Abstract
Purpose
The primary aim of our study was to evaluate retinal microvascular anomalies recorded with optical coherence tomography angiography (OCTA) and the retinal metabolic function measured with retinal oximetry (RO) in patients with retinitis pigmentosa (RP). The secondary aim of the study was to link the presence of macular edema to microvascular and metabolic parameters in RP.
Methods
OCTA and RO were performed on 94 eyes: 64 eyes diagnosed with RP with (ME-RP) and without (no-ME-RP) macular edema were compared with 30 control eyes. Study end points were as follows: mean superficial (FAZ-S) and deep foveal avascular zone (FAZ-D) determined by OCTA. In addition, we evaluated the mean arterial (A-SO2; %), venular (V-SO2; %) oxygen saturation, their difference (A-V SO2; %), as well as the corresponding mean diameter of the retinal arterioles (D-A; μm) and venules (D-V; μm).
Results
RP patients differed from controls by enlarged FAZ-S and FAZ-D (p ≤ 0.001), attenuated retinal vessels (p ≤ 0.001), and increased retinal vessel oxygen saturation (p ≤ 0.010). Subgroup analyses within RP patients revealed more pronounced alterations of microvascular parameters and metabolic function in the presence of macular edema. In the no-ME-RP subgroup, significant interactions were present between FAZ-S, A-SO2, and V-SO2, whereas in the ME-RP subgroup, we found significant correlations between FAZ-D and D-A.
Conclusion
A combined microvascular structure–metabolic function approach enhances our understanding of inherited retinal diseases. The presence of macular edema in RP seems to be a result of more altered microvascular–metabolic function. Macular edema should thus be taken into consideration when evaluating microvascular and/or metabolic changes in RP.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Retinitis pigmentosa (RP) represents a heterogeneous group of inherited retinal diseases marked by progressive photoreceptor cell degeneration [1,2,3]. Apart from pigmentary changes with a perivascular “bone spicule” configuration and the pale, prominent appearing optic disc, a universal clinical feature in eyes with RP is the attenuation of retinal vessels [3,4,5,6,7,8]. It is thought to reflect decreased metabolic demand of the degenerating retina [6, 7, 9,10,11].
Invasive measurements with microelectrodes in animals under normal conditions confirmed regional variations in the intraretinal oxygen distribution. Three oxygen consumption zones were identified and corresponded to the superficial and deep retinal capillary and choroidal beds [12]. According to published experiments on the oxygen metabolism of the retina, the metabolic supply of rod photoreceptors is mainly supported by choroidal vessels and to lesser extent by deep retinal capillaries [12,13,14,15,16]. Under dark adapted conditions, the metabolic activity of the highly energy-demanding rod photoreceptors is supported further by oxygen diffusion from the superficial retinal capillary vessels [16].
In vivo research in animal models for outer retinal degeneration revealed a reduction of the outer retinal oxygen consumption. Even if degenerated photoreceptors use less oxygen, the oxygen metabolism of the inner retina remained unaffected. These changes of the oxygen environment are thought to play a role in the progression of the degenerative process and neurovascular remodelling [17,18,19]. Furthermore, histopathological studies reported about sclerosis and atrophy of the retinal vasculature in advanced cases [20, 21]. In addition, in regions with destructed retinal pigment epithelium (RPE), a lack of choroidal capillaries was detected [21,22,23,24].
Optical coherence tomography angiography (OCTA) is a novel, contact-free and non-invasive imaging technique that allows for a depth-resolved visualization of both the retinal and the choroidal microvasculatures. It is based on motion contrast via mapping erythrocyte movement over time by comparing sequential OCT B-scans in a given cross section [25]. Several studies conducted with OCTA have reported about a reduction of retinal and choroidal blood flow, choroidal vascular defects, and an increase in the size of the foveal avascular zone (FAZ) in eyes of patients suffering RP [26,27,28,29]. However, some other studies reported contradictory results [30, 31].
Retinal vessel oximetry (RO) allows for contact-free in vivo measurements of the retinal oxygen saturation and thus the exploration of metabolic alterations [13, 32,33,34,35]. Recent RO studies on patients suffering from RP showed a significant increment of oxygen saturation in the retinal arterioles and venules, presumably the consequence of reduced oxygen consumption [6, 7, 36,37,38,39,40,41]. Furthermore, the oxygen saturation alterations correlated well with structural and functional changes [7, 8, 37, 38]. Thus, as the highest amount of oxygen is used by retinal photoreceptors, a reduction in retinal oxygen demand with secondary increase in the retinal vessel saturation values following cellular apoptosis has been hypothesized to explain the observed vascular oxygen alterations in RP patients [6, 7, 36,37,38,39,40,41]. Notably, the application of transcorneal electrical stimulation in patients with RP induced a measurable increase in oxygen consumption, the latter supposed to be the result of an improvement in oxygen demand of the preserved photoreceptors [42].
