in the moth Pseudoplusia includens. J Insect Physiol 45:113–126
Glupov VV, Khvoshchevskaya MF, Shchepetkin IA, Kryukova NA (1997) Morphofunctional structure of the hemocyte population in Galleria mellonella L. (Lepidoptera: Pyralidae) during infection. Biol Bull 24:529–536
Hillyer JF, Christensen BM (2002) Characterization of hemocytes from the yellow fever mosquito, Aedes aegypti. Histochem Cell Biol 117:431–440
Hillyer JF, Schmidt SL, Christensen BM (2003) Hemocyte-mediated phagocytosis and melanization in the mosquito Armigeres subalbatus following immune challenge by bacteria. Cell Tissue Res 313:117–127
Hoffmann JA, Reichhart JM, Hetru C (1996) Innate immunity in higher insects. Curr Opin Immunol 8:8–13
Inoue N, Hanada K, Tsuji N, Igarashi I, Nagasawa H, Mikami T, Fujisaki K (2001) Characterization of phagocytic hemocytes in Ornithodoros moubata (Acari: Ixodidae). J Med Entomol 38:514–519
Iwasaki Y (1930) Researches on the larval blood corpuscles of Bombyx mori and nine other Lepidoptera. Bull Kagoshima Imp Coll Agr Forest 8:172–284
Kawasaki H (1989) Methods for culture of Bombyx mori wing discs. J Tissue Culture Methods 12:31–33
Kawasaki H (1995) Ecdysteroid concentration inducing cell proliferation brings about the imaginal differentiation in the wing disc of Bombyx mori in vitro. Dev Growth Differ 37:575–580
Kiguchi K, Agui N (1981) Ecdysteroid levels and developmental events during larval moulting in the silkworm, Bombyx mori. J Insect Physiol 27:805–812
Kiguchi K, Agui N, Kawasaki H, Kobayashi M (1985) Developmental time-table for the last larval and pharate pupal stages in the silkworm, Bombyx mori, with special reference to the correlation between the developmental events and haemolymph ecdysteroid levels. Bull Sericult Exp Sta 30:83–100
Lackie AM (1988) Immune mechanisms in insects. Parasitol Today 4:98–105
Lavine MD, Strand MR (2002) Insect hemocytes and their role in immunity. Insect Biochem Mol Biol 32:1295–1309
Ling E, Fukamoto K, Xu S, Shirai K, Kanekatsu R, Kobayashi Y, Tu Z, Funayama T, Watanabe H, Kiguchi K (2003a) Regeneration of hemopoietic organs in the silkworm, Bombyx mori, after locally targeted irradiation with heavy ion beams. J Insect Biotechnol Sericol 72:95–100
Ling E, Shirai K, Kanekatsu R, Kobayashi Y, Tu Z, Funayama T, Watanabe H, Kiguchi K (2003b) Why does hemocyte density rise at the wandering stage in the silkworm, Bombyx mori? J Insect Biotechnol Sericol 72:101–109
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Mullett H, Ratcliffe NA, Rowley AF (1993) The generation and characterisation of anti-insect blood cell monoclonal antibodies. J Cell Sci 105:93–100
Nittono Y (1960) Studies on the blood cells in the silkworm, Bombyx mori, L. Bull Sericult Exp Sta 16:171–266
Shirae M, Saito Y (2000) A comparison of hemocytes and their phenoloxidase activity among Botryllid ascidians. Zool Sci 17:881–891
Strand MR, Johnson JA (1996) Characterization of monoclonal antibodies to hemocytes of Pseudoplusia includens. J Insect Physiol 42:21–31
Wago H (1991) Phagocytic recognition in Bombyx mori. In: Gupta AP (ed) Immunology of insects and other arthropods. CRC Press, Boca Raton, pp 215–235
Willott E, Trenczek T, Thrower LW, Kanost MR (1994) Immunochemical identification of insect hemocyte populations: monoclonal antibodies distinguish four major hemocyte types in Manduca sexta. Eur J Cell Biol 65:417–423
Wyllie A, et al (1998) Apoptosis and cell proliferation, 2nd edn. Boehringer Mannheim, Biochemica
Yamashita M, Iwabuchi K (2001) Bombyx mori prohemocyte division and differentiation in individual microcultures. J Insect Physiol 47:325–331
Acknowledgements
We thank Dr. A. Teramoto, Shinshu University, for his guidance on using the Radiance 2000 confocal scanning laser microscope.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ling, E., Shirai, K., Kanekatsu, R. et al. Classification of larval circulating hemocytes of the silkworm, Bombyx mori, by acridine orange and propidium iodide staining. Histochem Cell Biol 120, 505–511 (2003). https://doi.org/10.1007/s00418-003-0592-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-003-0592-6