Abstract
Background
The minimally invasive ab-interno trabeculectomy (AIT) via electro-ablation with the Trabectome has been on the European market since 2009. Many studies have proven the safety and efficacy of the procedure. Up until now, studies investigating the long-term effect of AIT have been sparse. In this study, we present long-term results of AIT in patients with primary and secondary open-angle glaucoma.
Methods
In a retrospective monocentric study, the data of all the patients having undergone the procedure in 2010 at our institution were recorded. Data was collected during routine examinations at our institution. In total, 81 eyes of 74 patients (46 patients with primary open-angle glaucoma (POAG), 28 patients with pseudoexfoliative glaucoma (PEXG)) were included. At every examination, the intraocular pressure (IOP) was measured using Goldmann applanation tonometry and the number of IOP-lowering medication was registered. Statistical analysis was done using the Kaplan-Meier analysis or Dunnett’s t test, respectively.
Results
For both groups (POAG and PEXG), we found a significant lowering of the IOP (28% for POAG and 26% for PEXG) and a significant reduction of the number of IOP-lowering medication (32% for POAG and 29% for PEXG) after a median follow-up period of 3.5 years.
Conclusion
In patients with open-angle glaucoma and especially pseudoexfoliative glaucoma, ab-interno trabeculectomy is an effective surgical procedure to significantly lower the intraocular pressure on a long-term basis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glaucoma is one of the leading causes for blindness and irreversible visual field loss worldwide [1]. It is estimated that 64.3 million people, or 3.54% of the world’s population, have glaucoma [2]. Lowering the intraocular pressure (IOP) remains the only effective therapy to slow down or eventually halt the process of optic nerve atrophy [3,4,5]. This is primarily achieved through the use of topical IOP-lowering medication. However, in many patients, the sole and exclusive use of medication proves to be insufficient. For these patients, laser treatment or surgical therapy provides an alternative modality for reduction of the IOP. The gold standard in surgical glaucoma therapy is a filtration surgery by means of modern trabeculectomy (TET) as introduced by Cairns [6]. TET or episcleral aqueous drainage implants are the most effective methods to reduce the intraocular pressure but come at the cost of some serious undesirable effects such as cicatrization, prolonged hypotony, (late) bleb infection, cataract formation, and a high rate of revision surgeries [7,8,9]. This has led to an ongoing effort to develop minimally invasive pressure-lowering surgeries that avoid these pitfalls.
The Trabectome® (Neomedix Corp., Tustin, CA) is a surgical instrument used for minimally invasive ab-interno trabeculectomy (AIT). It has been on the European market since 2009. The device removes the trabecular meshwork through an electro-ablative procedure, hereby eliminating the presumed main outflow barrier in primary and secondary open-angle glaucoma [10]. Many past studies have shown the safety and effectiveness of the Trabectome as a surgical procedure for lowering the intraocular pressure (IOP) in patients with primary and secondary open-angle glaucoma (reviewed in [11]).
Most of the past studies investigating the effectiveness of the Trabectome show a mean observation period between 6 and 48 months [12]. The only exceptions are the studies by Mosaed et al. [13] and Esfandiari et al. [14], the former being the largest study (n = 5435 eyes were included) up to date and providing an observation period of up to 90 months, the latter reporting on 5-year follow-up results. For their study, Mosaed et al. used the Trabectome Study Group Database, a global database, maintained in cooperation with Neomedix Corp. In this study, an internal database on Trabectome patients was used which is maintained independently. To get further insight in the long-term results after AIT with the Trabectome, we reviewed data on patients having undergone this surgery in 2010 in our institution.
Methods
We investigated all patients who were treated at our institution with an AIT using the Trabectome in the year 2010. Of the 81 performed surgeries, 26 were combined AIT with cataract surgery (see Table 1 for the detailed demographic data on all patients).
The collection and review of data were performed in 2016 to allow all eyes included in our analysis to have a follow-up period of up to 5 years.
A total number of 81 eyes of 74 patients suffering from primary open-angle glaucoma (POAG) or pseudoexfoliative glaucoma (PEXG) were included in this single-center retrospective study. Of these 74 patients, 46 suffered from POAG and 28 from PEXG.