Given the known microvascular and metabolic alterations in RP, it is conceivable that also the superficial and deep retinal vascular structures may exhibit metabolic dysfunction. Thus, the main goal of this prospective observational study was to investigate the superficial and deep foveal avascular zones and RO parameters of eyes affected by RP and to compare these measurements to healthy control eyes. Furthermore, the presence of macular edema in RP has been found to reflect a stronger impairment of metabolic function and indicated a different relationship between altered oxygen saturation parameters against functional and structural parameters [39, 40]. Therefore, our secondary objective of the study was to link the presence of macular edema to retinal microvascular anomalies and metabolic functional parameters.
Methods
Prospective, observational study of 94 eyes was performed: 64 eyes diagnosed with RP and 30 healthy control eyes were examined from January 2018 to September 2019 in the University Eye Hospital Basel, Switzerland. This study was approved by the local IRB (Ethics Commission of Central and Northern Switzerland, EKNZ Basel Switzerland; trial number EKNZ BASEC 2017-00937).
Subjects
All patients underwent all required standard evaluations by experienced fellowship-trained specialists for inherited retinal diseases (M.G.T and H.P.N.S.) and presented with a typical clinical picture of retinitis pigmentosa. All participants underwent a detailed ophthalmic examination, which included among others refraction, best corrected visual acuity (BCVA) obtained under standardized Early Treatment Diabetic Retinopathy Study (ETDRS) conditions, intraocular pressure examination by applanation, biomicroscopy, and funduscopy. The clinical phenotype of RP patients was characterized following a detailed ophthalmic examination as described above and additional OCT of the macula, fundus autofluorescence, visual field testing with semi-automated kinetic perimetry (V4e, III4e, I4e, III3e isopters tested with Octopus 900®, Haag-Streit AG Bern, Switzerland), and full-field electroretinography (ffERG, Diagnosys LLC Espion system; ISCEV standard [43]). The RP group was divided in two subgroups: with (ME-RP) and without appearance of macular edema (no-ME-RP), defined by the presence of intraretinal spaces situated within and around the foveal center. The inclusion criteria for patients and controls included the following: stable fixation and refractive error (spherical equivalent) of < 6 diopters. Patients and controls who had previous ocular surgery or ocular and systemic pathology (as for instance, diabetes mellitus or neurological disease) that may influence the OCTA and RO measurements were not included in this study. Additional exclusion criteria were images with inadequate quality or an expressed unwillingness to participate in the study. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional/national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Prior to OCTA measurements, pupils were dilated (Tropiphen 10 ml eye drops prepared in our institutional pharmacy as a combination of tropicamide 0.5% and phenylephrine 1%).
Retinal vessel oximetry acquisition
RO measurements were performed with the spectrophotometric oximetry unit for retinal vessel oximetry (IMEDOS Systems UG, Jena, Germany; Fundus Camera FF450, Carl Zeiss Meditec AG, Jena, Germany) (Fig. 1). Fundus images were recorded using a camera system, DCC Digital Camera KY-F75 (JVC Inc., Yokohama, Japan), coupled to the Zeiss fundus camera from a camera angle of 50°. The software operating the system (VISUALIS; IMEDOS Systems UG) differentiates simultaneously between oxygenated and deoxygenated hemoglobin based on the differential light imaging characteristics at different wavelengths, measuring the oxygen saturation level in the examined retinal vessel. In summary, RO was performed at two different wavelengths: at the green channel (548 ± 10 nm) to record the oxygen insensitive image and at the red channel (610 ± 10 nm) to record the oxygen sensitive image [33, 44]. An optic disc-centered image protocol was used where two concentric rings were created in the peripapillary area: one with a radius of 1.0 optic disc diameters and the other with a radius of 1.5 optic disc diameters. The region between these two circles defined the area of interest where all measurements were obtained. Four test–retest fundus images for each eye were performed [8, 36, 37, 45]. One image with optimal illumination, red channel illumination < 160 steps of the scale, and green channel illumination > 60 steps of the scale was further selected for analysis, as described in details in one of our previous publications [45]. For oximetry analyses, all main arterioles and venules were selected manually within the area of interest. The software (Vesselmap) calculates then the optical density ratio (ODR) of the two images and, thus, the mean oxygen saturation of the evaluated retinal vessel. The global mean oxygen saturation in retinal arterioles (A-SO2) and venules (V-SO2) was measured, and their difference, the A-V SO2, was calculated. In addition, we determined the diameters of the retinal arterioles (D-A) and venules (D-V).
