Abstract
Purpose
Treatment of secondary glaucoma in uveitis patients is challenging. Owing to the young age of these patients, sufficient lowering of the intraocular pressure (IOP) is essential to prevent progression of visual field loss. However, because of the chronic inflammatory stimulus, filtration surgery has an increased risk of failure, especially in patients who have previously undergone surgery. Therefore, minimally invasive glaucoma surgery is a valuable alternative.
Methods
The clinical records of 24 consecutive patients with uveitic secondary glaucoma who underwent trabeculectomy ab interno with the Trabectome® at the Eye Center of the Albert-Ludwigs University of Freiburg between June 2009 and June 2014 (registered in the Freiburg trabectome database) were retrospectively analyzed. The general baseline information for each patient included age, gender, glaucoma type, ocular medication and current IOP. The postoperative IOP and number of antiglaucomatous medications were recorded at each visit. Statistical analyses were performed using the Kaplan-Meier estimator and Dunnett’s t-test.
Results
The mean IOP before surgery was 31 ± 6.7 mmHg (median 32 mmHg). Both the IOP and the number of medications significantly decreased over the various follow-up intervals after trabeculectomy ab interno with the Trabectome®. Patients with follow-ups continuing past one year showed an IOP-reduction of approximately 40 % and a medication number reduction from 2 to 0.67. The failure rate (necessitating further glaucoma surgery) was N = 3 (12.5 %) patients.
Conclusions
Trabeculectomy ab interno with the Trabectome® is a minimally invasive and effective method for controlling IOP in uveitic secondary glaucoma.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uveitic glaucoma was first described by Joseph Beer in 1813 [1]. The mean age at first diagnosis of uveitis is 39 years [2]. The prevalence of secondary glaucoma in patients with uveitis is approximately 20 % [3]. Owing to the young age of these patients, sufficient control of IOP is necessary for the prevention of progressive visual field deterioration. The success of trabeculectomy ab externo is lower in uveitis patients then in patients with primary open angles glaucoma, as both young age and autoimmune inflammation cause an accelerated and intensified wound-healing response [4, 5]. Glaucoma drainage devices are an alternative for lowering IOP in uveitis patients [6–9], although using these requires a complex surgical intervention.
In uveitic glaucoma, the outflow resistance is primarily increased as a result of alterations in the trabecular meshwork [3, 10, 11]; therefore, removal of the altered meshwork should lead to sufficient IOP reduction. Since 2006, the Trabectome®, a device used to perform an ab interno trabeculectomy, has been used in glaucoma surgery [12, 13]. The trabecular meshwork is removed by electroablation, resulting in an increased aqueous outflow to the opened Schlemm’s canal.
In this study, we investigated the effectiveness and safety of surgery with the Trabectome® in uveitic patients.
Methods
Patient selection
The study protocol was approved by the ethics committee of the Albert LudwigsUniversity Freiburg (No. 235/10_160678). The study followed the regulations of the Good Clinical Practice (GCP) Guidelines and the Declaration of Helsinki.
The clinical records of consecutive patients who underwent trabeculectomy ab interno with the Trabectome® at the University Eye Hospital Freiburg between June 2009 and June 2014 (registered in the Freiburger Trabectome database) were retrospectively analyzed. All patients with uveitis and a follow-up > 60 days were included. A total of 24 eyes from 24 patients with anterior or intermediate uveitis were included. Indication for glaucoma surgery was based on clinical observation. All patients suffered from elevated IOP under maximal tolerated antiglaucomatous therapy and showed an open angle. In 11 of the 24 patients a preoperative visual field examination was available. The mean defect (MD) was 4.92 ± 4.69 (mean ± standard deviation). Surgery was performed by three experienced glaucoma surgeons (JJ, MN, AA).
Evaluation of outcomes
Baseline information for each patient included age, gender, glaucoma type, antiglaucomatous and other ocular medication, and current IOP level.
Surgical procedure
Surgery was performed using the Trabectome® system, including the Trabectome single-use handpiece including an irrigation-aspiration (I/A) system (Neomedix Inc., Tustin, USA). After topical anaesthesia a 1.7 mm clear cornea tunnel was made; local anaesthesia was instilled into the anterior chamber. Surgery was performed under gonioscopic control using the modified Swan-Jacob-Lens. The trabecular meshwork was removed over 90 to 120° (described in detail by Jordan et al. [14]). None of the eyes had active inflammation for at least 6 weeks at the time of surgery. At the end of surgery, dexamethasone 4 mg was applied intracamerally.