All surgeries were performed by two surgeons (JFJ and MN) using the Trabectome system. In cases of a combined surgery, the Trabectome surgery was performed prior to phacoemulsification. For topical anesthesia, proxymetacainehydrochloride eye drops (Proparakain-POS 0,5%®, Ursapharm, Germany) were applied. After clear corneal incision (1.7 mm), lidocaine 1% (Xylocaine®, AstraZeneca, Germany) was instilled into the anterior chamber for intraocular anesthesia.
The procedure was performed as previously described by Jordan et al. in 2013 [15]. During surgery, we used different types of viscoelastics: hypromellose 2% (dispensary of the University Medical Center, Freiburg, Germany; 57 eyes), Ocucoat® (Bausch & Lomb Berlin, Germany; 22 eyes), or Acri.Healon® (Carl Zeiss, Oberkochen, Germany; 2 eyes) to stabilize the anterior chamber.
Postoperatively, all patients were given pilocarpine 2% three times per day for the first week after surgery and twice per day for four more weeks. Prednisolone 1% was applied five times per day or more, depending on the postoperative signs of inflammation and reflux bleeding. The topical steroid was reduced gradually over 5 weeks after surgery. If transient postoperative IOP spikes occurred, additional topical and/or systemic medication to lower intraocular pressure was given, depending on the postoperative IOP levels.
All follow-up visits consisted of a measurement of the intraocular pressure using Goldmann applanation tonometry as well as recording the number of current IOP-lowering medication.
The statistical analysis was done using Dunnett’s t test. The long-term success of the procedure was analyzed using a Kaplan-Meier analysis for right-censored data. GNU R and additional packages were used for computing statistical tests [16,17,18,19].
Absolute success was defined as an IOP < 21 mmHg and a reduction of the IOP from the baseline of at least 20% without the need of additional medication. The qualified success of the procedure was defined as an IOP < 21 mmHg and 20% reduction of baseline IOP, permitting the additional use of IOP-lowering medication. The criteria for success were selected according to the World Glaucoma Association (WGA) [5].
Results
In the following text, the term “eye drops” refers to the active pharmaceutical ingredients.
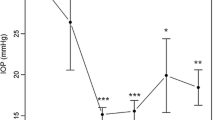
The mean follow-up time was approximately 3.5 years (1267 ± 704 days). All following postoperative results refer to this follow-up time. Mean IOP before surgery in patients with POAG was 23.0 ± 5.8 mmHg and dropped to 16.5 ± 4.1 (− 28%) at mean follow-up. For patients with PEXG, IOP before surgery was 23.1 ± 5.1 mmHg and was reduced to 17.2 ± 6.1 mmHg (− 26%), respectively (Fig. 1). Before surgery, patients with POAG used 2.8 ± 1.0 different IOP-lowering eye drops. After surgery, this number fell to 1.9 ± 1.1 (− 32%). For patients with PEXG, 2.4 ± 1.0 different IOP-lowering eye drops were used before surgery. This figure declined to 1.7 ± 1.3 (− 29%) (Fig. 2 and Table 2).
Mean intraocular pressure (IOP) readings at follow-up visits. Follow-up visits (see Table 2) were clustered (0 = preoperative, 1 to 29, 30 to 364, 365 to 729, 730 to 1064, 1065 to 1459, 1460 to 1824, 1825 to 2177 days). If a single patient had more than one follow-up visit during a given timeframe, mean IOP values for this patient and timeframe were calculated first. IOP reduction from baseline was statistically significant (p < 0.05 or less) for all time points
Mean number of IOP-lowering medication (eye drops) at follow-up visits. Clustering of follow-up visits was identical to Fig. 1 and Table 2. The reduction of number of IOP-lowering eye drops was statistically significant (p < 0.05) for all time points in the POWG subgroup and time points 4 and 7 in the PEXG subgroup
The Kaplan-Meier analysis showed a qualified success rate of 44.6% for POAG and 67.5% for PEXG after the mean follow-up time of 3.5 years. The differences in long-term survival between POAG and PEXG Kaplan-Meier survival analyses (Fig. 3) revealed to be statistically significant using a log-rank test (p = 0.01). “Survival” of the procedure was defined as Δ IOP ≥ − 20% from baseline and not higher than 21 mmHg, no medication (absolute success); or Δ IOP ≥ −20% from baseline and not higher than 21 mmHg, topical medication allowed (qualified success).
