Abstract
The presence and changes of estradiol nuclear binding and related functions in uterine luminal and glandular epithelium were studied before and after blastocyst implantation using receptor autoradiography with 3H-estradiol-17β in association with 3H-thymidine incorporation and immunocytochemical binding of antibody to estrogen receptor ER-α. 3H-estradiol nuclear binding is present but variable during days 1.5–7.5 of pregnancy. Sites of strong nuclear binding of 3H-estradiol exhibit strong immunocytochemical staining with ER-α antibody. Qualitative and quantitative evaluation of autoradiograms reveal that there is a general increase of nuclear 3H-estradiol binding during the first 3 days after fertilization in both luminal and glandular epithelium. The binding of estradiol is stronger in glandular epithelium from day 2.5 to day 7.5, paralleled by a rise in 3H-thymidine incorporation on day 2.5. By comparison, in the epithelium of the uterine lumen 3H-estradiol nuclear binding is low, but relatively high in epithelial cells at lateral branching of the lumen where the increase in 3H-estradiol binding corresponds to an increased labeling index with 3H-thymidine. A highly differentiated binding of 3H-estradiol to luminal and glandular epithelium was demonstrated with region- and time-specific changes of related effects on cell proliferation, differentiation, and secretion, probably involving involution and remodeling. The strong 3H-estradiol binding to glandular epithelium suggests that estradiol exerts pronounced effects on glandular activities in the periimplantation period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
After conception, extensive morphological and functional changes take place in the uterus, which can be followed through the preimplantation and periimplantation stages (review in Abrahamshon and Zorn 1993). Estradiol is one of the ovarian hormones that play an important role in the proliferation, differentiation, and function of uterine cells during early pregnancy (for review see Weitlauf 1994).
Estradiol acts on the uterine luminal and glandular epithelia by promoting changes in cell shape and cell surface components, on the number and shape of glands, and the glandular–stromal ratio. Moreover, estradiol induces synthesis of many secretory molecules and may facilitate their secretion (Martin et al. 1973; Weitlauf 1994; Chen et al. 1999).
The action of estradiol is mediated through intracellular estrogen receptors (ERs), which regulate gene transcription via the estrogen-responsive element (Mangelsdorf et al. 1995). Estrogen receptors have been localized by "in situ" radiolabeled hormone binding and by immunohistochemistry in the epithelial, stromal, and myometrial compartments of the uterus in different species (Stumpf 1968; Prasad et al. 1974, 1976; Sartor 1977; Ward et al. 1978; Ennis and Stumpf 1988; Yamashita et al. 1989; Brenner et al. 1991; Li et al. 1992; Tibbets et al. 1998; Tan et al. 1999; Tessier et al. 2000). Each one of these studies was focused on a particular stage of pregnancy so that the picture of the period of pregnancy remained fragmented.
Changes in ER levels in the endometrium during early pregnancy and pseudopregnancy have been quantified by biochemical methods (Talley et al. 1977; Ward et al. 1978; Logeat et al. 1980; Martel and Psychoyos 1981; Moulton and Koenig 1981). In most cases, however, the ER quantitative evaluations have been made with the whole uterus and neither the dynamic morphological adaptation of the pregnant uterus nor the ER distribution in different compartments or individual cellular populations of the endometrium were considered (Martel and Psychoyos 1981; Weitlauf 1994). This may explain the difficulties in establishing a comprehensive relationship between the ER concentration and modifications observed in the different compartments of the uterus during the postfertilization and blastocyst implantation periods.
In this study, we followed the distribution and changes in ER levels in the mouse uterine luminal and glandular epithelium during the preimplantation and periimplantation periods. The binding of 3H-estradiol to nuclear receptor was demonstrated by receptor microautoradiography. 3H-estradiol nuclear binding was characterized by time-related topographic and quantitative analysis of autoradiograms, by comparisons with patterns of 3H-thymidine incorporation, as well as the localization of antibody to ER-α. Receptor microautoradiography has been applied because of its high sensitivity and resolution, its quantitative nature, and its informative value demonstrated in previous studies with radiolabeled estradiol and other steroid hormones (Stumpf et al. 1981; Shughrue et al. 1992).
Materials and methods
Animals and tissue preparation
Forty-nine adult virgin female Swiss albino mice, aged 3–4 months, ranging in weight from 30 to 37 g, were used. The animals were housed in a 12 h/12 h light/dark schedule at 22°C, with food and water available at all times. To know the precise time of pregnancy, females were mated during a 2-h period and then examined for copulation plugs. When plugs were found, this was considered zero hour postcoitum and the beginning of pregnancy. Animals at 1.5, 2.5, 3.5, 4.5, 5.5, 6.5, and 7.5 days of pregnancy were used, with at least three each for receptor microautoradiographic studies and two each for immunocytochemical studies. The chemical identity of nuclear radioactivity as being 3H-estradiol has been established in previous experiments (Stumpf 1971; Martel and Psychoyos 1981; Moulton and Koenig 1981). National and international principles of laboratory animal care were followed, and experiments were approved by the Institute of Biomedical Sciences' Animal Ethics Committee.
