Abstract
Purpose
T helper 17 (Th17) cells are believed to play a critical role in the chronic inflammatory and immune response in streptozotocin (STZ)-induced retinopathy. The purpose of our study was to investigate the effect of the IL-23–Th17–IL-17A pathway via the blood–retinal barrier on STZ-induced diabetic retinopathy in rats.
Methods
The ratio of IL-17A+CD4+ T cells in peripheral blood mononuclear cells of STZ-treated and wild-type rats was determined using flow cytometry. The IL-17A mRNA levels in the retinas were measured using real-time PCR. The protein expression of IL-17A in the peripheral blood and retinas was measured using an ELISA kit. The retinal structure in the wild-type and STZ-treated rats was examined using hematoxylin and eosin (H&E) staining. Additionally, the permeability of the blood–retinal barrier was quantified using the Evans blue technique.
Results
The ratio of IL-17A+CD4+ T cells in peripheral blood mononuclear cells was markedly increased in rats treated with STZ compared to the wild-type group. IL-17A protein levels in the peripheral blood and retinas were also significantly elevated in STZ-treated rats. However, when the anti-IL 23Rp19 antibody was injected into the vitreous cavity in the eyes of STZ-treated rats for a period of one week, retinal pigment epithelium cells became markedly tighter, and micrangium and endothelial cells were significantly reduced. The expression of IL-17A mRNA and protein in the retina also decreased significantly compared with the placebo-treated group.
Conclusions
This study provided further insight into the function of the IL-23–Th17–IL-17A pathway in STZ-induced diabetic retinopathy in rats. Local injection of the anti-IL-23Rp19 antibody may improve the structure of the blood–retinal barrier, thus offering the potential for treatment using intravitreal anti-IL-23Rp19 antibodies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic retinopathy (DR), a common disease-specific complication of diabetes, is the leading cause of visual impairment and acquired blindness amongst adults worldwide [1]. In 2012, globally, there were approximately 93 million cases of DR, of which 17 million were individuals with proliferative retinopathy [2]. A number of factors are involved in the development of DR, including inflammation [3], oxidative stress [4], and advanced glycation end products (AGEs) [5]. In recent years, evidence has accumulated suggesting that chronic inflammation and immune response promote the development of DR, particularly retinopathy associated with type 1 diabetes, which is related to various immune cells and cytokines [6, 7]. There has been heightened interest recently in the function of T helper 17 (Th17) cells, which have been implicated in the pathogenesis of type 1 diabetes [8].
Extensive studies of Th17 cells have reported their involvement in the development of inflammatory and autoimmune diseases such as rheumatoid arthritis, inflammatory bowel disease, multiple sclerosis, and type 1 diabetes [9]. As a novel independent subset of CD4+ T cells, Th17 cells are primarily characterized by their production of IL-17A, IL-17F, and IL-22. IL-17A is a key cytokine responsible for the recruitment, activation, and migration of neutrophils, and can induce the secretion of proinflammatory mediators by nonimmune cells such as fibroblasts, endothelial cells, and epithelial cells [10]. This leads to the proliferation and accumulation of neutrophils in the innate immune system and the integration of innate and adaptive immunity in vivo. Nadeem et al. [11] demonstrated increased IL-17A serum concentrations in patients with diabetic retinopathy. IL-17A also promotes blood–retinal barrier disruption and retinal inflammation by inducing the secretion of inflammatory factors in endothelial cells and by the downregulation of tight-junction proteins [12]. Increased levels of IL-17A can lead to local inflammation and immune response in the retina, which in turn may promote the development of DR [13].
