Abstract
Purpose
Previously, we measured retinal large vessels and capillaries separately on optical coherence tomography angiography (OCTA). In the present study, we aim to evaluate the role of these parameters in association to diabetic macular edema (DME) and ellipsoid zone disruption (EZD).
Methods
In this cross-sectional study, 54 eyes from 31 patients (10 females, 31 Asians) with severe non-proliferative diabetic retinopathy (25 eyes) or proliferative diabetic retinopathy (PDR, 29 eyes) were enrolled. All eyes underwent 3 × 3 mm OCTA scans centered on the fovea. Perfusion density (PD), vessel length density (VLD), and vessel diameter index (VDI) were calculated for retinal large vessels and superficial capillaries separately. Other OCTA findings included suspended scattering particles in motion (SSPiM), number of microaneurysms (MA) in all retinal layers, and the area of foveal avascular zone (FAZ) of superficial capillary plexus. DME and EZD were evaluated on B-scans. Both univariate and multivariate analysis were performed.
Results
Of the 54 study eyes, 31 (57%) had DME and 21 (40%) had EZD. Multivariate regression model showed that PDR (β = 27.8, 95% confidence interval (CI): 2.7–282.8, p = 0.005), more MA (β = 2.5, 95% CI: 1.3–4.5, p = 0.003), and increased VDI of larger vessels (β = 1.9, 95% CI: 1.0–3.5, p = 0.047) were risk factors for DME. As for EZD, presence of SSPiM (β = 5.5, 95% CI: 1.2–26.1, p = 0.032) and increased VDI of capillaries (β = 3.9, 95% CI: 1.1–13.8, p = 0.034) were risk factors.
Conclusions
In eyes with diabetic retinopathy, dilation of retinal larger vessels was associated with macular edema, while dilation of retinal capillaries was associated with ellipsoid zone disruption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.

Introduction
Diabetic macular edema (DME) and ellipsoid zone disruption (EZD) are common causes of visual impairment in patients with diabetic retinopathy [1, 2]. In recent years, optical coherence tomography angiography (OCTA) has been widely used to evaluate the microvasculature of diabetic retinopathy [3].
Several diagnostic markers on OCTA have been reported to be correlated to the development of DME or EZD. One prospective study revealed that vessel density of superficial capillary plexus (SCP) predicted the development of DME [4]. While another study reported that the vessel density and foveal avascular zone (FAZ) of deep capillary plexus (DCP) were associated with DME [5]. In some cross-sectional studies, deep capillary nonperfusion [6] and vessel density measurements in both SCP and DCP [7] were found to be associated with disruption of outer retinal layers. Results related to SCP were not quite consistent. The retinal SCP includes capillaries and larger vessels (arterioles and venules) and these two types of vessels might act differently in association with DME or EZD.
In our previous study, retinal large vessels (arterioles and venules) and superficial capillaries were separately auto-segmented and measured based on OCTA. We found that perfusion density (PD) of retinal capillaries significantly decreased while the PD of retinal large vessels increased with more severe diabetic retinopathy [8]. Consequently, in this present study, we aim to investigate relevant DME and EZD prognostic markers with distinct evaluation of retinal larger vessels and capillaries on the OCTA en face images.
Methods
Image acquisition
In this cross-sectional study, patients diagnosed with severe non-proliferative diabetic retinopathy (sNPDR) or proliferative diabetic retinopathy (PDR) in one or both eyes were enrolled. Clinical diagnosis was made by dilated fundus examination performed independently by two retina specialists. Eyes were excluded if (1) refractive media was unclear to acquire a gradable image; (2) other confounding retinal diseases possibly affecting vascular or macular function were present, for example, retinal vein occlusion, retinal artery occlusion, advanced age-related macular degeneration, or macular hole; and (3) refractive error<− 6.0D or > 3.0D.
All eyes underwent at least one 3 × 3 mm OCTA scan (AngioPlex 10.0, Cirrus HD-5000, Carl Zeiss Meditec Inc., Dublin, CA) centered on the fovea. Scans were repeated if obvious motion artifacts, decentered macula, or a low signal strength (SS < 7) were detected. The study was approved by the Institutional Review Board of the First Affiliated Hospital of Xi’an Jiaotong University and conducted in accordance with the ethical standards stated in the Declaration of Helsinki. Written informed consent was obtained from all individuals prior to their participation in the study.