Autoradiography
3H-estradiol localization
[2,4,6,7,16,17-3H]–estradiol-17β (E2–3H), specific activity 140 Ci/mMol (New England Nuclear), was dissolved in ethanol–water 1:10 and, under ether anesthesia, injected into the tail vein, 0.2 μg/100 g body weight. Mice were killed 1 h afterwards. Uterine horns were excised and transversal and longitudinal fragments of 1–2 mm length were placed on tissue holders and freeze-mounted by immersion into isopentane cooled by liquid nitrogen. Four-micron frozen sections were cut in a cryostat (Microm 500) and thaw-mounted on emulsion-coated slides (Stumpf 1971). The mounted slides were stored in a desiccator box at −15°C for 35, 70, or 100 days. Short-exposure (35-day) autoradiograms were used for silver grain counting, and long-exposure autoradiograms for qualitative and low-magnification surveys. At the end of exposure, slides were fixed in buffered 2% paraformaldehyde for 30 s, then photographically processed, and stained with methyl green–pyronin, specific for RNA (red) and DNA (blue-green).
Quantitative evaluation of autoradiograms with 3H-estradiol
Silver grains over nuclei were counted interactively with a Computer System Pro Plus Scan connected to a Nikon photomicroscope fitted with a digital camera at 100× magnification.
During the process of embryo implantation, the uterine lumen changes markedly. Consequently, the epithelial cells that line the uterine crypt usually die by apoptosis. Because of this fact, quantitative evaluation was done only in the period in which the luminal epithelium was still present, from day 1.5 to day 4.5 of pregnancy.
For quantitation, regions of the luminal and glandular epithelium were selected from transverse sections according to their position in the uterus (see schematic representation of the selected areas in Fig. 1). Before counting, in the selected regions nuclear areas of individual cells were determined and the mean nuclear area was assessed. For silver grain counting, only nuclei with an area above the mean were selected. For each epithelial region, at least ten nuclei were evaluated per section in three slides each per animal in three animals. Silver grains over nuclei were counted, entered, and semiautomatically evaluated with a computer program.
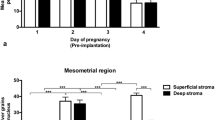
Schematic representation of a transverse section of uterine horns. Days 1–3 (a) and day 4 of pregnancy (b). M Mesometrial extensions of the lumen, AM antimesometrial extension of the lumen, SR straight region of the central part of the lumen, B branched extensions, SL glands in subluminal location, DS glands in a deep stromal location, E embryo
3H-thymidine incorporation
3H-thymidine (New England Nuclear), specific activity 60 Ci/mM, dissolved in distilled water, was injected i.p. 1 μCi/g body weight into two mice at the same periods of pregnancy as described above. One hour after the injection, the animals were killed and uterine tissues dissected and immersion-fixed in Methacarn (methanol, chloroform, glacial acetic acid; 6:3:1) for 3 h, washed, dehydrated, and embedded in Paraplast. Five micron sections were placed on glass slides, deparaffinized, hydrated, and then dipped in liquefied nuclear emulsion, air-dried and placed in desiccator boxes for exposure in a refrigerator. After exposure for 60 days, slides were photographically processed, stained with methyl green–pyronin and coverslipped.
The regional distribution of 3H-thymidine-labeled cells was compared to that of 3H-estradiol-labeled cells. The comparison was based on a qualitative analysis of the patterns of regional labeling without counting individual cells.
Immunohistochemistry
Affinity-purified rabbit polyclonal antibody for mouse ER-α isoform (MC-20; Santa Cruz Biotechnology, USA) was raised against a peptide mapping the C-terminus of the ER-α of mouse origin.
Small pieces of uterus were take from pregnant animals at the same stages mentioned for 3H-estradiol localization. Samples were fixed with Methacarn fixative, rinsed with absolute ethanol, and embedded in Paraplast. Five-micrometer sections were cut (Microm HM-200), adhered to glass slides with 0.1% poly-l-lysine (Sigma, USA), and then air-dried at room temperature. Each of the succeeding steps was followed by a thorough rinse with PBS.
