Abstract
Purpose
To investigate the predictive factors for recurrence of polypoidal choroidal vasculopathy (PCV).
Methods
The medical records of 78 consecutive patients (78 eyes) with treatment-naïve PCV who responded to first-line treatment and completed at least a 3-year follow-up after the first remission were retrospectively analyzed. In this comparative cohort study, baseline characteristics were compared between the patients who had at least one recurrence (the recurrence group) and those without recurrence (the non-recurrence group) during at least 3-year follow-up periods. In addition, possible predictive factors for recurrence of PCV were investigated by using Cox regression analysis.
Results
Within 3 years of the first remission, 50 eyes (64 %) showed at least one recurrence (mean 1.5; 1 to ∼2 times). There were no significant differences in the baseline characteristics between the recurrence group and the non-recurrence group. However, the largest polyp diameter was significantly different: the mean largest polyp diameter (524 ± 340 μm) was significantly larger in the recurrence group compared to that of the non-recurrence group (352 ± 173 μm; P = 0.038). Cox regression analysis showed that the largest polyp diameter at baseline significantly correlated with recurrence of PCV (B = 1.470, P = 0.015).
Conclusions
The largest polyp diameter at baseline may be predictive for PCV recurrence, as it was significantly larger in patients who had at least one recurrence.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polypoidal choroidal vasculopathy (PCV) is characterized by abnormal branching vascular networks and terminating polypoidal lesions, which are most evident by indocyanine green angiography (ICGA) [1–5]. PCV occurs frequently in Asian populations, accounting for up to 50 % of presumed exudative age-related macular degeneration (AMD) [4–8]. Currently, PCV is generally considered to be a peculiar subset of exudative AMD, especially type 1 choroidal neovascularization, showing abnormal branching vascular networks and terminating polyps beneath the retinal pigment epithelium [2, 9–11].
Although the clinical features and natural course of PCV are distinctively different from those of exudative AMD [1, 2, 4, 5, 7, 12, 13], the treatment strategies for PCV do not significantly differ from those of typical exudative AMD. The representative treatments for PCV include photodynamic therapy (PDT) and intravitreal injections of anti-vascular endothelial growth factor (VEGF). The PDT-based treatments (PDT monotherapy or combination PDT plus anti-VEGF therapy) have shown good long-term visual outcomes and high regression rates for polypoidal lesions [14–18]. Anti-VEGF monotherapy is the mainstay of treatment for exudative AMD, and is also widely used as the first-line treatment for PCV, as several studies have demonstrated favorable visual outcomes despite limited polyp regression [19–23]. Recently, evidence-based guidelines for PCV were reported [24]. They suggest that PDT monotherapy or combination therapy (combined PDT with intravitreal ranibizumab injections) as the initial treatment guided by ICGA. If polyp regression is incomplete, re-treatment with PDT monotherapy or combination therapy is again recommended [24]. If there is complete regression of polyps by ICGA, leakage from abnormal branching vascular networks, intravitreal ranibizumab injections are recommended as a re-treatment [24].
Although both PDT-based therapy and anti-VEGF therapy have shown good visual outcomes [16–23], recurrence of PCV is an another problem needing to be solved. Long-term follow-up studies with a 3-year minimum follow-up period showed PCV recurrence rates of approximately 69 % to 78 % after PDT-based therapies [14, 16, 25–27] and anti-VEGF monotherapy [28]. Because of the high recurrence rate of PCV, these studies recommend careful follow-up of patients [14, 15, 25–28]. Despite the high rates of long-term recurrence, little is known about the contributing factors. In this study, we investigated risk factors for PCV recurrence after treatment during long-term follow-up periods using ICGA.