Image processing and grading
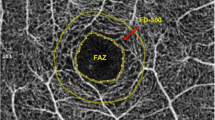
En face images of superficial capillary plexus (SCP, automatically segmented from the internal limiting membrane to the defined boundary of inner plexiform layer) were generated by the AngioPlex software (version 10. 0). Image-manipulating process has been described in our previous study [8]. Perfusion density (PD, defined as the percentage of the vessels within 1- to 3-mm ring centered on the fovea) and vessel length density (VLD, defined as the vessel length per unit area) of the retinal total vasculature and large vessels (PDlarge and VLDlarge) (Fig. 1) were generated separately using a proprietary software developed by our group [9]. Retinal large vessels were defined as the arcades and their first and second branches. The test of this segmentation accuracy has been reported in our previous study [8]. The PD and VLD of retinal superficial capillaries, specified as PDcap and VLDcap, were calculated by extracting the corresponding measurements of large vessels from those of the total vasculature. The vessel diameter indexes of retinal capillaries (VDIcap) and large vessels (VDIlarge) were calculated as PDcap/VLDcap and PDlarge/VLDlarge, respectively. Foveal avascular zone (FAZ) of SCP was manually segmented by a certified retina specialist (J. L.) and the area was calculated using Image J (version 2.0.0-rc-43; U.S. National Institutes of Health, Bethesda, MD).
OCTA en face image (3 × 3 mm, centered on the fovea) of superficial capillary plexus (left). Binarized image with large vessel segmentations from the left image (middle). Skeletonized image of the retinal large vessels of the same eye (right). The parameters PDlarge and VLDlarge were calculated within 1- to 3-mm ring (yellow circles) based on the middle and the right image, respectively
Additional OCTA parameters were also evaluated. One is the presence of suspended scattering particles in motion (SSPiM) described as unanimous patchy non-blood flow high signals (Fig. 2) on en face images of either SCP or deep capillary plexus (DCP, a layer between the defined boundaries of the inner and outer plexiform layers). Microaneurysms (MA) identified as small round or oval hyperreflective lesions [10] were counted from the whole retinal vasculature (layers composed of SCP, DCP, and the avascular layer). An example of MAs is shown in Fig. 3.
Both DME and EZD were graded on the OCTA B-scans corresponding to the 3 × 3 mm en face images. DME was defined as the presence of retinal thickening, intraretinal cyst, or subretinal fluid on B-scans. The minimal length of the ellipsoid zone disruption to qualify as a definite EZD was 250 microns. All image gradings were performed by two independent retina specialists (J.L. & L.C.). Discrepancies were resolved by open adjudication to reach a consensus.
Statistics
Spearman’s rho test was used to evaluate correlations between each candidate factor and DME or EZD. The factors included age, gender, stage of diabetic retinopathy (sNPDR or PDR), and the features on OCTA en face images (PDcap, VLDcap, VDIcap, PDlarge, VLDlarge, VDIlarge, area of FAZ, number of MA, and SSPiM). Then, binary logistic regression was applied to perform multivariate analysis, in which independent variables were examined separately from statistically significant ones in the univariate analysis. If PD, VLD, and VDI were significantly correlated simultaneously in the univariate analysis, only VLD and VDI were included in the multivariate analysis. SPSS was used for all the statistics (IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY: IBM Corp). p value of < 0.05 was considered statistically significant.
Results
Demographics
A total of 54 eyes from 31 patients (10 females, 31 Asians) were enrolled in this study. Mean age of all patients was 56 ± 10 (32–71) years. Among the 54 eyes, 25 were diagnosed with sNPDR and the rest 29 eyes were diagnosed with PDR. Fifty-seven percent of all imaged eyes (31/54) had DME and 40% (21/54) were confirmed with EZD. SSPiM were found in 24 eyes (44%, 24/54), which were all found to have DME. Table 1 illustrates the findings related to the number of MA, area of FAZ, PDlarge, VLDlarge, VDIlarge, PDcap, VLDcap, and VDIcap.
