Abstract
Background
Inadequate screening of treatment-warranted retinopathy of prematurity (ROP) can lead to devastating visual outcomes. Especially in resource-poor communities, the use of an affordable, portable, and easy to use smartphone-based non-contact fundus photography device may prove useful for screening for high-risk ROP. This study evaluates the feasibility of screening for high-risk ROP using a novel smartphone-based fundus photography device, RetinaScope.
Methods
Retinal images were obtained using RetinaScope on a cohort of prematurely born infants during routine examinations for ROP. Images were reviewed by two masked graders who determined the image quality, the presence or absence of plus disease, and whether there was retinopathy that met predefined criteria for referral. The agreement between image-based assessments was compared to the gold standard indirect ophthalmoscopic assessment.
Results
Fifty-four eyes of 27 infants were included. A wide-field fundus photograph was obtained using RetinaScope. Image quality was acceptable or excellent in 98% and 95% of cases. There was substantial agreement between the gold standard and photographic assessment of presence or absence of plus disease (Cohen’s κ = 0.85). Intergrader agreement on the presence of any retinopathy in photographs was also high (κ = 0.92).
Conclusions
RetinaScope can capture digital retinal photographs of prematurely born infants with good image quality for grading of plus disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Retinopathy of prematurity (ROP) is primarily a vasoproliferative disorder affecting low birth weight premature infants. Despite advances in treatment strategies, including laser photocoagulation or anti-VEGF agents, ROP continues to be a leading cause of childhood blindness in the world due to inadequate screening [1]. Barriers to inadequate screening are many, including shortage of ophthalmologists trained and willing to screen for ROP and the lack of patient access to trained ophthalmologists in resource-poor communities.
Although binocular indirect ophthalmoscopy (BIO) by an experienced ophthalmologist remains the gold standard for staging ROP, alternative methods such as retinal imaging and telemedicine have been proposed to improve the screening for treatment-warranted ROP. Commercially available wide-field retinal photography devices, such as the RetCam (Clarity Medical Systems, Pleasanton, CA) or Phoenix Icon, have been used in several large clinical trials to evaluate the utility of retinal photography for ROP screening. For example, in the Stanford University Network for the Diagnosis of Retinopathy of Prematurity (SUNDROP) [2,3,4] initiative, 608 premature newborns requiring ROP screening were evaluated via remote retinal photography using RetCam II/III over a 6-year period. The images were interpreted by a masked ROP specialist for treatment-warranted disease. During the 6 years of the study, no cases of treatment-warranted ROP went undetected as confirmed by bedside BIO and no adverse anatomical outcomes were observed. Similarly, the e-ROP study was a multicenter clinical trial where trained non-physician imagers acquired photographs of the retina using RetCam and independent graders evaluated the retinal photographs for evidence of ROP and the image-based assessment was compared with clinical examination [5]. Of the 1257 infants screened, 19.4% infants had characteristics of referral-warranted ROP and the sensitivity of remote grading of images was 81.9% and specificity 90.1%.
These studies established the accuracy of telemedicine as a screening tool using ophthalmoscopy as the reference standard. However, many other studies have also suggested that significant variability exists in ROP grading among experienced examiners [6,7,8]. The Imaging and Informatics in ROP Research Consortium evaluated whether telemedicine or ophthalmoscopy is more accurate in diagnosing clinically significant ROP when compared to a reference standard diagnosis [9]. In this study, a reference standard diagnosis was generated by integration of the telemedicine diagnosis by 3 independent image graders and the ophthalmoscopic diagnosis. There was no difference in sensitivity between ophthalmoscopy and telemedicine for detecting type 2 or worse ROP.
These results support the use of telemedicine for the diagnosis of clinically significant ROP. Although RetCam is able to acquire high-quality, wide-field retinal photographs, there are several design features that may limit its accessibility in resource-poor communities. These include the high cost of the device, the need for an experienced photographer or technician to operate the device, and the need to make contact with the ocular surface in order to acquire an image. We have developed a novel, portable, non-contact, handheld smartphone-based retinal camera (RetinaScope) capable of capturing high-quality, wide-field fundus images [10, 11]. The use of the mobile phone platform creates a fully embedded system for acquisition, storage, and wireless transfer of images for remote evaluation. Additionally, the familiarity of users with smartphone photography technology creates an intuitive and easy-to-use system for fundus photography. In this study, we investigated the utility of RetinaScope in screening high-risk treatment-warranted ROP, compared to the gold standard of dilated fundus examination with scleral depression by indirect ophthalmoscopy. We focus on accurate detection of plus disease, defined as arterial tortuosity and venous dilation in the posterior pole, because it is a critical component of the International Classification of ROP (ICROP) [12]. The presence of plus disease is a necessary feature for threshold disease and sufficient feature for type I ROP, both of which have been shown to warrant prompt treatment with either laser photocoagulation or cryotherapy. Therefore, early and accurate diagnosis of plus disease is essential to prevent vision-threatening disease.