Methods
Study subjects
We retrospectively reviewed the medical records of Korean patients with treatment-naïve PCV who fulfilled the inclusion and exclusion criteria. The patients were treated at the Vitreoretinal Service Clinic of Yonsei University Medical Center between October 2006 and October 2013. The first treatment for each patient was performed between October 2006 and September 2010. Written informed consent for each treatment was obtained at the time of each treatment. This comparative cohort study was approved by the Institutional Review Board of Yonsei University College of Medicine and was conducted in accordance with the tenets of the Declaration of Helsinki. Inclusion criteria were: (1) treatment-naïve symptomatic macular PCV with subfoveal leakage documented by fluorescein angiography (FA), (2) the presence of branching vascular networks and polypoidal lesions by ICGA, (3) completion of at least a 3-year follow-up after the first treatment (PDT, combination therapy of PDT with anti-VEGF therapy, or anti-VEGF monotherapy) for PCV, and (4) at least one remission after the first treatment phase (remission defined as the complete resolution of exudative fluid from PCV for at least 6 months after the end of the first treatment period). Exclusion criteria were: (1) PCV with persistent exudative fluid or leakage during the follow-up period, (2) other concomitant ocular diseases, such as diabetic retinopathy, high myopia, vein or artery occlusion, or epiretinal membrane, and (3) aphakia, absence of posterior capsule, or history of vitrectomy due to reduced anti-VEGF retention.
Examinations
All patients received complete ophthalmologic evaluations including best-corrected visual acuity (BCVA) testing using a decimal visual acuity chart, slit-lamp biomicroscopy, dilated fundus examination with indirect ophthalmoscopy, color fundus photography, digital FA, ICGA, and optical coherence tomography (OCT). OCT images were obtained by time-domain OCT (Stratus®; Carl Zeiss Meditec, Dublin, CA, USA) prior to 2009, and then by spectral domain OCT (SD OCT) subsequently (Spectralis; Heidelberg Engineering, Heidelberg, Germany). FA and ICGA were performed with the Heidelberg Retina Angiograph system (HRA-2; Heidelberg Engineering) and a confocal scanning laser ophthalmoscope.
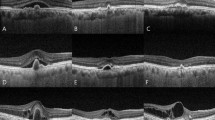
Polypoidal lesions were classified according to angiographic characteristics [29]. The locations of PCV lesions were subdivided into three categories: subfoveal, juxtafoveal (within 1–199 μm from the foveal center), or extrafoveal (200 μm or more from the foveal center). PCV was classified into two categories according to the number of discrete areas of polypoidal lesions: single or multiple. The representative figures of polypoidal lesions were shown in Fig. 1. The greatest linear dimension included the entire branching vascular network of vessels and polypoidal lesions at the early phase of ICGA; this was assessed with an electronic caliper using HRA-2 software (Heidelberg Engineering). The PCV lesion area was also measured manually with the same procedure. Pigment epithelial detachment (PED) was not considered if there was no underlying vascular component when the greatest linear dimension and PCV lesion area were measured. In addition, the presence of choroidal hyperpermeability was evaluated on late-phase ICGA images.
First-line treatment for PCV
Patients received one of three treatment modalities as the first-line treatment for PCV: a single session of PDT, anti-VEGF monotherapy (three monthly intravitreal injections of anti-VEGF agents), or combination therapy of PDT with anti-VEGF therapy (a single session of PDT and three monthly intravitreal injections of anti-VEGF agents). During the study period, the mainstay of treatments for exudative AMD, including PCV, shifted from PDT-based therapy to anti-VEGF monotherapy in our clinic. Thus, three treatment modalities could be assessed during this period.
For PDT, all patients received a 6-mg/m2 infusion of verteporfin (Visudyne; Novartis AG, Bulach, Switzerland) over a period of 10 minutes. Then, 15 minutes after the end of verteporfin infusion, a diode laser at 689 nm was applied to the PCV lesion. The entire lesion was covered with 50-J/cm2 light energy and a light dose rate of 600 mW/cm2 for 83 seconds. The laser spot size included an additional 500 μm to cover the borders on each side. If the exudative fluid from the PCV was not completely resolved after PDT, additional PDT was applied at 3-month intervals until the exudative fluid resolved.
Anti-VEGF therapy was administered as three monthly intravitreal injections of ranibizumab (0.5 mg/0.05 mL; Lucentis®; Genentech, Inc, South San Francisco, CA, USA). Topical anesthesia was applied, and 10 % povidone-iodine was used to scrub eyelids and lashes before intravitreal injections. Povidone-iodine eye drops (1.25 %) were applied, and a sterile lid speculum was inserted between the eyelids. The anti-VEGF agent was injected into the vitreous cavity through the superior sclera using a 30-gauge needle at a position 3.5 mm posterior to the corneal limbus in phakic eyes or 3.0 mm posterior in pseudophakic eyes. Pressure was applied to the injection site using a sterile cotton swab for 1 minute to prevent leakage. Patients were instructed to apply antibiotic eye drops for 1 week following the injections.