In total, 31 (= 38.3%) of the cases reported here needed further surgery during follow-up, with 8 cases requiring more than one further surgery. In 21 cases, a trabeculectomy was performed; other treatments included a second AIT (n = 2), selective laser trabeculoplasty (n = 4), or cyclophotocoagulation (n = 10). The mean time between Trabectome surgery and second surgery was 3.4 years (range 8 days to 5.7 years).
In 95% of the cases, an intraoperative or early postoperative reflux bleeding from the collector channels was noticed, most of these being mild to moderate. A transient early postoperative (≤ 3 months postop.) IOP elevation over 30 mmHg was observed in 18.5% of the cases. No severe adverse events such as endophthalmitis or loss of vision were observed.
We further analyzed the effectiveness of the combined surgery (Trabectome + phacoemulsification + intraocular lens implantation), as well as the effectiveness of the Trabectome in patients with PEXG in comparison to POAG. A Cox proportional hazards model (corrected for age and lens state) shows a nearly two-times increased risk for failure for POAG patients compared with that for PEXG (p = 0.04) (Table 3).
Discussion
The results of our data suggest that AIT using the Trabectome provides a minimally invasive technique, with an effective long-term lowering of the IOP and reduction of the number of IOP-lowering medication.
In 2014, Mosaed et al. presented the largest published data pool, consisting of 5435 eyes with a follow-up time of 90 months [13]. Fifty-six percent of these cases received the Trabectome procedure alone, 41% received the Trabectome procedure combined with cataract extraction, and 2% received the Trabectome combined with other surgical procedures. After 90 months of observation, average IOP was reduced from a baseline of 23.1 to 17.2 mmHg (26%), and the average number of glaucoma medications was reduced from 2.6 to 1.1 (58%). In this data report, a mixed population of different forms of glaucoma was included. A separate analysis of the different subgroups was not provided. It also remains unclear how many patients were still included after 90 months of observation. A survival analysis was not provided. While the relative reduction of the IOP in the work of Mosaed et al. is comparable with our results, the saving of eye drops clearly exceeds our outcomes. Due to the rather summarizing presentation of the results in the work of Mosaed et al., a more detailed comparative analysis of the results is unfortunately not possible.
In 2012, Ting et al. compared the results of the Trabectome in PEXG to those in primary OAG in a retrospective, central database analysis [20]. For PEXG, in the 67 Trabectome-only eyes, IOP was reduced from a baseline of 29.0 to 16.1 mmHg (45%); in the 45 cases of combined surgery, IOP was reduced from a baseline of 21.7 to 14.2 mmHg (35%) after 1 year. The number of glaucoma medications was reduced by 29% and 36%, respectively. For POAG, after 1 year, the IOP of the 450 Trabectome-only eyes was reduced from 25.5 to 16.8 mmHg (34%); the IOP for the 263 combined-surgery eyes was reduced from 19.9 to 15.6 mmHg (22%), reducing glaucoma medication by 18.5% and 29%, respectively. These results are in concordance with our findings, showing that the initial reduction of IOP and medication after 1 year can still be seen after a longer follow-up period of about 3.5 years.
Further single-center studies in 2013 by Ahuja et al. and Jordan et al. described similar IOP-lowering results for patients undergoing surgery with the Trabectome or combined Trabectome/cataract surgery. Ahuja et al. analyzed POAG patients and compared Trabectome-only patients to combined-surgery patients, reporting an IOP reduction after 24 months of 35.1% and 22.8%, respectively [21]. In 2013, our group described the difference between POAG and PEXG patients undergoing Trabectome or combined surgery, hereby similarly to this study not separating the results of the two types of procedures. In this analysis of our database, we concentrated on short-term outcome of patients having undergone AIT. We found that for patients with POAG, IOP was improved by 26% and glaucoma medications reduced by 43%. For patients with PEXG, IOP was improved by 28% with a glaucoma medication reduction of 45%. The mean follow-up periods were 6.7 ± 7.8 months (204 ± 238 days) and 6.6 ± 9.1 months (200 ± 278 days), respectively [15]. When comparing the results of our group in 2013 to our current results, we must acknowledge the fact that some patients were included in both studies. The difference, however, lies in the point of analysis. In 2013, we used the last available data point for every patient, therefore resulting in a relatively short follow-up period. In our current study, we concentrated on the long-term follow-up, resulting in the different follow-up periods as mentioned above.