Methods
Ethical considerations
This study was approved by the Institutional Review Board at the University of Michigan and Sparrow Hospital, Michigan State University. All research was conducted in compliance with human subjects regulations and adhered to the tenets of the Declaration of Helsinki. This study was registered on ClinicalTrials.gov, identifier NCT03076697.
Participants and photography protocol
Infants who met the American Academy of Pediatrics and American Academy of Ophthalmology ROP screening criteria were recruited from the neonatal intensive care unit and the outpatient clinic at the C.S. Mott Children’s Hospital at the University of Michigan (Ann Arbor, Michigan) and Sparrow Hospital (Lansing, Michigan). Patients’ parents or legal guardians provided informed consent for photography. The study was conducted from May 2018 to September 2018. Each patient underwent a dilated fundus examination with scleral depression by an ophthalmologist experienced in ROP screening (PL, CB). A single drop of mydriatic and anesthetic agents consisting of phenylephrine 2.5%, cyclopentolate 0.5%, tropicamide 1%, and proparacaine 0.5% was used for dilation. An eyelid speculum was used for clinical examination and fundus photography. Following clinical examination, photographs of the retina were acquired with RetinaScope by an ophthalmology resident (TPP) and a research assistant (MTA) from standard 5 fields of view—central (posterior pole), nasal, temporal, superior, and inferior. Briefly, the RetinaScope device weighs less than a third of a kilogram and consists of a 3D-printed plastic housing containing optics for illuminating and imaging the retina using the camera of a smartphone (iPhone 5s, Apple, Cupertino, CA) [13]. The device was powered by a rechargeable lithium battery (Fig. 1A, B). The retina was imaged through a 54-diopter ophthalmic lens (Ocular Instruments OI54-A, Bellevue, WA) which forms an intermediate image that was then relayed by a 20-mm focal length achromatic lens (Edmund Optics 47661, Barrington, NJ) to the camera of an iPhone 5s. The auto-focus feature of the iPhone camera was used to correct for refractive error. Each photograph captured approximately 50° of the retina with a resolution of 52.3 pixels/retinal degree. In many cases, a continuous video recording of the fundus was acquired as the child spontaneously shifted gaze. The user then scanned through the video and selected useful frames for image grading.
During exams in the NICU, the nurses closely monitored infant’s vital signs, cardiopulmonary status, and oxygen saturations. If bradycardia, apnea, or other abnormalities developed during retinal imaging, the examination was halted and the NICU staff immediately stabilized the infant. The demographic data, clinical findings, and diagnoses of all participants were recorded in a Health Insurance Portability and Accountability Act (HIPAA) compliant database.
Image grading
All photographs were graded by two independent masked pediatric retina specialists who had not participated in the patients’ clinical care (VSD, CX). No additional information such as birth weight, systemic comorbidities, or postmenstrual age was provided to ensure that the graders focused only on retinal features. The images were presented in a randomized order. The graders assessed the overall photograph quality (excellent, acceptable, and not gradable) using the following criteria: a photograph was considered excellent if it was in focus and the entire posterior pole was visualized and the grader could easily discern the dilation or tortuosity of vessels. A photograph was considered acceptable if it was overexposed, underexposed, or out of focus but adequate to determine the presence or absence of vessel dilation or tortuosity. An ungradable photograph was one where the image was out of focus or obscured by glare or motion artifact.
For each photograph, graders determined the presence or absence of plus disease, defined as the presence of sufficient vascular dilation and tortuosity present in at least 2 quadrants of the eye as compared to a standard photograph. A photograph was deemed equivocal if the grader could not definitively make a determination of plus disease. Photograph was deemed “referable” if graders noted evidence of a ridge or retinal hemorrhages, regardless of location. Cohen’s kappa coefficient was computed to determine the agreement between photographic assessment and clinical assessment of plus disease, as well as intergrader agreement whether a photograph met criteria for referral.