For combination therapy of PDT with anti-VEGF therapy, patients were treated with one session of PDT and three monthly intravitreal injections of bevacizumab (1.25 g/0.05 mL; Avastin®; Genentech, Inc). PDT was performed as described above. Intravitreal bevacizumab injections were administered immediately after PDT on the same day and in the same manner as previously described.
Follow-up and recurrence of PCV
Follow-up visits were arranged 1 week after each baseline treatment and monthly thereafter. Examinations included BCVA, dilated fundus examination with indirect ophthalmoscopy, and OCT. Three months after the first-line treatments, FA and ICGA were performed to evaluate treatment outcomes. Additional FA and ICGA were performed during monthly follow-up visits when PCV recurrence was suspected due to findings, such as the reappearance of new polyps, subretinal hemorrhage, or subretinal exudation on fundus examination, the reappearance of subretinal fluid on OCT, or decreased vision. Remission was defined as the complete resolution of subretinal exudative fluid and hemorrhages by fundus examination and SD OCT evaluation, regardless of regression of polyps and/or abnormal branching vascular networks. PCV recurrence was defined as the reappearance of active PCV lesions on ICGA with subfoveal leakage on FA and reappearance or development of subretinal exudative fluid on SD OCT examination after at least 6 months after the remission.
We investigated the angiographic data for patients in whom polyp regression occurred after each treatment modality. After evaluating polyp regression, PCV recurrence was also investigated. If active PCV with leakage was identified prior to 6 months after treatment, the original lesion was considered to be persistent rather than recurrent. The recurrence pattern of PCV was determined using FA and ICGA images that were taken at the time of diagnosis, 3 months after the first treatment, and at the time of recurrence. If polyps regressed after the first treatment, the recurrence pattern was further classified as an exudation from an abnormal branching vascular network without polyps or as the development of new polyps. If polyps did not regress, and recurrent exudation from non-regressing polyps and abnormal branching vascular networks occurred, we defined this type of recurrence as a persistent polyp. After the first treatment period, we treated recurrent PCV with monthly injections of intravitreal anti-VEGF agents, mainly bevacizumab, until the exudative fluid resolved according to OCT.
Statistical analyses
Patient characteristics, including age at initial diagnosis, sex, and BCVA at baseline, were retrieved from medical charts. BCVA results were converted to a logarithm of the minimum angle of resolution (logMAR) value for statistical analysis. In addition to these parameters, the angiographic characteristics of PCV were also retrieved. Data regarding the largest polyp diameter, greatest linear dimension of choroidal vascular networks, area of the entire PCV lesion, presence of PED (larger than the area of one optic disc diameter), polyp location (subfoveal, juxtafoveal, or extrafoveal), and area of discrete polyp lesion (single or multiple) were retrieved and recorded. The first recurrence was defined as our primary outcome measure for statistical analysis.
IBM SPSS 18.0 software for Windows (SPSS/IBM Corporation, Chicago, IL, USA) was used for statistical analyses. We evaluated baseline and clinical factors with respect to the recurrence rates. Hereby the nonparametric analyses were performed with a Mann–Whitney U test and Kruskal–Wallis analysis for the continuous variables and chi-square test for the categorical variables. To minimize the effect of different intervals between the first remission and recurrence, Cox regression analysis was performed. To minimize the possible interaction between the possible risk factors for recurrence of PCV, two-way interactions for possible risk factors were included in Cox regression analysis. Differences with P < 0.05 were considered statistically significant.