An example for retinal oximetry (RO) measurements in left eyes of (a) a healthy subject and (b) a patient suffering from retinitis pigmentosa (RP). RO parameters were calculated in all main first and second branch retinal arterioles and venules within 1.0–1.5 optic disc diameter distances from the optic disc margin
Optical coherence tomography angiography acquisition
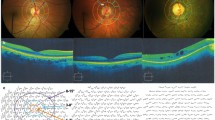
We performed 9 × 15 mm OCTA scans centered around the fovea for an evaluation of both, the superficial (FAZ-S), and the deep retinal foveal avascular zone (FAZ-D) with a PLEX Elite 9000 OCTA device (Carl Zeiss Meditec AG, Jena). The superficial retinal foveal avascular zone was defined as the vessel-free zone within the superficial vascular plexus localized between the inner limiting membrane (ILM) and the inner plexiform layer (IPL) of the retina. The retinal foveal avascular zone was defined as the vessel-free zone within the deep vascular plexus localized between IPL and the outer plexiform layer (OPL) of the retina. The measurement of the avascular area in square millimeters was performed via the software’s ruler tool of ImageJ (1.52a, Rasband, W.S., ImageJ, U.S. National Institutes of Health, Bethesda, MD, USA). All scans were recorded masked by one experienced operator (M.D.V.W.). The FAZ measurements were performed independently by two experienced retina specialists (M.D.V.W. and M.G.T.). Cases with non-agreement were discussed thoroughly and brought into alignment. Within these measurements we aimed to find both similarities and differences between diseased and healthy eyes. Furthermore, central retinal thickness (CRT) was measured automized in micrometers from the ILM to the RPE by using the Zeiss ETDRS retina thickness algorithm (version 0.1) within the central sector of a standardized ETDRS chart centered over the fovea with a diameter of 1 mm. In addition, the maximal retinal thickness was measured manually in micrometers from the ILM to the RPE in the ME-RP subgroup with the software’s ruler tool (Fig. 2).
Image examples of the superficial and deep perfusion trace obtained with 9 × 15 mm OCTA scans centered around the fovea showing the superficial (FAZ-S, left side) and deep retinal foveal avascular zones (FAZ-D, right side) in right eyes of a healthy control subject and two patients suffering from retinitis pigmentosa both in absence (no-ME-RP) or presence (ME-RP) of cystoid macular edema. The red marker represents the measurement of the avascular area in mm2
In order to avoid the effect of continuous light exposure on retinal vessel diameter and oxygen saturation, the RO was performed before the OCTA measurement.
Study end points were as follows: the mean superficial (FAZ-S) and deep retinal foveal avascular zone (FAZ-D) determined by optical coherence tomography angiography (OCTA). In addition, we evaluated the mean arterial (A-SO2; %) and venular (V-SO2; %) oxygen saturation, their difference (A-V SO2; %), as well as the mean diameter of the retinal arterioles (D-A; μm) and venules (D-V; μm).
Statistical analysis
Retinitis pigmentosa may be presented asymmetrically or even unilaterally [46, 47]. Therefore, for statistical evaluation, ANOVA-based linear mixed effects models were performed with SPSS® (IBM SPSS Statistics®, version 22.0.0.0) which allows taking the dependency of the left and right eye in the same subject into account. The model is thus suitable for repeated measurements also when considering both eyes in the analysis. P < 0.05 was defined as statistically significant. For statistical evaluation, normal distribution of all parameters was ensured with histograms and Shapiro–Wilk test. In order to compare subgroups against one another, ANOVA with all pairwise comparisons was performed; all presented p values were Bonferroni adjusted. To predict the effect of the underlying disease on OCTA and RO measurements, the eye and the group were treated as a fixed factor and the subject as a random factor. In order to exclude an age- or refraction-related bias, we took into account the spherical equivalent and the age as covariates. In order to predict the interaction effect of structural OCTA parameters on metabolic functional RO measurements, linear mixed effects models were applied. In this context, a significant interaction indicates a different relationship of structural OCTA parameters with the RO variables between subgroups. Results are presented as arithmetic mean and standard deviation (± SD) over all examined subgroups, each with their corresponding p values.
Results
Altogether, 94 eyes were enrolled in the study: 64 eyes of 32 patients diagnosed with RP (15 ♀ 17 ♂; 49.7 ± 15.4 years, range 20–81 years) and 30 age-matched control eyes from 15 healthy participants (8 ♀ 7 ♂; 43.8 ± 16.6 years, range 21–71 years). We divided the RP group into two subgroups: with (ME-RP) and without appearance of macular edema (no-ME-RP), defined by the presence of intraretinal spaces situated within the foveal center. In the no-ME-RP subgroup, 29 patients participated with 53 eyes (27 right and 26 left) without macular edema, and in the ME-RP subgroup, 8 patients participated with 11 eyes (5 right and 6 left) with macular edema. Five patients were part of both subgroups, each having one eye with ME (2 right and 3 left eyes). Depending on the presence of macular edema, mean CRT was reduced in the no-ME-RP subgroup (220.00 ± 37.80 μm), while it was increased in the ME-RP subgroup (312.55 ± 39.54 μm) when compared with controls (247.08 ± 17.16 μm, p < 0.001; Table 1). In the ME-RP subgroup, the average maximal retinal thickness was 394.55 ± 70.54 μm. All demographic characteristics of our controls and RP patients are summarized in Table 1.
Comparison of FAZ-S and FAZ-D from OCTA measurements in RP against controls and within RP subgroups with and without macular edema
In general, compared with controls, eyes suffering from RP showed significantly enlarged FAZ-S (p = 0.001) and FAZ-D (p < 0.001; Table 2). However, evaluated in subgroups, only the ME-RP differed from controls by both parameters: FAZ-S and FAZ-D (p < 0.001). When evaluated between RP subgroups, ME-RP showed significantly wider FAZ-S and FAZ-D than no-ME-RP (p < 0.001; Table 2).