Statistical analysis
Statistical analysis was performed using JMP version 13 (SAS Institute, Cary, NC). All variables were graphically examined for normal distributions and outliers to determine the appropriate statistical tests. Measures of central tendency and variation were used to describe the study population. Infants with plus disease were compared with non-plus cohort with respect to baseline characteristics using t test and chi-squared analysis as appropriate. Statistical significance level was set as a two-tailed test with alpha < 0.05. The graders’ assessments were compared to each other and to the gold standard clinical exam using Cohen’s kappa statistic with 95% confidence intervals (CI), which corrects the observed percentage of agreements between the raters for the effect of chance. A value of 0 implies no agreement beyond chance, whereas a value of 1 corresponds to a perfect agreement between the two graders. Clinical examination with indirect ophthalmoscopy served as the gold standard.
Results
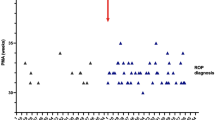
The primary outcome measure of this study was to determine whether a handheld, portable, non-contact, smartphone-based fundus photography device can acquire photographs of diagnostic value for assessment of plus disease in premature infants undergoing ROP screening. A total of 27 premature infants (14 males, 13 females) were recruited in this study (Table 1). The mean birth weight of all patients was 838 g (standard deviation (SD) 318 g); mean gestational age was 27 weeks and 6 days (SD 3.3 weeks). All of the consented patients were included in the analysis. No patients were excluded. No patients developed bradycardia, apnea, or other abnormalities during smartphone photography requiring premature termination of the photography session.
Of the 27 infants examined, 2 patients (3 eyes) were classified as having plus disease and 5 patients (9 eyes) had ROP in zone I or II based on clinical examination by indirect ophthalmoscopy. The two infants with plus disease had a lower birth weight (645 ± 177 g) and earlier gestational age (25.7 ± 2.8 weeks) compared to the cohort of infants without plus disease.
Fundus photographs or video recordings of both eyes of each infant were acquired with RetinaScope. Average time for image acquisition was 2.2 ± 1.1 min. A single image acquired with RetinaScope captures approximately 50° of the retina. The images from different fields of view were aligned and stitched together to generate an approximately 90° wide-field montage photograph on the smartphone as described before (Fig. 2A) [11]. The overall image quality of RetinaScope was similar to commercially available RetCam3 (Fig. 2B). Representative images acquired with RetinaScope of normal, retinopathy with plus disease, and vascular abnormalities such as presence of ridge and retinal hemorrhage are shown in Fig. 3.
Image quality as assessed by the two masked graders was 2% and 5% as not gradable, 49% and 49% as acceptable, and 49% and 46% as excellent (Table 2). Images deemed “not gradable” were excluded from subsequent analysis. No infant with clinically determined plus disease was missed by the masked graders (false negative 0 for each grader). Cohen’s κ as a measure of interrater agreement between the two methods of screening for plus disease (photograph-assessment vs. indirect ophthalmoscopy examination) was 1.0 and 0.85 for the two graders, respectively. Similarly, the intergrader agreement on the presence or absence of any retinopathy in photographs was high (κ = 0.92). There were 5 and 2 eyes where the graders noted posterior pole pathology that did not meet their criteria for plus disease. Three patients had intraretinal hemorrhages in zone 2 and one patient had a ridge in zone 2. Both graders accurately noted these pathologies on photographs. Two patients had a ridge in zone 3, which was not well photographed with RetinaScope and neither grader was able to identify.
Discussion
Retinopathy of prematurity screening is a global health problem. Fundus photography and remote grading by experienced ophthalmologists have the potential to drastically increase access to care for many patients in resource-poor communities. While several studies have been conducted using the RetCam system, the high cost of the device, the need for experienced photographer to operate the device, and the need to make contact with the ocular surface to acquire an image may limit its accessibility in resource-poor communities. We have developed a portable, cost-effective, smartphone-based fundus imaging device capable of acquiring diagnostic quality retinal photographs for screening of plus ROP disease in prematurely born infants. Prior work demonstrated the use of Volk Pictor device (Volk Optical, Mentor, OH) for ROP screening [14]. Our work adds to the growing body of literature on the feasibility and utility of portable non-contact fundus photography devices for ROP screening. Although RetinaScope has a smaller field of view (50°), compared to RetCam (130°), the view afforded by RetinaScope was adequate to evaluate the posterior pole for the presence of vessel dilation and tortuosity consistent with plus disease. There may be instances where abnormal vessel morphology outside the posterior pole, especially near a ridge, may meet criteria for plus disease for a clinical examiner. These cases would be missed by a grader assessing vessel morphology in the posterior pole. However, we did not encounter this scenario in this small study sample. Furthermore, a wider field image can be generated by montaging retinal photographs from different fields of view which allowed assessment of hemorrhages or ridge beyond the posterior pole. Due to worse image quality of the peripheral retina, we did not attempt to localize the transition between avascular and vascularized retina.