Results
Overall characteristics of patients
Seventy-eight eyes of seventy-eight patients met the study criteria and were retrospectively analyzed. Among the study population, 55 patients (71 %) were male, and the mean age at diagnosis was 67 ± 9 years. The mean duration of the first treatment period to achieve the first remission was 6 ± 2 months (3 months to 8 months). At the time of diagnosis, the mean greatest linear diameter was 3 ± 1 mm (0.5 to ∼7 mm), and the mean largest polyp diameter was 467 ± 380 μm (120 to ∼1,650 μm). The mean area of PCV lesion was 6 ± 5 mm2 (0.5 to ∼23 mm2). The location of PCV was subfoveal (44 patients, 56 %), juxtafoveal (20 patients, 26 %), and extrafoveal (14 patients, 18 %). The discrete area of PCV was 1 in 71 patients (91 %) and multiple in 7 patients (9 %). Choroidal hyperpermeability was present in 24 patients (31 %), and PED was present in 36 patients (46 %). The mean BCVA was 0.64 ± 0.41 logMAR (20/87 Snellen equivalent). During the follow-up period, the mean total number of PDTs was 2 ± 1 times, and the mean total number of anti-VEGF injections was 9 ± 7 times.
Recurrence of PCV
Within 3 years after the first remission of PCV, 50 eyes (64 %) showed at least 1 recurrence. The mean number of recurrence was 1.5 (1 to ∼2 times) in the eyes with at least one recurrence during follow-up periods. After assessment of recurrence rate, we investigated the regression of polyps and the pattern of recurrence in these patients. Among these 50 eyes, 35 eyes (70 %) showed polyp regression, and 15 eyes (30 %) showed persistent polyps at the time of the first remission. Despite treatment, none of the eyes showed complete regression of abnormal branching vascular networks. In the eyes with recurrence, the recurrence pattern was classified as follows: reactivation of persistent polyps, new development of polyps, and leakage from abnormal branching vascular networks without polyps Fig. 2.
Comparison of patients with recurrent and without recurrent PCV after the first treatment
The baseline characteristics and polyp regression at the first remission were compared between patients with at least one recurrence (the recurrence group) and those without recurrence (the non-recurrence group). There were no significant differences in baseline characteristics, except in the largest polyp diameter. The mean largest polyp diameter was 524 ± 340 μm (170 to ∼1,650) in the recurrence group and 352 ± 173 μm (120 to ∼570) in the non-recurrence group (P = 0.038). Distribution map of the largest polyp size at baseline is shown as Fig. 3. Comparisons of the baseline characteristics are described in Table 1.
Distribution map of the largest polyp size at baseline. The mean largest polyp diameter was 524 ± 340 μm in the recurrence group and 352 ± 173 μm in the non-recurrence group. The mean largest polyp diameter at baseline was significantly larger in the recurrence group than that of the non-recurrence group (P = 0.038)
First-line treatment strategies and recurrence of PCV
Among 78 patients, 12 patients were treated with PDT monotherapy (PDT group). Thirty-four patients received anti-VEGF monotherapy (anti-VEGF group), and 32 patients received combination therapy with PDT plus anti-VEGF therapy (combination group). When we compared baseline characteristics among the three treatment groups, there were no significant differences, including for age at diagnosis (P = 0.135), sex (P = 0.522), the baseline mean BCVA (P = 0.275), and PCV characteristics (location of PCV, P = 0.541; discrete area of PCV, P = 0.436; the greatest linear diameter, P = 0.526, the largest polyp diameter, P = 0.494; area of PCV lesion, P = 0.311; choroidal hyperpermeability, P = 0.051; and presence of PED, P = 0.171). However, the polyp regression rate at the time of first remission was significantly different among the three treatment groups (P = 0.005). Significantly higher polyp regression rates were observed in the PDT and combination groups compared to that of the anti-VEGF group. Although the recurrence rates after each treatment were not significantly different among the three groups (P = 0.758), the recurrence pattern was significantly different (P = 0.004).
Predictive factors for recurrence of PCV
We investigated predictive factors for the recurrence of PCV. Baseline characteristics, such as age at the time of diagnosis, sex, and baseline BCVA, were included, as were PCV characteristics, including the largest polyp diameter at baseline, the greatest linear diameter, location, and type of PCV. Treatment strategy (PDT, anti-VEGF therapy, and combination therapy) was also included in the statistical analysis. Because the interval between the first remission and recurrence varied among patients, we performed Cox regression analysis to confirm predictive factors for PCV recurrence (Table 2). Cox regression analysis showed that the largest polyp diameter at baseline was predictive for PCV recurrence (B = 1.470, P = 0.015).