Postoperative management
All patients received steroid eye drops (prednisolone 1 %) every hour for the first few days. The dosage was adapted to the individual intraocular inflammation level. To prevent anterior synechia, pilocarpine (2 %) was administered to all eyes 3 times a day for the first 6 weeks after surgery, and twice a day thereafter for 6 more weeks. IOP-lowering medication was given topically or systemically according to the individual’s postoperative IOP levels. No change in basic therapy was done. All patients went on taking their individual immunosuppressive basic therapy including steroids, if needed.
Statistics
Time points of follow-up visits were clustered in follow-up intervals. The IOP and the number of antiglaucomatous medications were analyzed at each follow-up: follow-up interval 1, 1–30 days post-operatively (n = 10); follow-up interval 2, 31–90 days post-operatively (n = 12); follow-up interval 3, 91–180 days post-operatively (n = 10); follow-up interval 4, 181–365 post-operatively (n = 8); and follow-up interval 5, 366–1046 days post-operatively (n = 9).
Statistical analysis was performed using the Kaplan-Maier estimator [15] and Dunnett’s t-test [16, 17] for multiple comparisons.
Results
A total of 24 eyes from 24 patients were included. Baseline data for the patients are shown in Table 1. During follow up time no relaps of uveitic inflammation was seen in any of the patients (Mean 394 ± 341 days, range 60 – 1046 days).
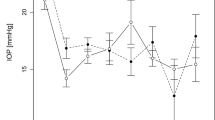
The baseline mean IOP was 31 ± 6.7 mmHg (median 32 mmHg). The mean individual postoperative IOP at the last documented visit was 20 ± 11.4 mmHg (median 16 mmHg). The mean postoperative IOP in follow-up interval 1 was 26 ± 18.4 mmHg (median = 16 mmHg); in follow-up interval 2, 15 ± 2.9 mmHg (median = 16 mmHg); in follow-up interval 3, 16 ± 4.1 mmHg (median = 17 mmHg); in follow-up interval 4, 20 ± 12.7 mmHg (median = 18 mmHg); and in follow-up interval 5, 18 ± 6.6 mmHg (median = 16 mmHg). The IOP results during follow-up are presented in Fig. 1. Figure 2 shows the IOP results at the final documented visit compared to baseline for each patient. Dunnett’s t-test confirmed a significant difference between baseline and follow-up (significance level p < 0.05).
Mean intraocular pressure was significantly lower during follow-up than before surgery. The ordinate indicates IOP [mmHg], whereas the abscissa is the time after surgery in days. Significance level: ***p < 0.001, **p < 0.01, *p < 0.05. Follow up visits were grouped by time after surgery to compensate for inhomogeneous timepoints of follow up. If a single patient was seen several times during a defined timeframe, mean IOP values for this patient and timeframe were calculated first. During the first follow-up interval (1–30 days) ten patients showed up for one follow up visit. During the second follow-up interval (31–89 days) a total of twelve patients showed up for follow up visit. Nine of these patients showed up for one visit, one patient for two visits and two patients for three visits. During the third follow-up interval (90–180 days) a total of ten patients showed up for follow up visit. Nine of these patients showed up for one visit and one patient for two visits. During the fourth follow up interval (181–364 days) a total of eight patients showed up for follow up visit. Five of these patients for one visit, two patients for two visits and one patient for three visits. During the fifth follow-up interval (365 – 1046 days) a total of nine patients showed up for follow up visit. Six of these patients showed up for one visit and three patients for two visits
IOP reduction between baseline and the last visit. Scatter plot of preoperative IOP on the abscissa compared to postoperative IOP on the ordinate at the last documented visit. Each dot indicates a patient. Points below the oblique line define a lower postoperative IOP than baseline. The dotted line represents a 20 % reduction of the IOP from baseline
The mean number of glaucoma medications at the preoperative visit was 2 ± 1.4 (range = 0–4, median = 2). The mean number of postoperative medications was 1 ± 0.82 in follow-up interval 1; 1.1 ± 0.37 in follow-up interval 2; 1.1 ± 0.88 in follow-up interval 3; 0.92 ± 0.85 in follow-up interval 4; and 0.67 ± 0.83 in follow-up interval 5. The mean number of medications during follow-up is presented in Fig. 3.
The mean number of medications was lower during the whole follow-up than before surgery. The ordinate indicates the number of antiglaucomatous substances, whereas the abscissa is the time after surgery in days. Significance level: **p < 0.01. Same timeframes as in Fig. 1 were used
In 87.5 % (21 of 24) of the eyes, the IOP was controlled after trabeculectomy ab interno with the Trabectome® (20 % IOP reduction and IOP < 21 mmHg) with additional medication (relative success). None of the patients achieved absolute success (20 % IOP reduction and IOP < 21 mmHg without additional medication).Three eyes, or 12.5 %, needed further glaucoma surgery for IOP control. One eye received a Baerveldt device at day 7. In 2 eyes, we performed a trabeculectomy with mitomycin C: 1 at day 11 and 1 at day 389. Qualified success, defined as sufficient IOP lowering using antiglaucomatous medications, is demonstrated in the Kaplan-Meier survival plot in Fig. 4.