Comparison of RO parameters in RP against controls and within RP subgroups with and without macular edema
Compared with controls, eyes suffering from RP showed significant differences, when the A-SO2 and V-SO2 parameters were taken into account: Both in the presence (ME-RP) and in the absence (no-ME-RP) of cystoid macular edema, the A-SO2 and V-SO2 in RP patients were significantly increased (p < 0.03). Even showing slightly higher V-SO2 values in the ME-RP, neither A-SO2 nor V-SO2 reached statistically significant difference between the ME-RP and no-ME-RP subgroups (p > 0.17; Table 2).
Comparison of retinal vessel diameters in RP against controls and within RP subgroups with and without macular edema
In comparison with controls, eyes suffering from RP showed significantly attenuated retinal vessels, when the D-A and D-V were taken into account: Both in the presence (ME-RP) and in the absence (no-ME-RP) of cystoid macular edema, the D-A and D-V were significantly thinner (p < 0.001). Again, even showing slightly more attenuated D-A in the ME-RP, there was no statistically significant difference for neither D-A nor D-V between the RP subgroups (p > 0.35; Table 2).
Interactions between retinal vessel structure (OCTA parameters) and metabolic function (RO parameters) in retinitis pigmentosa patients with and without macular edema
RP eyes without macular edema (no-ME-RP) showed significant structure–function correlations between the FAZ-D and the arterial and venular oxygen saturations (A-SO2 and V-SO2). This means that an enlarged FAZ-D correlated positively not only with increased A-SO2 (p = 0.004; with the slope of 48.951 [CI 99.12–102.52]) but also with increased V-SO2 (p = 0.012; with the slope of 48.954 [CI 59.234–65.961]). In RP, eyes with macular edema (ME-RP) interactions were significant between the FAZ-D and the diameter of the retinal arterioles (D-A). This means that enlarged FAZ-D correlated positively with attenuated retinal arterioles (p = 0.039; with the slope of 69.668 [CI 65.818–73.519]).
Interactions between OCTA parameters and visual acuity in retinitis pigmentosa patients with and without macular edema
When analyzed with linear mixed effects models, we found significant interactions between the logMAR BCVA and the FAZ-S in no-ME-RP eyes (p = 0.003; Table 3). However, for ME-RP eyes, we found no significant interactions. Also, the FAZ-D showed no significant impact on the logMAR BCVA in patients suffering from RP.
Thus, both the superficial and deep foveal avascular zone measurements were correlated with oxygen metabolic function in RP.
Discussion
The present study confirms not only an altered metabolic function in RP but also a different metabolic pattern in those RP patients suffering from macular edema. In addition, we detected microvascular alterations in RP which were presented differently depending on the presence or absence of macular edema. Furthermore, we found that both the superficial and deep foveal avascular zone measurements correlate with the oxygen metabolic function in retinitis pigmentosa. Also, these correlations seem to depend on the presence of macular edema.
There are several possible explanations for our findings: Altered retinal metabolism in RP is proposed to be the main cause for compromised vasculogenesis and neurovascular remodelling of the degenerated retina. As the intraretinal oxygen is mainly used by the highly energy-demanding rod photoreceptors, a reduction in retinal oxygen consumption with secondary increase in the retinal vessel saturation values following cellular apoptosis has been proposed to explain the observed vascular oxygen alterations in RP patients [6, 7, 36,37,38,39,40,41]. Oxidative stress following apoptosis of rod photoreceptors is currently discussed to be the trigger for the secondary loss of cones and inner retinal cells [48,49,50]. Therefore, the presence of macular edema in RP is supposed to represent a more advanced stage of photoreceptor degeneration [39, 40]. In agreement, we found in our study more altered metabolic function in ME-RP patients.
Consistent with the neurovascular remodelling occurring with degeneration, attenuated vessels are regarded as a clinical hallmark in eyes with RP [3,4,5,6, 51]. The degree of retinal vessel attenuation seems to correlate with the remaining central retinal function as measured by multifocal ERG [6] and BCVA [39].
However, not only vessel diameters but also ocular hemodynamics are altered in RP. Ocular blood flow alterations, either retrobulbar, choroidal or retinal, have been confirmed in RP. For instance, hemodynamic studies in RP patients, using laser Doppler velocimetry [9], color Doppler imaging [52, 53], laser speckle flowgraphy [54], and retinal functional imaging [55], have revealed significant reduction of retinal blood flow. Based on MRI imaging, reduced retino-choroidal blood flow has been noted [56]. Furthermore, choroidal hemodynamic disturbance has shown to correlate with worsening in the retinal function measured by full-field ERG, multifocal ERG, or perimetry in RP [53, 54, 57].
Regarding retinal vessel attenuation and ocular blood flow alterations in RP, it is not an unexpected finding that RP patients present with microvascular anomalies, which can be documented nowadays using OCTA, where the reduction of retinal and choroidal blood flow and an increase in the size of the foveal avascular zone (FAZ) have been reported [26,27,28,29].