Representative figures are shown in Fig. 4.
A 62-year-old male was referred due to decreased vision in his left eye for 1 month. Best-corrected visual acuity (BCVA) of the left eye was 0.7 logMAR (logarithm of the minimum angle resolution; 20/100 Snellen visual acuity). Fluorescein angiography (FA) showed irregular hyperfluorescence at an early phase (a), from which profuse leakage was shown in a late phase (b). Optical coherence tomography (OCT) showed subretinal fluid with vascularized pigment epithelial detachment (c). Indocyanine green angiography (ICGA) showed polyps with abnormal branching vascular networks (d). Combination therapy was performed as for the first-line treatment: a single session of photodynamic therapy (PDT) with three monthly intravitreal injections of bevacizumab (1.25 mg/0.05 mL; Avastin®; Genentech, Inc). After treatment, hyperfluorescence at an early phase (e) did not show profuse leakage at a late phase (f) on FA, and OCT showed resolution of subretinal fluid (g). ICGA showed polyp regression (h), and BCVA improved to 0.3 logMAR (20/39 Snellen visual acuity). After 9 months, BCVA of the left eye decreased to 0.5 logMAR (20/63 Snellen visual acuity); FA showed irregular leakage from the early (i) to late phase (j) on FA. OCT showed subretinal fluid with pigment epithelial detachment (k). Persistent abnormal branching vascular networks were seen on ICGA without definite polyps (l)
Discussion
In this study, we focused on the predictive factor for PCV with at least a 3-year follow-up after the first remission of treatment-naïve PCV. During 3 years after the first remission, 50 patients (64 %) showed at least 1 recurrence. When we compared the baseline characteristics between the recurrence group and non-recurrence group, there were no significant differences except the mean largest polyp diameter at baseline. The mean largest polyp diameter at baseline was significantly larger in the recurrence group when compared to the non-recurrence group. In addition, subsequent Cox regression analysis showed that the largest polyp size at baseline significantly correlated with PCV recurrence.
Baseline lesion sizes, such as the greatest linear diameter and the largest polyp size, have been suggested as possible prognostic factors for visual outcome after treatment of PCV [15, 28, 30–34]. Based on these studies and the current study, the largest polyp size at baseline may represent the disease activity of treatment-naïve PCV, predicting treatment outcome and recurrence. However, the range of the largest polyp size should be further considered. In this study, the results suggest that the mean largest polyp size is significantly large in the recurrence group, and statistical analysis showed the largest polyp size at baseline as a predictive factor for recurrence of PCV. However, there were some patients to be considered. Some patients had recurrence of PCV although the largest polyp size was relatively smaller than the mean largest polyp size of the recurrence group. Some patients also did not show recurrence although the largest polyp size was relatively larger than the mean largest polyp size of the non-recurrence group. These occurrences suggest that there may be other factors affecting the recurrence of PCV. Previous studies have suggested the predictive value of the greatest linear diameter of PCV, although they did not include baseline polyp size [30, 34]. Our results suggest that the largest polyp size represents disease activity and can be used as a predictive factor for PCV recurrence even after the regression of polyps. However, we also think that there may be other factors associated with recurrence of PCV which were not found in our study. Thus, future prospective studies with larger populations may be helpful for confirming the predictive value of the largest polyp size and for further investigation of other predictive factors in patients with PCV.
In addition to predictive factors for PCV recurrence, we investigated the recurrence pattern after three treatment modalities used in this study. PDT-based treatment appears to be more effective for the regression of polypoidal lesions when compared to that of anti-VEGF therapy. The polyp regression rate is 73 % to 99 % after PDT-based therapy [14–18, 35–37], whereas that of anti-VEGF therapy is usually 22 % to 43 % [20, 28, 38, 39]. In this study, polyp regression at the time of the first remission differed significantly among treatment groups. Polyp regression at the time of the first remission was significantly higher in patients who received PDT and combination therapy compared to that of patients who received anti-VEGF monotherapy; this was consistent with previous studies [14–18, 20, 28, 35–39]. The recurrence pattern was significantly different among treatment groups, possibly in accordance with differences in the polyp regression rates. PDT and combination therapy showed higher rates of polyp regression; the development of new polyps or leakage from abnormal branching vascular networks without new polyps were more dominant in these groups than with anti-VEGF monotherapy. However, the polyp regression rate and recurrence patterns after each treatment strategy were not significantly associated with recurrence of PCV in this study. The recurrence rates after three treatment modalities were not significantly different in this study, consistent with previous studies [14, 15, 25–27].