Graders were asked to determine whether posterior pole vessel morphology appeared abnormal but did not meet their definition of plus disease; these images were labeled “equivocal.” In a screening model, these infants would require a clinical examination by an ophthalmologist. The presence of posterior pole vessel pathology has been associated with the development of treatment-requiring ROP [15] and similarly, normal posterior pole vessel morphology is a reliable marker for the absence of stage 3 ROP [16].
Smartphone technology has advanced significantly with better cameras, faster processors, and intuitive easy-to-use software design. The availability of smartphones has increased in both developed and developing countries, which makes smartphone-based screening for ophthalmic diseases a particularly attractive target. Preventable causes of blindness, such as cataract, glaucoma, diabetic retinopathy, and ROP may benefit from the use of teleophthalmology with smartphones [17, 18]. In addition, the health care cost of ROP screening for infants born at rural and regional hospitals is exceedingly high [19], while telemedicine-based remote screening has been shown to be a more cost-effective option [20, 21]. To our knowledge, this is the first study to report the feasibility and accuracy of screening for plus ROP using smartphone-based fundus photography.
The number of ophthalmologists willing and able to manage ROP is insufficient for a number of reasons, including low reimbursement [22], high liability, and specialized training required to properly screen ROP [23]. Meanwhile, the incidence of ROP worldwide is rising because of advances in neonatology [24]. Fraught with these challenges, there is a need to develop quantitative, reliable, affordable, and easy to use approach to ROP diagnosis [25]. Additionally, clinical diagnosis of plus disease is highly variable, and there is a high interobserver variability on the diagnosis even among ROP experts [7, 26]. This is because the diagnosis of plus disease is based on interpretation of venous dilation and arteriolar tortuosity, which are both continuous variables. The grader relies on an internal threshold to transform these continuous variables into a categorical outcome. Different graders may have a variable operating threshold for what they consider to be plus disease and may focus on different pathologic features or have different interpretations of the same features. Imaging and automated analysis will play a critical role in the diagnosis of ROP. Pour et al. described an algorithm for automated assessment of plus disease by mathematically computing vessel curvature and tortuosity [27]. Recently, Brown et al. [28] reported a deep learning–based algorithm for automated detection of plus disease using retinal images from premature infants with ROP. Their algorithm diagnosed plus disease with comparable or better proficiency than ROP experts. Incorporation of such automated system into a smartphone-based photography device could provide advice at the point of care and has the potential to improve the quality, cost, and accessibility of ROP screening.