According to the results of Ma et al., reduced retinal blood flow and attenuated vessel diameters are related to increased oxygen tension in the retinal extracellular space [5]. Other studies suggested the higher extracellular oxygen level to lead to further vasoconstriction and diminished blood flow in retinal arteries [57] with further alterations in hemodynamics [53, 58,59,60]. Thus, enlarged FAZ in RP and reduced arteriole diameters as determined in our study could well be linked to a more progressive stage of outer retinal degeneration with the following: loss of photoreceptors, reduced barrier function of the RPE, and corresponding retinal and choroidal vascular remodelling.
Therefore, we studied further the relationship between metabolic and microvascular alterations in RP patients and we found that these are correlated. Furthermore, in RP without macular edema, there were significant interactions between the arterial and venular oxygen saturations (A-SO2 and V-SO2) and the superficial FAZ. As RO especially measures, the oxygenation in retinal vessels located in the inner retina our results confirm an altered metabolic function of the inner retina resulting from a degenerative process of the outer retina in adults suffering from RP. Thus, this result seems to have a higher impact on the superficial vascular layer, as measured in our study with the superficial FAZ.
Contrary to the results of the studies cited before [26,27,28,29], other publications reported to have no significant changes in the size of the FAZ between diseased and healthy eyes [30, 31]. Based on the results of the present study in a genetically heterogenic RP group with different age and stage of degeneration, one reason for discrepancy in FAZ findings in RP might probably be the presence of macular edema. The latter could be assumed not only because of the artifacts due to scattering and enlargement of the evaluated macular space as a result of edema but also due to a different altered metabolic stage of degeneration.
Furthermore, in RP eyes with macular edema, we found the vessel diameter of the retinal arterioles (D-A) to present with significant interactions with the deep FAZ. Here, more attenuated arterial vessels in ME-RP presented with a higher level of FAZ-D enlargement. These results are in accordance with recent studies that found the arterial vessel diameters to be significantly rarefied in RP [7, 40]. Those findings were even more pronounced in the presence rather than in the absence of macular edema and correlated well with the degree of the residual central retinal function as measured by multifocal ERG [7]. This vessel attenuation seems to have a significant impact on the deep rather than the superficial FAZ in ME-RP. Furthermore, even if rods are thought to be the main players in the pathogenesis of outer retinal degeneration, cones are also involved even if occurring later in the course of degeneration. Taking the normal human topographic distribution of photoreceptors with higher cone density in the fovea [61] into account, it is plausible that the presentation of macular edema in RP may reflect the compromised resistance of cones to oxidative stress.
In addition, we found the FAZ-S to have a significant effect on the visual acuity in no-ME-RP patients. This phenomenon could only be found for the superficial but not for the deep FAZ. Also, these findings are in agreement with current studies [62, 63] which found the FAZ-S but not the FAZ-D to correlate with visual function in RP.
Our analyses revealed significantly enlarged superficial and deep FAZ in RP only in eyes in presence, however, not in the absence of macular edema. Thus, our findings might explain why recent OCTA studies in RP found controversial results regarding the FAZ: While several studies reported about an increase in the size of the FAZ in eyes of patients suffering from RP [26,27,28,29], others published results contrary to these findings [30, 31].
Furthermore, RP patients without macular edema presented with less FAZ enlargement and slightly better BCVA. Also, the FAZ-S correlated well with BCVA. These results are in agreement with the study of Konieczka et al. where no-ME-RP patients showed a clear relationship between the residual BCVA and structure–function parameters evaluated by OCT and multifocal ERG [39]. Thus, one may suppose more preserved cone photoreceptors and less vascular remodelling in no-ME-RP patients. On the contrary, in the presence of macular edema (ME-RP subgroup), these results might be influenced by scattering artifacts [64] and therefore bias the evaluation of retinal microvasculature and its effect on BCVA.
The study has limitations that include the genetic heterogeneity of our RP patients, possible artifacts due to the refractive error, and the small sample size in the ME-RP subgroup.
In conclusion, our study showed that RP patients with macular edema show more pronounced alterations in microvascular structure and metabolic function. Increased retinal vessel oxygen saturation, narrower retinal vessels, and more disrupted FAZ seem to be linked to the clinical appearance of macular edema. As photoreceptors die during progression of the degenerative process, the normal microvascular structure and metabolic homeostasis in the RP retina seem to suffer from a significant alteration with enlargement of FAZ. This probably leads to higher levels of free oxygen radicals in the intercellular space, causing persistent macular thickening, clinically identified as macular edema. Whether a significant cone decimation in advanced stages of RP is also marked by a higher grade of neurovascular remodelling and, therefore, leads to a severe metabolic dysfunction remains to be investigated. Furthermore, studies are needed to evaluate to what extent the altered retinal and choroidal perfusion in RP patients may contribute to the metabolic dysfunction and to the presence of macular edema in the future.