All steps were performed in a humid chamber at room temperature and care was taken to avoid drying. Sections were treated with 3% H2O2 (Merck) in PBS for 30 min to block endogenous peroxidase activity. Non-specific reaction was blocked by incubating the sections for 30 min with normal goat serum diluted 1:1 in PBS-10% bovine serum albumin followed by incubating overnight with undiluted Super Block (Pierce) blocking buffer. After this, sections were incubated overnight at 4°C in primary antibody ER-α diluted 1:100 in PBS–0.3% Tween 20. Sections were then washed thoroughly with PBS followed by incubation with biotinylated goat anti-rabbit IgG (Vector) diluted 1:100 in PBS for 1 h at room temperature. After rinsing in PBS, sections were treated with Vectastain ABC kit (Vector) for 1 h at room temperature. The reaction was visualized using 0.03% 3,3′-diaminobenzidine (Sigma) plus 0.03% H2O2 in PBS. Control sections were prepared omitting the primary antibody. After immunostaining, sections were lightly stained with Mayer's hematoxylin and examined with a Nikon Eclipse E800, and images were captured with Image Pro Plus software (Media Cybernetics, L.P. Maryland, USA) and subsequently printed with an Epson Stylus 777.
Results
Morphology and 3H-estradiol nuclear distribution
Preimplantation period
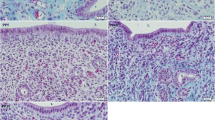
In this period, a single layer of tall columnar epithelium lined the uterine lumen. On day 1.5 of pregnancy, the uterine lumen was very branched and distended and contained cell debris and mucus, and the cells displayed many luminal protrusions of apical cytoplasm (Fig. 2a). On this day and on day 2.5, the apical region of the epithelium was intensely pyronin (RNA)-positively stained (Fig. 2a, b). Qualitative and quantitative evaluations showed that the nuclear concentration of 3H-estradiol was weak on day 1.5 and increased from day 2.5 to day 3.5 of pregnancy (Figs. 2a–c, 4a). The binding of 3H-estradiol was always conspicuous in certain areas of the epithelial layer, particularly in the extremities of luminal branches (Figs. 2a, 4a). On day 3.5 the increases in the 3H-estradiol binding by luminal epithelial cells situated in the mesometrial pole of the uterus were notable (Figs. 2c, 4a).
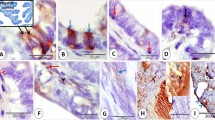
3H-estradiol concentration on days 1.5 (a, b), 2.5 (c, d), and 3.5 (e, f) after fertilization. In the luminal epithelium, nuclear labeling is higher at luminal branches and at mesometrial and antimesometrial extensions (a, c, e). The concentration of 3H-estradiol is higher in glands than in luminal epithelium (a–f). Among glands, epithelial height, diameter of lumen, and nuclear silver grain density vary (c, d, f). Asterisks indicate low-labeled glandular profiles. G Gland, GD glandular duct, L uterine lumen, bv blood vessel. Exposure times: 70 days for c–f; 100 days for a, b
Glands were numerous and well developed particularly on day 1.5 of pregnancy. On this day and in the following days, the majority of the glands were present in the middle and antimesometrial stroma. Qualitative and quantitative evaluation showed that nuclear concentration of radioactivity was clearly stronger in the glands than in the luminal epithelium throughout that period (Figs. 2a–f, 4a, b). Moreover, 3H-estradiol binding increased from day 1.5 to day 2.5 (compare Fig. 2d and e, and see Fig. 4b). In serial sections, the heterogeneity of uterine glands was apparent. Some of these profiles seemed to indicate strong secretory activity. Some of the glands were strongly distended with low cuboidal epithelium, while some of the larger glands even had squamous epithelium. The retention of 3H-estradiol was likewise heterogeneous among the various glandular profiles particularly on day 3.5 (Fig. 2f). Squamous epithelial cells observed mainly on day 1.5, sometimes were weakly labeled or unlabeled. On the contrary, on day 2.5 many intensely labeled glandular segments were observed near or in contact with the uterine lumen (Fig. 2b). Qualitative and quantitative evaluation showed that on days 1.5 and 2.5 nuclear labeling was stronger in the glands situated in the deep stroma than in the glands situated in the superficial stroma (Fig. 4b)
Periimplantation period
On day 4.5 of pregnancy, a few hours before the embryo implantation starts, the architecture of the uterine lumen changed markedly. On this day, no branched extensions were observed in the uterine lumen and blastocysts were lodged in deep crypts formed into the antimesometrial stroma (Fig. 3). Qualitative and quantitative evaluation showed that the 3H-estradiol binding in the epithelium decreased compared to that on day 3.5. The binding was very low, particularly in epithelial cells contacting trophoblast cells and increased toward the mesometrial region of the lumen (Figs. 3, 4a).