This study has several limitations, including the retrospective nature and relatively small study population. The retrospective nature of this study lead to several limitations, including the heterogeneous treatment strategy for treatment-naïve PCV. However, we demonstrated that treatment strategy did not show significant differences in recurrence rates and did not significantly correlate with PCV recurrence. In addition, the baseline characteristics of three treatment modalities were not significantly different, showing fairly random distribution. Another limitation was in the use of anti-VEGF agents, which might affect the outcome. If this study was conducted in a prospective manner, uniform use of anti-VEGF agents could be achieved. However, in our real practice, we should consider various factors, including the Korean National Health Insurance (NHI) system and the patients’ economic statuses. Either PDT or ranibizumab is covered by the NHI system; usually, PDT is covered by the NHI system and bevacizumab is used for combined anti-VEGF therapy to reduce patients’ economic burden. However, a recent study indicates that there are no significant differences in efficacy or safety for ranibizumab and bevacizumab [40]. Thus, we believe that any potential bias from the difference in anti-VEGF agents was minimal. We believe that a future, long-term study with a prospective design and uniform treatment strategy is needed to strengthen our findings.
In conclusion, the largest polyp size at baseline significantly correlated with PCV recurrence, showing increased risk of recurrence with a larger polyp size at baseline. Thus, patients with larger polyps should be monitored more carefully due to higher risk of recurrence even after successful treatment of PCV.
References
Yannuzzi LA, Sorenson J, Spaide RF, Lipson B (1990) Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina 10:1–8
Immamura Y, Engelbert M, Iida T, Freund KB, Yannuzzi LA (2010) Polypoidal choroidal vasculopathy: a review. Surv Ophthalmol 55:501–515
Spaide RF, Yannuzzi LA, Slakter JS, Sorenson J, Orlach DA (1995) Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina 15:100–110
Yannuzzi LA, Ciadella A, Spaide RF, Rabb M, Freund KB, Orlock DA (1997) The expanding clinical spectrum of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol 115:478–485
Yannuzzi LA, Wong DW, Sforzolini BS, Goldbaum M, Tang KC, Spaide RF, Freund KB, Slakter JS, Guyer DR, Sorenson JA, Fisher Y, Maberley D, Orlock DA (1999) Polypoidal choroidal vasculopathy and neovascularized age-related macular degeneration. Arch Ophthalmol 117:1503–1510
Byeon SH, Lee SC, Oh HS, Kim SS, Koh HJ, Kwon OW (2008) Incidence and clinical patterns of polypoidal choroidal vasculopathy in Korean patients. Jpn J Ophthalmol 52:57–62
Sho K, Takahashi K, Yamada H, Wada M, Nagai Y, Otsuji T, Nishikawa M, Mitsuma Y, Yamazaki Y, Matsumura M, Uyama M (2003) Polypoidal choroidal vasculopathy. Incidence, demographic features, and clinical characteristics. Arch Ophthalmol 121:1392–1396
Maruko I, Iida T, Saito M, Nagayama D, Saito K (2007) Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am J Ophthalmol 144:15–22
Khan S, Engelbert M, Imamura Y, Freund KB (2012) Polypoidal choroidal vasculopathy: simultaneous indocyanine green angiography and eye-tracked spectral domain optical coherence tomography findings. Retina 32:1057–1068
Sato T, Kishi S, Watanabe G, Matsumoto H, Mukai R (2007) Tomographic features of branching vascular networks in polypoidal choroidal vasculopathy. Retina 27:589–584