Kaplan-Meier survival plot for probability of qualified success. Qualified success was defined as a 20 % IOP reduction and an IOP <21 mmHg with antiglaucomatous therapy. Failure was assumed when the target criteria (20 % IOP reduction and IOP <21 mmHg with antiglaucomatous therapy) could not be achieved on two consecutive follow-up visits
One patient had a significant anterior chamber hemorrhage postoperatively (although this patient was not on anticoagulative medication); thus, it was necessary to perform an anterior chamber lavage. No eyes showed endophthalmitis or uveitis reactivation following surgery with the Trabectome®.
Discussion
In this retrospective, noncomparative study, surgery with the Trabectome® seems to be an effective, minimally invasive method for lowering the IOP in uveitic glaucoma. The IOP-lowering effect is approximately 40 %, with a simultaneous significant reduction in antiglaucomatous medication use. No serious side effects were seen.
The pathophysiology of secondary IOP elevation in patients with uveitis is multifactorial and is not completely understood [10]. Chronic or long-term use of topical steroids leads to a steroid-induced alteration of the trabecular meshwork in one-third of uveitis patients [11]. In particular, young age seems to be a risk factor for steroid-induced glaucoma [18, 19]. Furthermore, in uveitis patients, cell debris causes mechanical obstruction and reduced aqueous outflow during acute inflammation. Additionally, the trabecular meshwork swells as result of the acute inflammation; therefore, outflow resistance increases. Secondary scarring due to chronic inflammation in the trabecular meshwork leads to increased outflow resistance and, as a result, the IOP becomes elevated [3]. Therefore, the primary cause of elevated IOP is reduced outflow following alterations in the trabecular meshwork. Initially, the increased IOP should be treated by topical administration of antiglaucomatous drugs [20]. If medical therapy is ineffective, surgical intervention is required. Various surgical options are available to lower the IOP in patients with medically uncontrolled uveitic secondary glaucoma. Irrespective of the method used, intraocular surgery should only be performed in eyes with controlled uveitis, with no signs of acute inflammation [21].
Trabeculectomy ab externo has been described as more effective when using additional an antimetabolite medication such as Mitomycin C or 5-Fluorouracil [4, 22–24]. The results for trabeculectomy with an antimetabolite medication in uveitic glaucoma seem to be comparable to those for primary open angle glaucoma, in cases of inactive uveitis without previous surgery [25]. However, in patients who have previously undergone surgery, the risk of failure through conjunctival scarring increases. Drainage valves are an alternative option. Previous studies describe success rates of up to 90 % [7, 26–28], with the best results for Baerveldt-devices [7]. However, drainage implantation is a complex surgical procedure with a risk of serious, vision-threatening complications [29, 30].
Goniotomy, or opening of the altered trabecular meshwork, has been reported to lower the IOP in young uveitis patients, with a success rate of up to 75 % and few complications [31]. Classic goniotomy, as described in the past, uses the same surgical access as the Trabectome®, but it is inferior because of overlapping wound edges, which could lead to secondary scarring. In contrast, trabeculectomy ab interno with the Trabectome® requires the removal of the trabecular meshwork by electroablation such that no overlapping wound edges exist. In particular, considering the chronic inflammation process, this approach could result in even better success rates then goniotomy. Following these pathophysiological considerations, we investigated the results of trabeculectomy ab interno with the Trabectome®. In our study, we showed sufficient IOP lowering and reduction of medication use, with a failure rate of 17 %. Owing to the use of clear cornea incisions, the patients’ conjunctivas were not affected; thus, later trabeculectomy ab externo [32] or drainage implantation procedures were possible. This method is therefore an option to delay cyclodestruction. The early postoperative results could be influenced by the steroid therapy. However, considering that the trabecular meshwork which contributes to outflow resistance in steroid-response glaucoma is removed during surgery, the influence of steroid induced IOP variations in the early postoperative period seems so be negligible.