More recent studies from 2015 by Pahlitzsch et al. and Werth et al. reported further similar results concerning the IOP-lowering effect and reduction of glaucoma medication, both using a 1-year follow-up period. Werth et al. described a higher IOP reduction in patients with secondary glaucoma compared with POAG; the difference between the groups, however, did not prove to be statistically significant [22, 23].
Pahlitzsch et al. provided a further study in 2018 reporting on the 3-year-results of AIT performed on 366 patients (268 POAG and 98 PEXG) [24]. Similar to our results, IOP was reduced from 19.10 ± 4.11 mmHg to 14.27 ± 2.93 mmHg (POAG) and glaucoma medication was decreased from 2.40 ± 0.92 to 1.77 ± 1.00 (POAG) after 36 months. In PEXG, IOP decreased from 22.49 ± 9.40 mmHg to 14.57 ± 5.05 mmHg and medications dropped from 2.31 ± 1.02 to 1.75 ± 0.91 after 36 months. However, the overall complete success showed a low survival rate (for IOP ≤ 21 mmHg: 13.5% in POAG and 7.9% in PEXG; for IOP ≤ 18 mmHg: 12.8% in POAG and 5.9% in PEXG).
Most recently in 2018, Esfandiari et al. published a single-center study showing the IOP-lowering effect and reduction of IOP-lowering medications following a combined surgery of phacoemulsification and AIT after a follow-up period of 5 years [14]. The IOP was decreased significantly from 20.0 ± 5.6 mmHg at baseline to 15.6 ± 4.6 mmHg after a 5-year follow-up. The baseline number of IOP-lowering medications was 1.8 ± 1.2, which decreased to 1.0 ± 1.2 medications at 5 years. The study also showed a higher success rate for PEXG. While the initial intraocular pressure was higher in our group, we achieved a comparable relative pressure reduction. In contrast, the relative drug reduction in our patient population was significantly lower. This is probably due to the higher initial level of pressure-reducing eye drops used in our population.
The abovementioned published results are in agreement with the results from this study regarding the overall reduction of IOP and glaucoma medications. The data presented here show that this reduction can still be observed after an extended follow-up period of approximately 3.5 years.
While not providing a follow-up period as long as Esfandiari et al. (5 years) or a patient group as large as Pahlitzsch et al. in their 3-year follow-up study, our results provide additional insight on the subgroup comparison of patients with PEXG and POAG as well as the comparison of patients who received AIT only with patients who underwent combined surgery (AIT and phacoemulsification). Thus, this work underlines and supports central statements of the preceding studies and simultaneously expands this clinically relevant question by central aspects.
The Cox proportional hazards model corrected for lens state and age could confirm the superiority of the Trabectome when performed in patients with PEXG over patients with POAG. The hazard ratio for failure of the Trabectome surgery was 1.96 for POAG. This is in concordance with findings of former studies [20, 21, 23], examining the effect of AIT in patients with PEXG.
Obviously, the limitations of a retrospective study must be kept in mind as well, and additional studies including larger patient numbers and even longer follow-up periods could provide further insight into the longevity of this minimally invasive procedure.
For many patients suffering from POAG and PEXG in particular with moderate IOP elevation, the Trabectome proves to be a valid surgical device for lowering the IOP and reducing the number of medication needed.