Tsujikawa A, Sasahara M, Otani A, Gotoh N, Kameda T, Iwama D, Yodoi Y, Tamura H, Mandai M, Yoshimura N (2007) Pigment epithelial detachment in polypoidal choroidal vasculopathy. Am J Ophthalmol 141:102–111
Uyama M, Wada M, Nagai Y, Matsubara T, Matsunaga H, Fukushima I, Takahashi K, Matsumura M (2002) Polypoidal choroidal vasculopathy: natural history. Am J Ophthalmol 133:639–648
Laude A, Cackett PD, Vithana EN, Yeo IY, Wong D, Koh AH, Wong TY, Aung T (2010) Polypoidal choroidal vasculopathy and neovascular age-related macular degeneration: same or different disease? Prog Retin Eye Res 29:19–29
Kang HM, Kim YM, Koh HJ (2013) Five-year follow-up results of photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol 115:438–447
Kang HM, Koh HJ, Lee CS, Lee SC (2014) Combined photodynamic therapy with Intravitreal Bevacizumab injections for polypoidal choroidal vasculopathy: long-term visual outcome. Am J Ophthalmol 157:598–606
Chan WM, Lam DS, Lai TY, Liu DT, Li KK, Yao Y, Wong TH (2004) Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy: one-year results of a prospective case series. Ophthalmology 111:1576–1584
Gomi F, Ohji M, Sayanagi K, Sawa M, Sakaguchi H, Oshima Y, Ikuno Y, Tano Y (2008) One-year outcomes of photodynamic therapy in age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese patients. Ophthalmology 115:141–146
Tomita K, Tsujikawa A, Yamashiro K, Ooto S, Tamura H, Otani A, Nakayama Y, Yoshimura N (2012) Treatment of polypoidal choroidal vasculopathy with photodynamic therapy combined with Intravitreal injections of Ranibizumab. Am J Ophthalmol 153:68–80
Oishi A, Kojima H, Mandai M, Honda S, Matsuoka T, Oh H, Kita M, Nagai T, Fujihara M, Bessho N, Uenishi M, Kurimoto Y, Negi A (2013) Comparison of the effect of Ranibizumab and verteporfin for polypoidal choroidal vasculopathy: 12-month LAPTOP study results. Am J Ophthalmol 156:644–651
Hikichi T, Higuchi M, Matsushita T, Kosaka S, Matsushita R, Takami K, Ohtsuka H, Ariga H (2012) One-year results of three monthly Ranibizumab injections and as-needed reinjections for polypoidal choroidal vasculopathy in Japanese patients. Am J Ophthalmol 154:117–124
Hikichi T, Higuchi M, Matsushita T, Kosaka S, Matsushita R, Takami K, Ohtsuka H, Kitamei H, Shioya S (2013) Results of 2 years of treatment with as-needed Ranibizumab reinjection for polypoidal choroidal vasculopathy. Br J Ophthalmol 97:617–621
Cheng CK, Peng CH, Chang CK, Hu CC, Chen LJ (2011) One-year outcomes of Intravitreal Bevacizumab (Avastin) therapy for polypoidal choroidal vasculopathy. Retina 31:846–856
Inoue M, Arakawa A, Yamane S, Kadonosono K (2013) Long-term outcome of Intravitreal Ranibizumab treatment, compared with photodynamic therapy, in patients with polypoidal choroidal vasculopathy. Eye 27:1013–1020
Koh AH, Expert PCV Panel, Chen LJ, Chen SJ, Chen Y, Giridhar A, Iida T, Kim H, Yuk Yau Lai T, Lee WK, Li X, Han Lim T, Ruamviboonsuk P, Sharma T, Tang S, Yuzawa M (2013) Polypoidal choroidal vasculopathy: evidence-based guidelines for clinical diagnosis and treatment. Retina 33:686–716
Akaza E, Mori R, Yuzawa M (2008) Long-term results of photodynamic therapy of polypoidal choroidal vasculopathy. Retina 28:717–722
Akaza E, Yuzawa M, Mori R (2011) Three-year follow-up results of photodynamic therapy for polypoidal choroidal vasculopathy. Jpn J Ophthalmol 55:39–44
Leal S, Silva R, Figueira J, Cachulo ML, Pires I, de Abreu JR, Cunha-Vaz JG (2010) Photodynamic therapy with verteporfin in polypoidal choroidal vasculopathy: results after 3 years of follow-up. Retina 30:1197–1205