3H-estradiol concentration on day 4.5 of pregnancy is very low in the epithelial cells lining the implantation crypt and progressively increases toward the mesometrial extension of the lumen. The concentration of 3H-estradiol is higher in glands than in luminal epithelium. Note variations among individual cells and changes along the course of the gland. G Gland, E embryo, M mesometrial region. Exposure time: 70 days
Quantitative evaluation of autoradiograms. Silver grain counts over luminal (a) and glandular (b) epithelial cell nuclei at days 1.5–4.5 of pregnancy from three animals per time period and the mean (±SD) of over 30 cells per epithelial region per animal. M Mesometrial extensions, AM antimesometrial extensions, SR straight region of the central part of the uterine lumen, B branched extensions, SL glands in the subluminal stroma, DS glands in the deep stroma, d day
Glands were in general smaller compared to the previous day and formed by cuboidal epithelium. Glands were very few in the implantation sites (IS; Fig. 3) and abundant in the interimplantation sites (IIS). Although smaller, the glandular cells showed generally strong pyronin-positive staining. The binding of 3H-estradiol decreased compared to that on days 2.5 and 3.5 (Fig. 4b), however, strongly labeled glandular segments crossed the decidualized area of the stroma and established contact with a very weakly labeled luminal epithelium (Fig. 3). No significant difference was observed in the 3H-estradiol binding when glands situated in the superficial stroma were compared with those in the deep stroma (Fig. 4b)
On day 5.5 the blastocysts were already implanted into uterine crypts (Fig. 5a). In serial longitudinal sections of the uterine horns, the uterine lumen displayed multiple folds at the mesometrial pole, from which a main crypt lodging the implanted embryo was formed toward the antimesometrial region (Fig. 5a, b). After the embryo implantation, the surface of the implantation crypt was devoid of epithelial layer, whereas the epithelium was maintained at the mesometrial pole of the IS and in the lumen of the IIS. In these regions, the luminal epithelial cells were low-cubical. 3H-estradiol binding was strong in the luminal epithelium of IIS, moderate in the mesometrial region of IS, and decreasing markedly toward the implantation crypt (Fig. 5a, b). No binding of 3H-estradiol was observed in the embryo.
On day 5.5 of pregnancy (a–d) 3H-estradiol concentration in the luminal epithelium varies according to the position in the uterine horn. It is diminished toward the implantation chamber, but increased toward the mesometrium and the lateral interimplantation regions as seen in the longitudinal sections (a). Enlargement of the region in the rectangle (b). Strongly labeled ducts of glands cross the decidualized endometrium toward the embryo (c). Glands in the predecidua are small and show distinct degree of 3H-estradiol binding (d). On day 7.5 of pregnancy (e, f) 3H-estradiol concentration in the luminal epithelium is strong at the mesometrial pole of the uterus with a gradient of decreased labeling toward the implantation crypt (e). The luminal epithelium is strong at the surface of the interimplantation sites but weak or abolished along the adjacent decidua (f). IIS Interimplantation site, IS implantation site, E embryo, G gland, D mature decidua, PD predecidua, bv blood vessel, M mesometrial region, L uterine lumen, NL new lumen. Exposure time: 100 days
In the IS, glands were sparse or mostly absent in decidualized areas of the endometrial stroma. Examination of serial sections, however, showed long glandular tubes that cross the entire extension of the stroma and terminate very close to the implantation chamber (Fig. 5a, c). Glands situated in the mature decidua and predecidua were always strongly labeled (Fig. 5c, d). A small number of glands was present in the non-decidualized stroma situated near the myometrium. These glands were tortuous and morphologically heterogeneous showing a low binding of 3H-estradiol.
On days 6.5 and 7.5, in general, both the morphology of the uterus as well as the 3H-estradiol binding and distribution were similar. The majority of epithelial cells continued to retain 3H-estradiol. However, there were notable differences regarding the 3H-estradiol binding between epithelial sheets situated at different places of the uterus. In both IS and IIS the labeling by 3H-estradiol was higher at the mesometrial end of the uterine lumen (Fig. 5e). On day 7.5, serial longitudinal sections of the uterus clearly showed the formation of the new lumen, crossing the uterine wall from the mesometrial to antimesometrial direction and running parallel to the interface between IIS and IS (Fig. 5f). In the new lumen, the epithelial layer that covers the IIS side was strongly labeled, while the epithelial layer of the opposite side in contact with decidua was only weakly labeled (Fig. 5f). The number of glands decreased progressively from day 5.5 to day 7.5 in both IIS and IS. In the regions of the decidua and predecidua, glands were sparse and inconspicuous and, because of the alterations of the glandular epithelium, may not be recognizable, except in serial sections that permitted to follow them and recognize their connections. The nuclear labeling in glands, however, continued to be higher than in luminal epithelium.