The achievable target pressure following trabeculectomy ab interno with the Trabectome® is limited because of the outflow resistance across the collector channels and because of the complex downstream draining and vascular system [14]. Therefore, very low target pressures are not obtainable. However, the Trabectome® is a good option for early surgery in eyes with moderate optic disc damage. Minimally invasive glaucoma surgery shows an advantageous risk profile, and the postoperative treatment is less complex than that for fistulating surgery (trabeculectomy/drainage tubes) [33, 34]. Compared with other minimally invasive glaucoma surgeries (trabecular microbypass stent, Schlemm’s canal scaffold or suprachoroidal microstent), trabeculectomy ab interno with a Trabectome® does not require any device implantation. Because the implantation of extraneous material can lead to prolonged intraocular inflammation even in non-uveitic eyes [35, 36], this is another advantage of this particular method. Furthermore, small devices with a small lumen, such as the iStent®, CyPass® or Hydrus scaffold®, could be obstructed by inflammatory cell debris. However, there are no controlled studies testing the effectiveness of minimally invasive glaucoma surgery in patients suffering from secondary glaucoma due to uveitis.
Typically, miotics are avoided in therapy for uveitic glaucoma as they can induce posterior synechia, exacerbate the symptoms of inflammation caused by ciliary spasm and worsen inflammation by disrupting the blood aqueous barrier [21]. Furthermore, it is known that miotics cause postoperative inflammation [37]. However, to avoid anterior synechia in the area of the trabecular meshwork ablation, we administered pilocarpine 2 % in all patients for a minimum of 6 weeks postoperatively. In no patients did we see a reactivation of the uveitis.
This study has the limitations of a retrospective study. Furthermore, the number of cases included was too small to create subgroups for different types of uveitis.
Because of its minimally invasive character, favorable perioperative risk profile and uncomplicated post-surgical treatment, trabeculectomy ab interno with the Trabectome® seems to be an effective and feasible method for glaucoma surgery in patients with secondary uveitic glaucoma.
References
Beer G (1813) Die Lehrer v. d. Augenkrankheiten. Vienna
Mackensen F, Paulus WE, Max R, Ness T (2014) Ocular changes during pregnancy. Dtsch Arztebl Int 111:567–576. doi:10.3238/arztebl.2014.0567
Moorthy RS, Mermoud A, Baerveldt G et al (1997) Glaucoma associated with uveitis. Surv Ophthalmol 41:361–394
Stavrou P, Murray PI (1999) Long-term follow-up of trabeculectomy without antimetabolites in patients with uveitis. Am J Ophthalmol 128:434–439
Carreno E, Villaron S, Portero A et al (2010) Surgical outcomes of uveitic glaucoma. J Ophthalmic Inflamm Infect 1:43–53. doi:10.1007/s12348-010-0012-8
Hill RA, Nguyen QH, Baerveldt G et al (1993) Trabeculectomy and Molteno implantation for glaucomas associated with uveitis. Ophthalmology 100:903–908
Ceballos EM, Parrish RK 2nd, Schiffman JC (2002) Outcome of Baerveldt glaucoma drainage implants for the treatment of uveitic glaucoma. Ophthalmology 109:2256–2260
Da Mata A, Burk SE, Netland PA et al (1999) Management of uveitic glaucoma with Ahmed glaucoma valve implantation. Ophthalmology 106:2168–2172. doi:10.1016/S0161-6420(99)90500-6
Papadaki TG, Zacharopoulos IP, Pasquale LR et al (2007) Long-term results of Ahmed glaucoma valve implantation for uveitic glaucoma. Am J Ophthalmol 144:62–69. doi:10.1016/j.ajo.2007.03.013
Herbert HM, Viswanathan A, Jackson H, Lightman SL (2004) Risk factors for elevated intraocular pressure in uveitis. J Glaucoma 13:96–99
Armaly MF, Becker B (1965) Intraocular pressure response to topical corticosteroids. Fed Proc 24:1274–1278
Francis BA, See RF, Rao NA et al (2006) Ab interno trabeculectomy: development of a novel device (Trabectome) and surgery for open-angle glaucoma. J Glaucoma 15:68–73
Minckler DS, Baerveldt G, Alfaro MR, Francis BA (2005) Clinical results with the Trabectome for treatment of open-angle glaucoma. Ophthalmology 112:962–967. doi:10.1016/j.ophtha.2004.12.043