Change history
24 February 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00417-020-04975-3
References
Ammann F, Klein D, Franceschetti A (1965) Genetic and epidemiological investigations on pigmentary degeneration of the retina and allied disorders in Switzerland. J Neurol Sci 2:183–196
Hamel C (2006) Retinitis pigmentosa. Orphanet J Rare Dis 1:40
Hartong DT, Berson EL, Dryja TP (2006) Retinitis pigmentosa. Lancet 368:1795–1809
Merin S, Auerbach E (1976) Retinitis pigmentosa. Surv Ophthalmol 20:303–346
Ma Y, Kawasaki R, Dobson LP et al (2012) Quantitative analysis of retinal vessel attenuation in eyes with retinitis pigmentosa. Invest Ophthalmol Vis Sci 53:4306–4314
Lopez Torres LT, Türksever C, Schötzau A, Orgül S, Todorova MG (2015) Peripapillary retinal vessel diameter correlates with mfERG alterations in retinitis pigmentosa. Acta Ophthalmol 93:e527–e533
Bojinova RI, Türksever C, Schötzau A, Valmaggia C, Schorderet DF, Todorova MG (2017) Reduced metabolic function and structural alterations in inherited retinal dystrophies: investigating the effect of peripapillary vessel oxygen saturation and vascular diameter on the retinal nerve fibre layer thickness. Acta Ophthalmol 95:252–261
Todorova MG (2017) Metabolic, structural and functional alterations in patients with inherited diseases of the retina. Acta Ophthalmol 95, thesis: 7–16
Grunwald JE, Maguire AM, Dupont J (1996) Retinal hemodynamics in retinitis pigmentosa. Am J Ophthalmol 122:502–508
Marc RE, Jones BW (2003) Retinal remodeling in inherited photoreceptor degenerations. Mol Neurobiol 28:139–147
Cottet S, Schorderet DF (2009) Mechanisms of apoptosis in retinitis pigmentosa. Curr Mol Med 9:375–383
Cringle SJ, Yu DY, Yu PK, Su EN (2002) Intraretinal oxygen consumption in the rat in vivo. Invest Ophthalmol Vis Sci 43:1922–1927
Stefánsson E, Wolbarsht ML, Landers MB 3rd (1983) In vivo O2 consumption in rhesus monkeys in light and dark. Exp Eye Res 37:251–256
Linsenmeier RA (1986) Effects of light and darkness on oxygen distribution and consumption in the cat retina. J Gen Physiol 88:521–542
Haugh LM, Linsenmeier RA, Goldstick TK (1990) Mathematical models of the spatial distribution of retinal oxygen tension and consumption, including changes upon illumination. Ann Biomed Eng 18:19–36
Cringle SJ, Yu DY (2002) A multi-layer model of retinal oxygen supply and consumption helps explain the muted rise in inner retinal PO(2) during systemic hyperoxia. Comp Biochem Physiol A Mol Integr Physiol 132:61–66
Yu DY, Cringle SJ (2001) Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog Retin Eye Res 20:175–208
Yu DY, Cringle SJ (2005) Retinal degeneration and local oxygen metabolism. Exp Eye Res 80:745–751
Padnick-Silver L, Kang Derwent JJ, Giuliano E, Narfström K, Linsenmeier RA (2006) Retinal oxygenation and oxygen metabolism in Abyssinian cats with a hereditary retinal degeneration. Invest Ophthalmol Vis Sci 47:3683–3689
Blanks JC, Johnson LV (1986) Vascular atrophy in the retinal degenerative rd mouse. J Comp Neurol 254:543–553
Milam AH, Li ZY, Fariss RN (1998) Histopathology of the human retina in retinitis pigmentosa. Prog Retin Eye Res 17:175–205
Henkind P, Gartner S (1983) The relationship between retinal pigment epithelium and the choriocapillaris. Trans Ophthalmol Soc UK 103:444–447
Korte GE (1986) Retinal pigment epithelium and endothelium. Arch Ophthalmol 104:802–804
Penn JS, Li S, Naash MI (2000) Ambient hypoxia reverses retinal vascular attenuation in a transgenic mouse model of autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci 41:4007–4013
Cerquaglia A, Lupidi M, Fiore T, Iaccheri B, Perri P, Cagini C (2017) Deep inside multifocal choroiditis: an optical coherence tomography angiography approach. Int Ophthalmol 37:1047–1051
Ling L, Gao F, Zhang Q et al (2019) Optical coherence tomography angiography assessed retinal and choroidal microvasculature features in patients with retinitis pigmentosa: a meta-analysis. Biomed Res 2019:6723917
Wang XN, Zhao Q, Li DJ et al (2019) Quantitative evaluation of primary retinitis pigmentosa patients using colour Doppler flow imaging and optical coherence tomography angiography. Acta Ophthalmol 97:e993–e997
Sugahara M, Miyata M, Ishihara K et al (2017) Optical coherence tomography angiography to estimate retinal blood flow in eyes with retinitis pigmentosa. Sci Rep 7:46396
Battaglia Parodi M, Cicinelli MV, Rabiolo A et al (2017) Vessel density analysis in patients with retinitis pigmentosa by means of optical coherence tomography angiography. Br J Ophthalmol 101:428–432
Takagi S, Hirami Y, Takahashi M et al (2018) Optical coherence tomography angiography in patients with retinitis pigmentosa who have normal visual acuity. Acta Ophthalmol 96:e636–e642
Koyanagi Y, Murakami Y, Funatsu J et al (2018) Optical coherence tomography angiography of the macular microvasculature changes in retinitis pigmentosa. Acta Ophthalmol 96:e59–e67
Schweitzer D, Thamm E, Hammer M, Kraft J (2001) A new method for the measurement of oxygen saturation at the human ocular fundus. Int Ophthalmol 23:347–353
Hammer M, Thamm E, Schweitzer D (2002) A simple algorithm for in vivo ocular fundus oximetry compensating for non-haemoglobin absorption and scattering. Phys Med Biol 47:233–238
Hardarson SH, Harris A, Karlsson RA et al (2006) Automatic retinal oximetry. Invest Ophthalmol Vis Sci 47:5011–5016