Comparison between 3H-estradiol nuclear binding and 3H-thymidine incorporation
On day 1.5, exclusively epithelial cells of the luminal epithelium incorporated 3H-thymidine. Similar to 3H-estradiol, tritiated thymidine incorporation was present mostly in epithelial cells situated in the extremities of the branched areas of the uterine lumen (Fig. 6a). On day 2.5, besides the luminal epithelium a few cells of the uterine glands were also labeled (Fig. 6b). On day 3.5, 3H-thymidine incorporation stopped in both luminal and glandular epithelium and started in the stromal compartment (Fig. 6c). This pattern of 3H-thymidine incorporation was maintained during days 4.5, (Fig. 6d), 5.5, and 6.5. On day 7.5 3H-thymidine incorporation was observed in luminal epithelial cells and corresponded to that of 3H-estradiol binding. In both IS and IIS the number of labeled cells was highest in the mesometrial end of the uterine lumen (Fig. 6e). At the IIS site, similar to 3H-estradiol, the epithelial layer that covers the new lumen was strongly labeled with 3H-thymidine while in the epithelial layer in contact with the decidua, only rare cells were labeled (Fig. 6f). In glands, no incorporation of 3H-thymidine was observed whereas glandular cells maintained a sustained binding of 3H-estradiol during all periods.
Autoradiograms of endometrium of pregnant mice 1 h after injection of 3H-thymidine. Paraffin sections stained with methyl green–pyronin. On day 1.5 of pregnancy, only luminal epithelial cells are labeled. Note a concentration of labeled cells in the branched areas of the lumen (a). On day 2.5, both luminal epithelium and glandular epithelium are labeled (b). On day 3.5, many stromal cells are labeled while both luminal epithelium and glandular epithelium are unlabeled (c). On day 4.5, labeled cells continue to be observed only in the endometrial stroma (d). On day 7.5, at the mesometrial pole, many luminal epithelial cells are labeled (e). Epithelial cells lining the surface of decidua at the IS are not labeled while most of the epithelial cells lining the IIS surface are labeled (f). L Uterine lumen, G uterine glands, E embryo, IS implantation site, IIS interimplantation site. Exposure time: 60 days
Comparison between 3H-estradiol nuclear binding and immunolocalization of ER-α
The immunocytochemical result of ER-α distribution was in general similar to that obtained for 3H-estradiol binding using autoradiography. Positive reaction for ER-α was observed in both luminal and glandular epithelium during all days of pregnancy. Figure 7 shows a comparison between immunocytochemistry and autoradiographs of uteri on day 3.5 of pregnancy.
Comparison between autoradiograms of 3H-estradiol injection (a, c, e) and immunocytochemistry for estrogen receptor (ER)-α (b, d, f). At low magnification, no differences are observed between the distribution of 3H-estradiol and ER immunolocalization. In both images, glands appear strongly labeled while the luminal epithelium and stroma appears negative (a, b). High magnification autoradiograms (e), however, clearly show that the 3H-estradiol binding occurs in almost all cells of the luminal epithelium, some of which remain unstained with antibody (f). L Uterine lumen, G uterine glands. Exposure time: 70 days
The strongest radiolabeled glands also were strongly immunopositive, and the luminal epithelium with relatively weak radiolabeling was also weakly immunopositive. When results of immunocytochemistry were compared with those of autoradiography, differences in sensitivity and resolution became apparent. For instance: (a) immunocytochemistry did not reveal subtle but clear differences between superficial and deep glands, (b) immunocytochemistry did not reveal differences among different regions of the luminal epithelium, and (c) immunocytochemistry showed unstained nuclei in the luminal epithelium that appeared labeled with 3H-estradiol.
Discussion
For the identification of in vivo hormone target tissues, receptor microautoradiography provides cellular–subcellular resolution and high sensitivity that is impossible or difficult to achieve by biochemical and immunocytochemical approaches alone. After injection of tritium-labeled estradiol with high specific activity, sites of uptake and binding can be identified, qualitatively and quantitatively, and then further characterized. The localized radioactivity reflects the acting hormone that binds to specific nuclear receptors with different affinities and capacities related to tissue-specific functions (Stumpf 2003).
After injection of radiolabeled estradiol-17β, uptake and retention of the hormone occurs in cell nuclei of specific uterine tissues with varying intensities and changes during the pre- and periimplantation periods of the embryo. These changes are complex and characteristic not only for each tissue, but also within the same tissue according to its location, its association with other tissues, predominantly the blastocyst, and the regions of the mesometrium and antimesometrium. In the autoradiograms, nuclear uptake of 3H-estradiol is demonstrated and substantiated by quantitative evaluation. In all of these target cell populations, 3H-estradiol uptake increases during the first 3 days after fertilization. Throughout the whole period the 3H-estradiol binding is highest in glandular epithelium compared with luminal epithelium.
The results of our immunocytochemical studies from day 1.5 to day 7.5 of pregnancy as well as those published in the literature for days 4–7 (Tan et al. 1999) indicate that in the uterine tissues estradiol binds to the subtype ER-α. When the results obtained by autoradiography are compared with those obtained by immunocytochemistry, there appears to be general agreement in low magnification surveys. Careful and detailed examination, however, and especially quantification that is possible only with high-resolution autoradiograms, reveal differences that pertain to different sensitivity and resolution of these two approaches.