Jordan JF, Wecker T, van Oterendorp C et al (2013) Trabectome surgery for primary and secondary open angle glaucomas. Graefes Arch Clin Exp Ophthalmol 251:2753–2760. doi:10.1007/s00417-013-2500-7
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457
Dunnett CW (1955) A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc 50:1096–1121
Dunnett CW (1964) New tables for multiple comparisons with a control. Biometrics 20:482–491
Kiddee W, Trope GE, Sheng L et al (2013) Intraocular pressure monitoring post intravitreal steroids: a systematic review. Surv Ophthalmol 58:291–310. doi:10.1016/j.survophthal.2012.08.003
Marcus MW, Müskens RPHM, Ramdas WD et al (2012) Corticosteroids and open-angle glaucoma in the elderly: a population-based cohort study. Drugs Aging 29:963–970. doi:10.1007/s40266-012-0029-9
Siddique SS, Suelves AM, Baheti U, Foster CS (2013) Glaucoma and uveitis. Surv Ophthalmol 58:1–10. doi:10.1016/j.survophthal.2012.04.006
Bodh S, Kumar V, Raina U et al (2011) Inflammatory glaucoma. Oman J Ophthalmol 4:3. doi:10.4103/0974-620X.77655
Towler HM, McCluskey P, Shaer B, Lightman S (2000) Long-term follow-up of trabeculectomy with intraoperative 5-fluorouracil for uveitis-related glaucoma. Ophthalmology 107:1822–1828
Jampel HD, Jabs DA, Quigley HA (1990) Trabeculectomy with 5-fluorouracil for adult inflammatory glaucoma. Am J Ophthalmol 109:168–173
Patitsas CJ, Rockwood EJ, Meisler DM, Lowder CY (1992) Glaucoma filtering surgery with postoperative 5-fluorouracil in patients with intraocular inflammatory disease. Ophthalmology 99:594–599
Kaburaki T, Koshino T, Kawashima H et al (2009) Initial trabeculectomy with mitomycin C in eyes with uveitic glaucoma with inactive uveitis. Eye (Lond) 23:1509–1517. doi:10.1038/eye.2009.117-cme
Hill RA, Heuer DK, Baerveldt G et al (1991) Molteno implantation for glaucoma in young patients. Ophthalmology 98:1042–1046
Rachmiel R, Trope GE, Buys YM et al (2008) Ahmed glaucoma valve implantation in uveitic glaucoma versus open-angle glaucoma patients. Can J Ophthalmol 43:462–467. doi:10.3129/i08-082
Ozdal PC, Vianna RNG, Deschênes J (2006) Ahmed valve implantation in glaucoma secondary to chronic uveitis. Eye (Lond) 20:178–183. doi:10.1038/sj.eye.6701841
Barton K, Feuer WJ, Budenz DL et al (2014) Three-year treatment outcomes in the Ahmed Baerveldt comparison study. Ophthalmology 121:1547–1557. doi:10.1016/j.ophtha.2014.01.036, e1
Bailey AK, Sarkisian SR (2014) Complications of tube implants and their management. Curr Opin Ophthalmol 25:148–153. doi:10.1097/ICU.0000000000000034
Freedman SF, Rodriguez-Rosa RE, Rojas MC, Enyedi LB (2002) Goniotomy for glaucoma secondary to chronic childhood uveitis. Am J Ophthalmol 133:617–621
Jea SY, Mosaed S, Vold SD, Rhee DJ (2012) Effect of a failed trabectome on subsequent trabeculectomy. J Glaucoma 21:71–75. doi:10.1097/IJG.0b013e31820bcfda
Brandao LM, Grieshaber MC (2013) Update on minimally invasive glaucoma surgery (MIGS) and new implants. J Ophthalmol. doi:10.1155/2013/705915
Kaplowitz K, Schuman JS, Loewen NA (2014) Techniques and outcomes of minimally invasive trabecular ablation and bypass surgery. Br J Ophthalmol 98:579–585. doi:10.1136/bjophthalmol-2013-304256
Hueber A, Roters S, Jordan JF, Konen W (2013) Retrospective analysis of the success and safety of Gold Micro Shunt Implantation in glaucoma. BMC Ophthalmol 13:35. doi:10.1186/1471-2415-13-35
Unal M, Kocak Altintas AG, Koklu G, Tuna T (2011) Early results of suprachoroidal drainage tube implantation for the surgical treatment of glaucoma. J Glaucoma 20:307–314. doi:10.1097/IJG.0b013e3181e3d30f
Roberts CW (1993) Intraocular miotics and postoperative inflammation. J Cataract Refract Surg 19:731–734
Acknowledgments
The results of this study were presented at the DOG (German Ophthalmological Society) in September 2014 in Leipzig
Financial disclosure
None of the authors has any proprietary or financial interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anton, A., Heinzelmann, S., Neß, T. et al. Trabeculectomy ab interno with the Trabectome® as a therapeutic option for uveitic secondary glaucoma. Graefes Arch Clin Exp Ophthalmol 253, 1973–1978 (2015). https://doi.org/10.1007/s00417-015-3102-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-015-3102-3