Geirsdottir A, Palsson O, Hardarson SH, Olafsdottir OB, Kristjansdottir JV, Stefánsson E (2012) Retinal vessel oxygen saturation in healthy individuals. Invest Ophthalmol Vis Sci 13:5433–5442
Todorova MG, Türksever C, Schorderet DF, Valmaggia C (2014) Retinal vessel oxygen saturation in patients suffering from inherited diseases of the retina. Klin Monatsbl Augenheilkd 231:447–452
Türksever C, Valmaggia C, Orgül S, Schorderet DF, Flammer J, Todorova MG (2014) Retinal vessel oxygen saturation and its correlation with structural changes in retinitis pigmentosa. Acta Ophthalmol 92:454–460
Todorova MG, Türksever C, Schötzau A, Schorderet DF, Valmaggia C (2016) Metabolic and functional changes in retinitis pigmentosa: comparing retinal vessel oximetry to full-field electroretinography, electrooculogram and multifocal electroretinography. Acta Ophthalmol 94:231–241
Konieczka K, Bojinova RI, Valmaggia C, Schorderet DF, Todorova MG (2016) Preserved functional and structural integrity of the papillomacular area correlates with better visual acuity in retinitis pigmentosa. Eye (Lond) 30:1310–1323
Bojinova RI, Schorderet DF, Valmaggia C, Türksever C, Schoetzau A, Todorova MG (2018) Higher retinal vessel oxygen saturation: investigating its relationship with macular edema in retinitis pigmentosa patients. Eye (Lond) 32:1209–1219
Waizel M, Türksever C, Todorova MG (2018a) The effect of autoimmune retinopathy on retinal vessel oxygen saturation. Eye (Lond) 32:1455–1462
Della Volpe-Waizel M, Zuche HC, Müller U, Rickmann A, Scholl HPN, Todorova MG (2020) Metabolic monitoring of transcorneal electrical stimulation in retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol 258:79–87
McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R et al (2015) ISCEV standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 130:1–12
Hammer M, Vilser W, Riemer T et al (2009) Diabetic patients with retinopathy show increased retinal venous oxygen saturation. Graefes Arch Clin Exp Ophthalmol 247:1025–1030
Türksever C, Orgül S, Todorova MG (2015) Reproducibility of retinal oximetry measurements in healthy and diseased retinas. Acta Ophthalmol 93:e439–e445
Farrell DF (2009) Unilateral retinitis pigmentosa and cone-rod dystrophy. Clin Ophthalmol 3:263–270
Mukhopadhyay R, Holder GE, Moore AT, Webster AR (2011) Unilateral retinitis pigmentosa occurring in an individual with a germline mutation in the RP1 gene. Arch Ophthalmol 129:954–956
Shen J, Yang X, Dong A et al (2005) Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. J Cell Physiol 203:457–464
Komeima K, Rogers BS, Lu L, Campochiaro PA (2006) Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc Natl Acad Sci 103:11300–11305
Usui S, Oveson BC, Lee SY, Jo YJ, Yoshida T, Miki A et al (2009) NADPH oxidase plays a central role in cone cell death in retinitis pigmentosa. J Neurochem 110:1028–1037
Eysteinsson T, Hardarson SH, Bragason D, Stefánsson E (2014) Retinal vessel oxygen saturation and vessel diameter in retinitis pigmentosa. Acta Ophthalmol 92:449–453
Akyol N, Kükner S, Celiker U et al (1995) Decreased retinal blood flow in retinitis pigmentosa. Can J Ophthalmol 30:28–32
Cellini M, Lodi R, Possati GL et al (1997) Color Doppler ultrasonography in retinitis pigmentosa. Preliminary study. J Fr Ophtalmol 20:659–663
Murakami Y, Ikeda Y, Akiyama M et al (2015) Correlation between macular blood flow and central visual sensitivity in retinitis pigmentosa. Acta Ophthalmol 93:e644–e648
Beutelspacher SC, Serbecic N, Barash H et al (2011) Retinal blood flow velocity measured by retinal function imaging in retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol 249:1855–1858
Zhang Y, Harrison JM, Nateras OS et al (2013) Decreased retinal-choroidal blood flow in retinitis pigmentosa as measured by MRI. Doc Ophthalmol 126:187–197
Langham ME, Kramer T (1990) Decreased choroidal blood flow associated with retinitis pigmentosa. Eye 4:374–381
Falsini B, Anselmi GM, Marangoni D et al (2011) Subfoveal choroidal blood flow and central retinal function in retinitis pigmentosa. Invest Ophthalmol Vis Sci 52:1064–1069
Harris A, Ciulla TA, Chung HS et al (1998) Regulation of retinal and optic nerve blood flow. Arch Ophthalmol 116:1491–1495
Konieczka K, Flammer A, Todorova M et al (2012) Retinitis pigmentosa and ocular blood flow. EPMA J 3:17
Kostic C, Arsenijevic Y (2016) Animal modelling for inherited central vision loss. J Pathol 238:300–310
Tang PH, Jauregui R, Tsang SH, Bassuk AG, Mahajan VB (2019) Optical coherence tomography angiography of RPGR-associated retinitis pigmentosa suggests foveal avascular zone is a biomarker for vision loss. Ophthalmic Surg Lasers Imaging Retina 50:e44–e48
Jauregui R, Park KS, Duong JK, Mahajan VB, Tsang SH (2018) Quantitative progression of retinitis pigmentosa by optical coherence tomography angiography. Sci Rep 8:13130
Waizel M, Todorova MG, Terrada C, LeHoang P, Massamba N, Bodaghi B (2018b) Superficial and deep retinal foveal avascular zone OCTA findings of non-infectious anterior and posterior uveitis. Graefes Arch Clin Exp Ophthalmol 256:1977–1984
Acknowledgments
We thank Mr. Corrado della Volpe, MSc, for editing the manuscript and Mrs. Deborah Cosette and Mrs. Birgit Sandhoefner for their kind assistance with the PLEX Elite 9000 OCTA device.