After fertilization in the epithelium of the uterine lumen and glands morphological changes occur in rapid succession paralleled by differences in 3H-estradiol nuclear uptake. On days 1.5, 2.5, and 7.5 of pregnancy, in the luminal epithelium nuclear concentration of 3H-estradiol is correlated with 3H-thymidine incorporation. The concentration of proliferative activity at the edges of the uterine lumen may reflect remodeling of the lumen that occurs in particular during the preimplantation period but also subsequently. The gradual increases in the 3H-estradiol binding by luminal epithelial cells observed from day 1.5 to day 3.5 are probably related to successive morphological and functional changes optimizing conditions for reception and nidation of the conceptus (Parr and Parr 1989; Weitlauf 1994). During the early periimplantation period on day 4.5 of pregnancy, the decrease of 3H-estradiol binding is probably influenced by progesterone dominance in this phase (Psychoyos 1973), which has an inhibitory effect on the ER-α expression in the luminal epithelium (Wang et al. 1999). Our results indicate that the inhibitory effect on ER expression is not homogeneous throughout the epithelial cells since the distribution of 3H-estradiol receptor binding is not uniform but varies along the uterine lumen. The binding of 3H-estradiol by epithelial cells lining the uterine crypt was very low, probably because these cells will die by apoptosis several hours after embryo implantation (Parr and Parr 1989).
During the later periimplantation period, the pattern of 3H-thymidine incorporation by luminal epithelial cells is again very similar to that observed for 3H-estradiol. In both IS and IIS, labeled cells are concentrated mainly in the mesometrial end of the uterine lumen. The sustained proliferative activity exhibited by the luminal epithelial cells at the mesometrial pole of the uterus may be related to the formation of the new uterine lumen that starts on day 7 of pregnancy. The proliferative activity expressed by epithelial cells that line the new lumen differs from one side to the other. A similar distribution is observed for 3H-estradiol receptor binding in these regions. These results demonstrate the existence of a close association between cell proliferation and ER expression in the luminal epithelium during this period of pregnancy.
Classic studies by Finn and Martin (1967) showed that mitotic activity of endometrial glands in mice tends to be low before ovulation but increases 3 days later. Our results of 3H-thymidine incorporation largely agree and extend these former data and demonstrate that 3H-thymidine incorporation by glandular cells on day 2.5 of pregnancy parallels a strong 3H-estradiol binding in these cells. Thus, our results corroborate those from Finn and Martin (1973) who suggested that the proliferative activity in glands during that period is hormone dependent. However, from day 3.5 of pregnancy onward, 3H-thymidine incorporation is no longer observed in the glandular epithelium, while a strong 3H-estradiol concentration continues to exist. The results of the present studies provide several indications for strong secretory activity of uterine glands during the preimplantation and periimplantation periods. This is apparent in the intense pyronin (RNA)-positive stain of the cytoplasm of epithelial glandular cells. It is noteworthy that some of the observed occasional drastic changes in glandular morphology resemble those seen in apocrine mammary glands. These changes all are associated with strong nuclear concentration of 3H-estradiol, with the exception of some squamous cells in the interrupted lining of distended portions of, perhaps, exhausted portions of the glands.
Different studies published in the literature provide evidence for glandular secretory capabilities during the periimplantation period (reviewed by Given and Enders 1989) and that this secretory activity is under the control of estradiol (Aitken 1977; Pratt 1977; Surani 1977; Fishel 1979). In vitro studies indicate that the secretory activity of cultured endometrial cells changes if progesterone or estrogen is added to the incubation medium (Bell et al. 1986). Glycoproteins such as uteroglobin (Beir 1968; Atger et al. 1980) and insulin-like growth factor-I (Person et al. 1997) change in the uterine flush according to the hormonal profile of the animal.
Recent studies with adult uterine gland knockout ewes demonstrated that endometrial glands and, by inference, their secretion are required for periimplantation conceptus survival and development (Gray et al. 2001). Moreover, it is known that a cytokine, leukemia inhibitory factor (LIF) is expressed in the uterine endometrial glands of mice, specifically on day 4 of pregnancy. The LIF secretion is under maternal control and its gene expression is estrogen-dependent (Bhatt et al. 1991; Shen and Leder 1992; Stuart et al. 1992). There is direct evidence obtained from mice deficient in a functional LIF gene that this molecule is an absolute requirement for embryo implantation. According to Cullinan et al. (1996) LIF acts through an autocrine/paracrine interaction with its receptor at the luminal epithelium. The presence of highly 3H-estradiol-labeled glandular profiles in the decidua in close proximity to the implantation cavity is shown in the present work, reinforce the concept that the uterine glands may have a role in the regulation of embryo implantation and development.