Imaging biomarkers of DME
The results of univariate analysis are shown in Table 2. DR staging (r = 0.402; p = 0.003), presence of SSPiM (r = 0.770; p < 0.001), number of MA (r = 0.477; p < 0.001), PDlarge (r = 0.369; p = 0.006), VLDlarge (r = 0.299; p = 0.028), VDIlarge (r = 0.342; p = 0.011), and VLDcap (r = − 0.314; p = 0.021) were found to be correlated with DME. Since SSPiM results from DME, they were excluded from the multivariate analysis, which showed that the DR staging (β = 27.8, 95% CI: 2.7–282.8; p = 0.005), the number of MA (β = 2.5, 95% CI: 1.3–4.5; p = 0.003), and VDIlarge (β = 1.9, 95% CI: 1.0–3.5; p = 0.047) were independent predictors of DME.
Imaging biomarkers of EZD
Results of the univariate analysis are demonstrated in Table 2. DR staging (r = 0.284, p = 0.038), presence of SSPiM (r = 0.357, p = 0.008), area of FAZ (r = 0.303, p = 0.026), PDcap (r = − 0.323, p = 0.017), VLDcap (r = − 0.384, p = 0.004), and VDIcap (r = 0.403, p = 0.002) were found to be correlated with EZD. The multivariate analysis showed that VDIcap (β = 3.9, 95% CI: 1.1–13.8, p = 0.034) and SSPiM (β = 5.5, 95% CI: 1.2–26.1, p = 0.032) were independent predictors of EZD. However, FAZ had a marginal level of significance (β = 52.9, 95% CI: 1.0–2787.8, p = 0.050).
Discussion
In this study, we found that the development of DME has much closer relationship with retinal larger vessels than that of retinal superficial capillaries. The likelihood of DME development would increase by 1.9 times if the diameter of retinal large vessels enlarged by 1 μm, independent of the severity of diabetic retinopathy. While, as for EZD, the parameters of retinal capillaries were more relevant. The probability of EZD would rise by 3.9 times if the superficial capillaries dilated by 1 μm.
Sun et al. [4] demonstrated that vessel density of SCP predicted development of DME. While Lee et al. [5] reported significant differences in vascular flow density in DCP but not SCP between DME eyes and non-DME eyes. Another study found that, in areas of edema where the retinal thickness was more than 400 μm, 91.3 ± 9.1% of the microaneurysms were found in the DCP [11]. However, the measurements of DCP could usually be compromised due to the projection artifacts and segmentation errors, especially in the settings of prominent retinal edema. Thus, we did not measure the DCP and the counting of MA was based on the whole retinal layers. The retinal large vessels defined in this study were the third- and fourth-grade retinal arterioles and venules in the 3 × 3 mm en face images centered on the fovea. We hypothesize that arteriolar and venular dilation could be related to retinal hyper-perfusion which has high likelihood to cause macular edema. Previous studies based on color fundus photographs (CFP) have confirmed that retinal venular dilation was associated with DME [12, 13]. Compared to CFP, OCTA provides more accurate measurements, particularly for smaller vessels.
Ellipsoid zone disruption has been confirmed to contribute to macular malfunction [2, 14]. It has been suggested that the dropout of DCP partially contributed to the outer retina disruption [6, 15]. Ahuja et al. [16] reported central subfield thickness and cube average thickness as biomarkers for EZD in diabetic retinopathy. More recently, Borrelli et al. [17] reported the association between ellipsoid zone reflectivity and choroidal capillary perfusion density in NPDR, indicating a photoreceptor damage in retinal choroidopathy. The blood supply of outer retina comes from the choroidal capillaries. Interestingly, in our study, EZD was correlated with the measurements of superficial retinal capillaries, and it is the caliber rather than the amount of capillaries that was more relevant. We believe that the photoreceptors damage in diabetic retinopathy may not be simply explained by ischemia causing retinal pigment epithelium atrophy or choroidal neovascularization, which do not constitute frequent findings in diabetic retinopathy. We also hypothesize that Müller cells which span from the internal limiting membrane to the outer retina and are critical in maintaining the retinal structures might be more relevant to the pathogenesis.