References
Quigley HA, Broman AT (2006) The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 90:262–267. https://doi.org/10.1136/bjo.2005.081224
Tham Y-C, Li X, Wong TY et al (2014) Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121:2081–2090. https://doi.org/10.1016/j.ophtha.2014.05.013
Kass MA, Heuer DK, Higginbotham EJ et al (2002) The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol Chic Ill 1960 120:701–713 discussion 829-830
Garway-Heath DF, Crabb DP, Bunce C et al (2015) Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet Lond Engl 385:1295–1304. https://doi.org/10.1016/S0140-6736(14)62111-5
Shaarawy T, Grehn F, Sherwood M, World Glaucoma Association (2009) WGA guidelines on design and reporting of glaucoma surgical trials. Kugler Publications, The Hague
Cairns JE (1968) Trabeculectomy. Preliminary report of a new method. Am J Ophthalmol 66:673–679
Kwong A, Law SK, Kule RR et al (2014) Long-term outcomes of resident- versus attending-performed primary trabeculectomy with mitomycin C in a United States residency program. Am J Ophthalmol 157:1190–1201. https://doi.org/10.1016/j.ajo.2014.02.028
Klink T, Kann G, Ellinger P et al (2011) The prognostic value of the Wuerzburg bleb classification score for the outcome of trabeculectomy. Ophthalmol J Int Ophtalmol Int J Ophthalmol Z Augenheilkd 225:55–60. https://doi.org/10.1159/000314717
Landers J, Martin K, Sarkies N et al (2012) A twenty-year follow-up study of trabeculectomy: risk factors and outcomes. Ophthalmology 119:694–702. https://doi.org/10.1016/j.ophtha.2011.09.043
Tektas O-Y, Lütjen-Drecoll E (2009) Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp Eye Res 88:769–775. https://doi.org/10.1016/j.exer.2008.11.025
Wecker T, Jordan JF (2015) Minimalinvasive Kammerwinkelchirurgie mit dem Trabektom. Klin Monatsblätter Für Augenheilkd 232:303–309. https://doi.org/10.1055/s-0034-1383014
Kaplowitz K, Bussel II, Honkanen R et al (2016) Review and meta-analysis of ab-interno trabeculectomy outcomes. Br J Ophthalmol 100:594–600. https://doi.org/10.1136/bjophthalmol-2015-307131
Mosaed S (2014) The first decade of global Trabectome outcomes. Eur Ophthalmic Rev 08:113. https://doi.org/10.17925/EOR.2014.08.02.113
Esfandiari H, Shah P, Torkian P et al (2018) Five-year clinical outcomes of combined phacoemulsification and trabectome surgery at a single glaucoma center. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. https://doi.org/10.1007/s00417-018-4146-y
Jordan JF, Wecker T, van OC et al (2013) Trabectome surgery for primary and secondary open angle glaucomas. Graefes Arch Clin Exp Ophthalmol 251:2753–2760. https://doi.org/10.1007/s00417-013-2500-7
Wickham H (2007) Reshaping data with the reshape package. J Stat Softw 21:1–20
Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer, New York
Wickham H (2011) The split-apply-combine strategy for data analysis. J Stat Softw 40:1–29
R Core Team (2014) R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna
Ting JLM, Damji KF, Stiles MC, Trabectome Study Group (2012) Ab interno trabeculectomy: outcomes in exfoliation versus primary open-angle glaucoma. J Cataract Refract Surg 38:315–323. https://doi.org/10.1016/j.jcrs.2011.08.043
Ahuja Y, Ma Khin Pyi S, Malihi M et al (2013) Clinical results of ab interno trabeculotomy using the trabectome for open-angle glaucoma: the Mayo Clinic series in Rochester, Minnesota. Am J Ophthalmol 156:927–935.e2. https://doi.org/10.1016/j.ajo.2013.06.001
Pahlitzsch M, Gonnermann J, A-KB M et al (2015) Trabekulotomie ab interno (Trabectome) – Kumulierte klinische Ergebnisse eines großen Glaukomkollektivs. Klin Monatsblätter Augenheilkd 232:1198–1207. https://doi.org/10.1055/s-0041-105941
Werth JP, Gesser C, Klemm M (2015) Unterschiedliche Effekte der Trabektomoperation auf unterschiedliche Glaukomformen. Klin Monatsblätter Augenheilkd 232:72–78. https://doi.org/10.1055/s-0034-1383010
Pahlitzsch M, Davids AM, Zorn M et al (2018) Three-year results of ab interno trabeculectomy (Trabectome): Berlin study group. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol 256:611–619. https://doi.org/10.1007/s00417-017-3882-8
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Avar, M., Jordan, J.F., Neuburger, M. et al. Long-term follow-up of intraocular pressure and pressure-lowering medication in patients after ab-interno trabeculectomy with the Trabectome. Graefes Arch Clin Exp Ophthalmol 257, 997–1003 (2019). https://doi.org/10.1007/s00417-019-04259-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04259-5