In summary, the present data indicate strong estrogen action upon glandular epithelium, however, with various changes of emphasis on proliferation and differentiation earlier, then secretion, and involution and remodeling later. Incipient cell involution and death appear to be associated with a reduction or abolition of nuclear estradiol binding. What causes the target tissue-specific changes in estrogen binding, morphology, and function remains to be clarified, involving perhaps progesterone and local tissue factors as well. The present data together point to a hierarchy of estrogen effect on uterine epithelia in which the glandular epithelium appears to play a predominant role during the postfertilization–periimplantation period.
References
Abrahamshon PA, Zorn TMT (1993) Implantation and decidualization in rodents. J Exp Zool 266:603–628
Aitken RJ (1977) Changes in the protein content of mouse uterine flushing during normal pregnancy and delayed implantation, and after ovariectomy and oestradiol administration. J Reprod Fertil 50:29–36
Atger M, Perricaudet M, Toillais P, Milgrom E (1980) Bacterial cloning of the rabbit uteroglobulin structure gene. Biochem Biophys Res Commun 93:1082–1088
Bhatt H, Brunet LJ, Stewart CL (1991) Uterine expression of leukemia inhibitory factor coincides with the onset of blastocyst implantation. Proc Natl Acad Sci U S A 88:11408–11412
Beir HM (1968) Uteroglobulin: a hormone-sensitive endometrial protein involved in blastocyst development. Biochem Biophys Acta 160:289–291
Bell SC, Patel SR, Kirwan PH, Drife JO (1986) Protein synthesis and secretion by the human endometrium during the menstrual cycle and the effect of progesterone in vitro. J Reprod Fertil 77:221–231
Brenner RM, McClellan MC, West NB, Novy MJ, Haluska GJ, Sternfeld MD (1991) Estrogen and progestin receptors in the macaque endometrium. Ann N Y Acad Sci 622:149–166
Chen D, Ganapathy P, Zhu LJ, Xu X, Li Q, Bagchi IC, Bagchi MK (1999) Potential regulation of membrane trafficking by estrogen receptor alpha via induction of rab11 in uterine glands during implantation. Mol Endocrinol 13:993–1004
Cullinan EB, Abbondanzo SJ, Anderson PS, Pollard JW, Lessey BA, Steward CL (1996) Leukemia inhibitory factor (LIF) and LIF receptor expression in human endometrium suggest a potential autocrine/paracrine function in regulating embryo implantation. Proc Natl Acad Sci U S A 293:3115–3120
Ennis BW, Stumpf WE (1988) Differential induction of progestin-binding sites in uterine cell types by estrogen and antiestrogen. Endocrinology 123:1747–1753
Finn CA, Martin J (1967) Patterns of cell division in the mouse uterus during early pregnancy. J Endocrinol 39:593–597
Finn CA, Martin L (1973) Endocrine control of gland proliferation in the mouse uterus. Biol Reprod 8:585–588
Fishel SB (1979) Analysis of mouse uterine proteins at pro-oestrus, during early pregnancy and after administration of exogenous steroids. J Reprod Fertil 55:91–100
Given RL, Enders C (1989) The endometrium of delayed and early implantation. In: Wynn R, Jollie W (eds) Biology of the uterus. Plenum, New York, pp 176–232
Gray A, Taylor K, Ramsey W, Hill J, Bazer F, Bartol F, Spencer T (2001) Endometrial glands are required for implantation conceptus elongation and survival. Biol Reprod 64:1608–1613
Li W, Boomsma RA, Verhage HG (1992) Immunocytochemical analysis of estrogen and progestin receptors in uteri of steroid-treated and pregnant cats. Biol Reprod 47:1073–1081
Logeat F, Sartor P, Vu Hai MT, Milgrom E (1980) Local effect of the blastocyst on estrogen and progesterone receptors in the rat endometrium. Science 207:1083–1085
Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schitz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM (1995) The nuclear receptor superfamily: the second decade. Cell 83:835–839
Martel D, Psychoyos A (1981) Estrogen receptors in the nidatory sites of the rat endometrium. Science 211:1454–1455
Martin L, Finn CA, Trinder G (1973) Hypertrophy and hyperplasia in the mouse uterus after oestrogen treatment: an autoradiographic study. J Endocrinol 56:133–144
Moulton BC, Koenig BB (1981) Estrogen receptor in deciduoma cells separated by velocity sedimentation. Endocrinology 108:484–488
Parr M, Parr E (1989) The implantation reaction. In: Wynn R, Jollie W (eds) Biology of the uterus. Plenum, New York, pp 233–278