References
Gilbert C (2008) Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev 84:77–82. https://doi.org/10.1016/j.earlhumdev.2007.11.009
Fijalkowski N, Zheng LL, Henderson MT, Wang SK, Wallenstein MB, Leng T, Moshfeghi DM (2014) Stanford University network for diagnosis of retinopathy of prematurity (SUNDROP): five years of screening with telemedicine. Ophthalmic Surg Lasers Imaging Retina 45:106–113. https://doi.org/10.3928/23258160-20140122-01
Wang SK, Callaway NF, Wallenstein MB, Henderson MT, Leng T, Moshfeghi DM (2015) SUNDROP: six years of screening for retinopathy of prematurity with telemedicine. Can J Ophthalmol 50:101–106. https://doi.org/10.1016/j.jcjo.2014.11.005
Fijalkowski N, Zheng LL, Henderson MT, Wallenstein MB, Leng T, Moshfeghi DM (2013) Stanford University Network for Diagnosis of Retinopathy of Prematurity (SUNDROP): four-years of screening with telemedicine. Curr Eye Res 38:283–291. https://doi.org/10.3109/02713683.2012.754902
Quinn GE, Ying GS, Daniel E, Hildebrand PL, Ells A, Baumritter A, Kemper AR, Schron EB, Wade K, e ROPCG (2014) Validity of a telemedicine system for the evaluation of acute-phase retinopathy of prematurity. JAMA Ophthalmol 132:1178–1184. https://doi.org/10.1001/jamaophthalmol.2014.1604
Wallace DK, Quinn GE, Freedman SF, Chiang MF (2008) Agreement among pediatric ophthalmologists in diagnosing plus and pre-plus disease in retinopathy of prematurity. J AAPOS 12:352–356. https://doi.org/10.1016/j.jaapos.2007.11.022
Chiang MF, Jiang L, Gelman R, Du YE, Flynn JT (2007) Interexpert agreement of plus disease diagnosis in retinopathy of prematurity. Arch Ophthalmol 125:875–880. https://doi.org/10.1001/archopht.125.7.875
Campbell JP, Ryan MC, Lore E, Tian P, Ostmo S, Jonas K, Chan RVP, Chiang MF, Imaging, Informatics in Retinopathy of Prematurity Research C (2016) Diagnostic discrepancies in retinopathy of prematurity classification. Ophthalmology 123:1795–1801. https://doi.org/10.1016/j.ophtha.2016.04.035
Biten H, Redd TK, Moleta C, Campbell JP, Ostmo S, Jonas K, Chan RVP, Chiang MF, Imaging, Informatics in Retinopathy of Prematurity Research C (2018) Diagnostic accuracy of ophthalmoscopy vs telemedicine in examinations for retinopathy of prematurity. JAMA Ophthalmol 136:498–504. https://doi.org/10.1001/jamaophthalmol.2018.0649
Maamari RN, Keenan JD, Fletcher DA, Margolis TP (2014) A mobile phone-based retinal camera for portable wide field imaging. Br J Ophthalmol 98:438–441. https://doi.org/10.1136/bjophthalmol-2013-303797
Patel TP, Kim TN, Yu G, Dedania VS, Lieu P, Qian CX, Besirli CG, Demirci H, Margolis T, Fletcher DA, Paulus YM (2019) Smartphone-based, rapid, wide-field fundus photography for diagnosis of pediatric retinal diseases. Transl Vis Sci Technol 8:29. https://doi.org/10.1167/tvst.8.3.29
International Committee for the Classification of Retinopathy of P (2005) The international classification of retinopathy of prematurity revisited. Arch Ophthalmol 123:991–999. https://doi.org/10.1001/archopht.123.7.991
Kim TN, Myers F, Reber C, Loury PJ, Loumou P, Webster D, Echanique C, Li P, Davila JR, Maamari RN, Switz NA, Keenan J, Woodward MA, Paulus YM, Margolis T, Fletcher DA (2018) A smartphone-based tool for rapid, portable, and automated wide-field retinal imaging. Transl Vis Sci Technol 7:21. https://doi.org/10.1167/tvst.7.5.21
Prakalapakorn SG, Wallace DK, Freedman SF (2014) Retinal imaging in premature infants using the Pictor noncontact digital camera. J AAPOS 18:321–326. https://doi.org/10.1016/j.jaapos.2014.02.013
Wallace DK, Freedman SF, Hartnett ME, Quinn GE (2011) Predictive value of pre-plus disease in retinopathy of prematurity. Arch Ophthalmol 129:591–596. https://doi.org/10.1001/archophthalmol.2011.63
Saunders RA, Bluestein EC, Sinatra RB, Wilson ME, O'Neil JW, Rust PF (1995) The predictive value of posterior pole vessels in retinopathy of prematurity. J Pediatr Ophthalmol Strabismus 32:82–85
Mohammadpour M, Heidari Z, Mirghorbani M, Hashemi H (2017) Smartphones, tele-ophthalmology, and VISION 2020. Int J Ophthalmol 10:1909–1918. https://doi.org/10.18240/ijo.2017.12.19
Rathi S, Tsui E, Mehta N, Zahid S, Schuman JS (2017) The current state of teleophthalmology in the United States. Ophthalmology 124:1729–1734. https://doi.org/10.1016/j.ophtha.2017.05.026