Müller cell destruction from chronic hyperglycemia could result in both retinal capillary dilation and photoreceptor damage. Evidences from previous animal studies have shown that primary Müller cell loss could lead to photoreceptor degenerations[18] and retinal telangiectasia [19]. Results from a previous clinical study also suggest that the same pathogenic pathways that create conditions for inner retinal disorganization also disrupt outer retinal architecture [20]. We believe that the caliber of superficial capillaries could be a better biomarker for EZD compared with the metrics of deeper layer vessels due to the free-of-projection artifact, less segmentation artifact, and high repeatability and reproducibility of retinal superficial microvasculature measurements [21].
Another finding of our study is the association between SSPiM and EZD. Kashani et al. first discussed the SSPiM as a novel feature on OCTA in eyes with hyperreflective intraretinal fluid on structural OCT, and suggested that it was caused by more advanced breakdown of the blood-retinal barrier, so that large particles, most likely the lipid, entered the intraretinal space [22]. Breakdown of the blood-retinal barrier in DME could create optimum conditions for damaging the outer retina.
The major limitation of this study is its cross-sectional design. A longitudinal study is warranted to confirm the predictive value of microvascular changes on OCTA for the macular structural changes. Another limitation is the lack of data on blood pressure status of included patients, or their diabetic status (level of glycosylated hemoglobin, urea, and creatinine[23]).
In summary, density of microaneurysms and retinal large vessel dilation could be potential predictors for the development of DME. Dilation of retinal superficial capillaries and the presence of SSPiM on either SCP or DCP could also prove to be useful clinical predictors for EZD. Further longitudinal studies are needed to establish a prognostic assessment for these imaging biomarkers for patients undergoing prospective trials or receiving long-term follow-up in clinic.
Data availability
The data is available on demand.
References
Das A, McGuire PG, Rangasamy S (2015) Diabetic macular edema: pathophysiology and novel therapeutic targets. Ophthalmology 122:1375–1394
Yohannan J, Bittencourt M, Sepah YJ et al (2013) Association of retinal sensitivity to integrity of photoreceptor inner/outer segment junction in patients with diabetic macular edema. Ophthalmology 120:1254–1261. https://doi.org/10.1016/j.ophtha.2012.12.003
Kim AY, Chu Z, Shahidzadeh A et al (2016) Quantifying microvascular density and morphology in diabetic retinopathy using spectral-domain optical coherence tomography angiography. Investig Ophthalmol Vis Sci 57:OCT362–OCT370. https://doi.org/10.1167/iovs.15-18904
Sun Z, Tang F, Wong R et al (2019) OCT angiography metrics predict progression of diabetic retinopathy and development of diabetic macular edema: a prospective study. Ophthalmology 126:1675–1684. https://doi.org/10.1016/j.ophtha.2019.06.016
Lee J, Moon BG, Cho AR, Yoon YH (2016) Ophthalmology 123(11):2368–2375. https://doi.org/10.1016/j.ophtha.2016.07.010
Scarinci F, Nesper PL, Fawzi AA (2016) Deep retinal capillary nonperfusion is associated with photoreceptor disruption in diabetic macular ischemia. Am J Ophthalmol 168:129–138. https://doi.org/10.1016/j.ajo.2016.05.002
Uğurlu N, Taşlıpınar Uzel AG, Şengün A et al (2019) Evaluation of the correlation between quantitative measurement of the foveal avascular zone and retinal vessel density and outer retinal disruptions in diabetic patients. Turkish J Med Sci 49:1041–1046. https://doi.org/10.3906/sag-1901-22
Lei J, Yi E, Suo Y et al (2018) Distinctive analysis of macular superficial capillaries and large vessels using optical coherence tomographic angiography in healthy and diabetic eyes. Investig Ophthalmol Vis Sci 59. https://doi.org/10.1167/iovs.17-23676
Xu X, Chen C, Ding W et al (2019) Automated quantification of superficial retinal capillaries and large vessels for diabetic retinopathy on optical coherence tomographic angiography. J Biophotonics 12. https://doi.org/10.1002/jbio.201900103