Person E, Sahlin L, Marsiroi B, Dantzer V, Eriksson H, Rodriguez-Martine H (1997) Insulin-like growth factor-I in the porcine endometrium and placenta: localization and concentration in relation to steroid influence during early pregnancy. Anim Reprod Sci 46:261–281
Prasad MR, Sar M, Stumpf WE (1974) Autoradiographic studies on (3H) oestradiol localization in the blastocyst and uterus of rats during delayed implantation. J Reprod Fertil 36:75–81
Prasad MR, Sar M, Stumpf WE (1976) Regional differences between nuclear concentration of (3H) estradiol and (3H) progesterone and their action on the uterus of rat during delayed implantation. J Exp Zool 197:71–79
Pratt HP (1977) Uterine proteins and the activation of embryo from mice during delayed implantation. J Reprod Fertil 50:1–8
Psychoyos A (1973) Endocrine control of egg implantation. In: Greep RO, Astwood EB (eds) Handbook of physiology, sect 7. Endocrinology, vol II, part 2. American Physiological Society, Washington, DC, pp 187–215
Sartor P (1977) Exogenous hormone uptake and binding in the rat uterus at the time of ova-implantation. Acta Endocrinol 84:804–812
Shen MM, Leder P (1992) Leukemia inhibitory factor is expressed by the preimplantation uterus and selectively blocks primitive ectoderm formation in vitro. Proc Natl Acad Sci U S A 89:8240–8244
Shughrue PJ, Stumpf WE (1992) Progestin target cell distribution in forebrain and midbrain regions of the 8-day postnatal mouse brain. Endocrinology 130:3650–3659
Stuart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondazo SJ (1992) Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 359:76–79
Stumpf WE (1968) Subcellular distribution of 3H-estradiol in rat uterus by quantitative autoradiography: a comparison between 3H-estradiol and 3H-norethynodrel. Endocrinology 83:777–782
Stumpf WE (1971) Autoradioautographic techniques for the localization of hormones and drugs at the cellular and subcellular levels. Acta Endocrinol 153:205–222
Stumpf WE (2003) Drug localization in tissues and cells. IDDC Press, Chapel Hill, NC
Stumpf WE, Sar M, Zuber TJ, Soini E, Tuohimaa P (1981) Quantitative assessment of steroid hormone binding sites by thaw-mount autoradiography. J Histochem Cytochem 29:201–206
Surani MAH (1977) Radiolabelled rat uterine luminal proteins and their regulation by oestradiol and progesterone. J Reprod Fertil 50:289–296
Talley DJ, Tobert JA, Armstrong EG Jr, Villee CA (1977) Changes in estrogen receptor levels during deciduomata development in the pseudopregnant rat. Endocrinology 101:1538–1544
Tan J, Paria BC, Dey SK, Das SK (1999) Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology140:5310–5321
Tessier C, Deb S, Prignent-Tessier A, Ferguson-Gottschall S, Gibori GB, Shiu RPC, Gibori G (2000) Estrogen receptors α and β in rat decidual cells: cell-specific expression and differential regulation by steroid hormones and prolactin. Endocrinology 141:3842–3851
Tibbetts T, Mendoza-Meneses M, O'Malley B, Conneely O (1998) Mutual and intercompartmental regulation of estrogen receptor and progesterone receptor expression in the mouse uterus. Biol Reprod 59:1143–1152
Wang H, Masironi B, Eriksson H, Sahlin L (1999) A comparative study of estrogen receptors α and in the rat uterus. Biol Reprod 61:955–964
Ward WF, Frost AG, Orsini MW (1978) Estrogen binding by embryonic and interembryonic segments of the rat uterus prior to implantation. Biol Reprod 18:598–601
Weitlauf HM (1994) Biology of implantation. In: Knobil E, Neill JD (eds) The physiology of reproduction, 2nd edn. Raven, New York, pp 231–262
Yamashita S, Korach KS (1989) A modified immunohistochemical procedure for the detection of estrogen receptor in mouse tissues. Histochemistry 90:325–330
Acknowledgements
W.E. Stumpf is Visiting Professor, Department of Histology and Embryology, ICB, USP, supported by FAPESP (99/098-1). Dr. Soto-Suazo is supportd by a fellowship from CNPq (PD), Brazil. J.G. Oliveira is an undergraduate student supported by a fellowship from FAPESP (02/01249-8). We thank Dr. Alison Colqhoun for a critical reading of the manuscript. The project was supported by grants from FAPESO (00/00098-1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zorn, T.M.T., Soto-Suazo, M., Pellegrini, C.R. et al. Estradiol receptor binding to the epithelium of uterine lumen and glands: region- and time-related changes during preimplantation and periimplantation periods studied by autoradiography. Histochem Cell Biol 120, 1–12 (2003). https://doi.org/10.1007/s00418-003-0534-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-003-0534-3