Kang HM, Koh HJ (2013) Long-term visual outcome and prognostic factors after Intravitreal Ranibizumab injections for polypoidal choroidal vasculopathy. Am J Ophthalmol 156:652–660
Cackett P, Wong D, Yeo I (2009) A classification system for polypoidal choroidal vasculopathy. Retina 29:187–191
Tsujikawa A, Ojima Y, Yamashiro K, Nakata I, Ooto S, Tamura H, Nakanishi H, Hayashi H, Otani A, Yoshimura N (2011) Association of lesion size and visual prognosis to polypoidal choroidal vasculopathy. Am J Ophthalmol 151:961–972
Lee YA, Yang CH, Yang CM, Ho TC, Lin CP, Huang JS, Chen MS (2012) Photodynamic therapy with or without Intravitreal Bevacizumab for polypoidal choroidal vasculopathy: two years of follow-up. Am J Ophthalmol 154:872–880
Koizumi H, Yamagishi T, Yamazaki T, Kinoshita S (2011) Predictive factors of resolved retinal fluid after Intravitreal Ranibizumab for polypoidal choroidal vasculopathy. Br J Ophthalmol 95:1555–1559
Hikichi T, Ohtsuka H, Higuchi M, Matsushita T, Ariga H, Kosaka S, Matsushita R, Takami K (2011) Factors predictive of visual acuity outcomes 1 year after photodynamic therapy in Japanese patients with polypoidal choroidal vasculopathy. Retina 31:857–865
Saito M, Iida T, Kano M, Itagaki K (2014) Five-year results of photodynamic therapy with and without supplementary antivascular endothelial growth factor treatment for polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol 252:227–235
Lee SC, Seong YS, Kim SS, Koh HJ, Kwon OW (2004) Photodynamic therapy with verteporfin for polypoidal vasculopathy of the macula. Ophthalmologica 218:193–201
Silva RM, Figueira J, Cachulo ML, Duarte L, Faria de Abreu JR, Cunha-Vaz JG (2005) Polypoidal choroidal vasculopathy and photodynamic therapy with verteporfin. Graefes Arch Clin Exp Ophthalmol 243:973–979
Akaza E, Yuzawa M, Matsumoto Y, Kashiwakura S, Fujita K, Mori R (2007) Role of photodynamic therapy in polypoidal choroidal vasculoopathy. Jpn J Ophthalmol 51:270–277
Lai TY, Lee GK, Luk FO, Lam DS (2011) Intravitreal Ranibizumab with or without photodynamic therapy for the treatment of symptomatic polypoidal choroidal vasculopathy. Retina 31:1581–1588
Kokame GT, Yeung L, Lai JC (2010) Continuous anti-VEGF treatment with Ranibizumab for polypoidal choroidal vasculopathy: 6-month results. Br J Ophthalmol 94:297–301
Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group, Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, Toth C, Redford M, Ferris FL 3rd (2012) Ranibizumab and Bevacizumab for treatment of neovascular age-related macular degeneration. Two-year results. Ophthalmology 119:1388–1398
Financial disclosure
HJ Koh is a consultant/advisor for Allergan, Bayer, and Novartis Korea, Seoul, Republic of Korea. HJ Koh has received honoraria as a lecturer from Allergan, Bayer, and Novartis Korea, Seoul, Republic of Korea. The other authors (HM Kang and SC Lee) certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Contributions of authors
Design and conduct of study (HM Kang and SC Lee); collection of data (HM Kang); management, analysis, and interpretation of data (HM Kang, HJ Koh, and SC Lee); and preparation, review, and approval of the manuscript (HM Kang, HJ Koh, and SC Lee).
Other acknowledgments
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding/support
No funding was received for this research.
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Kang, H.M., Koh, H.J. & Lee, S.C. Baseline polyp size as a potential predictive factor for recurrence of polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol 254, 1519–1527 (2016). https://doi.org/10.1007/s00417-015-3241-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-015-3241-6