Funding
Margarita G. Todorova was partially supported by unrestricted grant from OPOS (Stiftung Ostschweizerische Pleoptik- and Orthoptik-Schule). Hendrik P. N. Scholl was supported by the Swiss National Science Foundation, National Center of Competence in Research Molecular Systems Engineering “Molecular Systems Engineering,” the Wellcome Trust, and the Foundation Fighting Blindness Clinical Research Institute. Maria della Volpe Waizel was supported by the SAMW (Schweizerische Akademie der Medizinischen Wissenschaften), the Bangerter Foundation, and the SNF (Swiss National Science Foundation) with ad personam grants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the local IRB (Ethics Commission of Central and Northern Switzerland, EKNZ Basel Switzerland; trial number EKNZ BASEC 2017-00937).
Disclaimer
The sponsor had no role in the design or conduct of this research.
Conflict of interest
Margarita G. Todorova was partially supported by unrestricted grant from OPOS (Stiftung Ostschweizerische Pleoptik- and Orthoptik-Schule). Dr. Maria della Volpe Waizel was generously supported by the SAMW (Schweizerische Akademie der Medizinischen Wissenschaften), the Bangerter Foundation, and the SNF (Swiss National Science Foundation) with ad personam grants. Dr. Hendrik Scholl is supported by the Swiss National Science Foundation, National Center of Competence in Research Molecular Systems Engineering “Molecular Systems Engineering,” the Wellcome Trust, and the Foundation Fighting Blindness Clinical Research Institute.
Dr. Scholl is a member of the Scientific Advisory Board of: Astellas Institute for Regenerative Medicine; Gensight Biologics; Ionis Pharmaceuticals, Inc.; Gyroscope Therapeutics Ltd.; Janssen Research & Development, LLC (Johnson & Johnson); Pharma Research & Early Development (pRED) of F. Hoffmann-La Roche Ltd.; Novartis Pharma AG (CORE); and Retinagenix LLC. Dr. Scholl is paid consultant of: Boehringer Ingelheim Pharma GmbH & Co; Gerson Lehrman Group; and Guidepoint.
Dr. Scholl is member of the Data Monitoring and Safety Board/Committee of ReNeuron Group Plc/Ora Inc. and member of the Steering Committee of Novo Nordisk (FOCUS trial).
Dr. Scholl is co-director of the Institute of Molecular and Clinical Ophthalmology Basel (IOB) which is constituted as a non-profit foundation and receives funding from the University of Basel, the University Hospital Basel, Novartis, and the government of Basel-Stadt.
These arrangements have been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. Johns Hopkins University and Bayer Pharma AG have an active research collaboration and option agreement. These arrangements have also been reviewed and approved by the University of Basel (Universitätsspital Basel, USB) in accordance with its conflict of interest policies.
Dr. Hendrik Scholl is principal investigator of grants at the USB sponsored by the following entities: IVERIC bio (Ophthotech Corporation), Kinarus AG, and Novartis Pharma AG. Grants at USB are negotiated and administered by the institution (USB) which receives them on its proper accounts. Individual investigators who participate in the sponsored project(s) are not directly compensated by the sponsor but may receive salary or other support from the institution to support their effort on the project(s).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised:The published online version contain mistake for the author Maria della Volpe Waizel name was incorrectly presented.
Electronic supplementary material
Supplemental Figure S1
presents the corresponding central macular b-scans of the examples shown in Fig. 1. (PNG 2794 kb)
Rights and permissions
About this article
Cite this article
Todorova, M.G., Scholl, H.P.N. & della Volpe Waizel, M. The impact of macular edema on microvascular and metabolic alterations in retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol 259, 643–652 (2021). https://doi.org/10.1007/s00417-020-04913-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-020-04913-3