Yu TY, Donovan T, Armfield N, Gole GA (2018) Retinopathy of prematurity: the high cost of screening regional and remote infants. Clin Exp Ophthalmol. https://doi.org/10.1111/ceo.13160
Isaac M, Isaranuwatchai W, Tehrani N (2018) Cost analysis of remote telemedicine screening for retinopathy of prematurity. Can J Ophthalmol 53:162–167. https://doi.org/10.1016/j.jcjo.2017.08.018
Kovacs G, Somogyvari Z, Maka E, Nagyjanosi L (2017) Bedside ROP screening and telemedicine interpretation integrated to a neonatal transport system: economic aspects and return on investment analysis. Early Hum Dev 106-107:1–5. https://doi.org/10.1016/j.earlhumdev.2017.01.007
Braverman RS, Enzenauer RW (2010) Socioeconomics of retinopathy of prematurity in-hospital care. Arch Ophthalmol 128:1055–1058. https://doi.org/10.1001/archophthalmol.2010.151
Wallace DK (2012) Fellowship training in retinopathy of prematurity. J AAPOS 16(1). https://doi.org/10.1016/j.jaapos.2011.11.003
Mora JS, Waite C, Gilbert CE, Breidenstein B, Sloper JJ (2018) A worldwide survey of retinopathy of prematurity screening. Br J Ophthalmol 102:9–13. https://doi.org/10.1136/bjophthalmol-2017-310709
Moshfeghi DM (2018) Systemic solutions in retinopathy of prematurity. Am J Ophthalmol DOI. https://doi.org/10.1016/j.ajo.2018.05.013
Gschliesser A, Stifter E, Neumayer T, Moser E, Papp A, Pircher N, Dorner G, Egger S, Vukojevic N, Oberacher-Velten I, Schmidt-Erfurth U (2015) Inter-expert and intra-expert agreement on the diagnosis and treatment of retinopathy of prematurity. Am J Ophthalmol 160:553–560 e553. https://doi.org/10.1016/j.ajo.2015.05.016
Pour EK, Pourreza H, Zamani KA, Mahmoudi A, Sadeghi AMM, Shadravan M, Karkhaneh R, Pour RR, Esfahani MR (2017) Retinopathy of prematurity-assist: novel software for detecting plus disease. Korean J Ophthalmol 31:524–532. https://doi.org/10.3341/kjo.2015.0143
Brown JM, Campbell JP, Beers A, Chang K, Ostmo S, Chan RVP, Dy J, Erdogmus D, Ioannidis S, Kalpathy-Cramer J, Chiang MF, Imaging, Informatics in Retinopathy of Prematurity Research C (2018) Automated diagnosis of plus disease in retinopathy of prematurity using deep convolutional neural networks. JAMA Ophthalmol. https://doi.org/10.1001/jamaophthalmol.2018.1934
Acknowledgments
We thank Sparrow Hospital (Lansing, MI) for participating in this study and allowing the authors to recruit patients.
Financial support
This work was supported by the Knights Templar Eye Foundation Career-Starter Research Grant (TPP, TNK, YMP), 1K08EY027458 (YMP), the University of Michigan Translational Research and Commercialization for Life Sciences (TNK, YMP), the University of Michigan Center for Entrepreneurship Dean’s Engineering Translational Prototype Research Fund (TNK, YMP), the QB3 Bridging the Gap Award from the Rogers Family Foundation (DAF), the Bakar Fellows Award (DAF), the Chan-Zuckerberg Biohub Investigator award (DAF), the National Eye Institute grant 4K12EY022299 (YMP), the University of Michigan Department of Ophthalmology and Visual Sciences, and unrestricted departmental support from Research to Prevent Blindness. The funding agency was not involved in the study design, collection, analysis, interpretation of data, or writing of the text.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Institutional Review Board at the University of Michigan and Sparrow Hospital, Michigan State University. All research was conducted in compliance with human subjects regulations and adhered to the tenets of the Declaration of Helsinki. This study was registered on ClinicalTrials.gov, identifier NCT03076697.
Conflict of interest
DAF is a co-founder of CellScope, Inc., a company commercializing a cellphone-based otoscope, and holds shares in CellScope, Inc. DAF and TNK are inventors on US patents and related applications pertaining to a “Retinal CellScope Apparatus.”
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Patel, T.P., Aaberg, M.T., Paulus, Y.M. et al. Smartphone-based fundus photography for screening of plus-disease retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 257, 2579–2585 (2019). https://doi.org/10.1007/s00417-019-04470-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04470-4