La Mantia A, Kurt RA, Mejor S et al (2019) Comparing fundus fluorescein angiography and swept-source optical coherence tomography angiography in the evaluation of diabetic macular perfusion. Retina 39:926–937. https://doi.org/10.1097/IAE.0000000000002045
Hasegawa N, Nozaki M, Takase N et al (2016) New insights into microaneurysms in the deep capillary plexus detected by optical coherence tomography angiography in diabetic macular edema. Investig Ophthalmol Vis Sci 57:OCT348–OCT355. https://doi.org/10.1167/iovs.15-18782
Muraoka Y, Murakami T, Nishijima K et al (2014) Association between retinal venular dilation and serous retinal detachment in diabetic macular EDEMA. Retina 34:725–731. https://doi.org/10.1097/IAE.0b013e3182a48732
Klein R, Myers CE, Lee KE et al (2012) Changes in retinal vessel diameter and incidence and progression of diabetic retinopathy. Arch Ophthalmol 130:749–755. https://doi.org/10.1001/archophthalmol.2011.2560
DaCosta J, Bhatia D, Talks J (2019) The use of optical coherence tomography angiography and optical coherence tomography to predict visual acuity in diabetic retinopathy. Eye (Lond). https://doi.org/10.1038/s41433-019-0606-9
Scarinci F, Jampol LM, Linsenmeier RA, Fawzi AA (2015) Association of diabetic macular nonperfusion with outer retinal disruption on optical coherence tomography. JAMA Ophthalmol 133:1036–1044. https://doi.org/10.1001/jamaophthalmol.2015.2183
Ahuja S, Saxena S, Meyer CH et al (2018) Central subfield thickness and cube average thickness as bioimaging biomarkers for ellipsoid zone disruption in diabetic retinopathy. Int J Retina Vitreous 4. https://doi.org/10.1186/s40942-018-0144-9
Borrelli E, Palmieri M, Viggiano P et al (2020) Photoreceptor damage in diabetic choroidopathy. Retina 40:1062–1069. https://doi.org/10.1097/IAE.0000000000002538
Shen W, Fruttiger M, Zhu L et al (2012) Conditional Müller cell ablation causes independent neuronal and vascular pathologies in a novel transgenic model. J Neurosci 32:15715–15727. https://doi.org/10.1523/JNEUROSCI.2841-12.2012
Zhao M, Andrieu-Soler C, Kowalczuk L et al (2015) A new CRB1 rat mutation links Müller glial cells to retinal telangiectasia. J Neurosci 35:6093–6106. https://doi.org/10.1523/JNEUROSCI.3412-14.2015
Das R, Spence G, Hogg RE et al (2018) Disorganization of inner retina and outer retinal morphology in diabetic macular edema. JAMA Ophthalmol 136:202–208. https://doi.org/10.1001/jamaophthalmol.2017.6256
Lei J, Durbin MK, Shi Y et al (2017) Repeatability and reproducibility of superficial macular retinal vessel density measurements using optical coherence tomography angiography en face images. JAMA Ophthalmol. https://doi.org/10.1001/jamaophthalmol.2017.3431
Kashani AH, Green KM, Kwon J et al (2018) Suspended scattering particles in motion: a novel feature of OCT angiography in exudative maculopathies. Ophthalmol Retina 2:694–702. https://doi.org/10.1016/j.oret.2017.11.004
Saxena S, Ruia S, Prasad S et al (2017) Increased serum levels of urea and creatinine are surrogate markers for disruption of retinal photoreceptor external limiting membrane and inner segment ellipsoid zone in type 2 diabetes mellitus. Retina 37:344–349. https://doi.org/10.1097/IAE.0000000000001163
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The study was approved by the Institutional Review Board of the First Affiliated Hospital of Xi’an Jiaotong University and conducted in accordance with the ethical standards stated in the Declaration of Helsinki.
Consent to participate
Written informed consent was obtained from all individuals prior to their participation in the study.
Consent for publication
Consent from all the authors was achieved.
Code availability
Custom code is available on demand.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lei, J., Xu, X., Chen, L. et al. Dilated retinal large vessels and capillaries associated with diabetic macular edema and photoreceptor loss respectively. Graefes Arch Clin Exp Ophthalmol 259, 1831–1837 (2021). https://doi.org/10.1007/s00417-020-05039-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-020-05039-2