A growing body of evidence suggests that cytokines such as transforming growth factor (TGF)-β, IL-6, IL-21, IL-1β, and IL-23, as well as transcription factors such as retinoic acid receptor-related orphan receptor (ROR)γt, RORα, and signal transducer and activator of transcription 3 (STAT3), play critical roles in the differentiation and maintenance of Th17 cells [14]. The most recent model shows that IL-1β and IL-6 induce the differentiation of Th17 cells from naïve T cells [15]. IL-21 is involved in Th17 differentiation in an autocrine manner via the RORγt signaling pathway [16]. Furthermore, deficiencies in RORα and RORγt, both of which play a dominant role in determining the fate of Th17 cells, have been associated with impaired Th17 cell differentiation [17]. Notably, IL-23 is responsible for the survival and amplification of Th17 cells as well as the production of IL-22 and IL-21 [18]. Hence, IL-23 may function at a late stage of Th17 cell differentiation after initial induction by other proinflammatory cytokines (e.g., IL-1β, TNF-α, IL-6, and IL-21) [16]. Rats deficient in IL-23 are completely resistant to EAE (experimental autoimmune encephalomyelitis) [19]. As such, the IL-23 function is indispensable in orchestrating the pathogenicity of Th17 cells. Toussirot et al. demonstrated the relevance of the IL-23/Th17 axis as a therapeutic target, as it plays a key role in the development of chronic inflammatory and autoimmune-mediated diseases [20].
Although the exact pathogenesis of DR has not been completely revealed, several theories exist. In this study, we investigate whether Th17 cells contribute to the progression of retinopathy in rats with streptozotocin (STZ)-induced type 1 diabetes, as well as the effect of blocking IL-23 on Th17 cells and the blood–retinal barrier.
Methods
Streptozotocin-induced type 1 diabetes in rats
Male Sprague–Dawley rats (200 ± 30 g, 8 weeks old, n = 40) were purchased from a local dealer and bred in the animal laboratory of Chongqing Medical University (Chongqing, China), where they were raised in a standard animal room with food and water. Type 1 diabetes was induced in the rats via a single intraperitoneal injection of high-dose streptozotocin (STZ, 60 mg/kg in 100 mmol/L citrate buffer [pH 4.5]). Age-matched rats serving as the normal control group received an equivalent amount of citrate buffer alone. Four weeks after STZ injection, rats with non-fasting blood glucose ≥250 mg/dL were regarded as diabetic. Throughout the experimental period, all rats were fed a regular diet, with unlimited water. All studies were approved by the Ethics Committee of Chongqing Medical University and conformed to guidelines set forth in the Statement for the Use of Animals in Ophthalmic and Vision Research.
STZ-treated rats with intravitreal injection
Three months after successful modeling of STZ-induced diabetes, the rats were injected with an anti-IL-23Rp19 antibody solution, which was administered with a sharp 30-gauge needle into the vitreous cavity through the corneal limbus. One eye of the STZ-treated rats was treated with a single injection of the anti-IL-23Rp19 antibody solution (5 μL, 200 μg/mL, in phosphate buffer solution [PBS, 0.01 mol/L, pH 7.2]; H-113, sc-50303, Santa Cruz Biotechnology); the other eye of the same STZ-treated rats received an equivalent amount of placebo (PBS, 0.01 mol/L, pH 7.2) alone. The duration of anti-IL-23Rp19 antibody treatment was determined based on the pharmacodynamics and drug metabolism cycle in vivo, and the dosage was determined according to the product manual. After successful anti-IL-23Rp19 antibody treatment for one week, the animals were euthanized.
Measurement of blood–retinal barrier permeability
The blood–retinal barrier (BRB) function was quantified using the Evans blue (EB) dye method. Briefly, after the rats were deeply anesthetized, EB dye (30 mg/mL in low saline; Sigma-Aldrich, St. Louis, MO, USA) was injected through the femoral vein over a period of 10 seconds at a dosage of 45 mg/kg. After the dye had circulated for two hours, the rats were perfused with 4 % paraformaldehyde through the left common carotid artery. Then, after dissecting, drying, and weighing the retina, EB was extracted via incubation of each retina in 200 μl formamide for 18 hours at 70 °C. The extract was ultracentrifuged at 14,000 rpm for 60 minutes at 4 °C, and 60 microliters of the supernatant was used for further spectrophotometric measurement at 620 and 740 nm.
Histological analysis
Rats were euthanized, and their eyes were immediately isolated. The retinas were immersed for 24 hours in 4 % paraformaldehyde and embedded in paraffin blocks; 5-μm sections were cut. The paraffin sections were stained using the hematoxylin and eosin (H&E) protocol. Microscopic images were digitally captured using a fluorescence microscope (Leica, DM6000).
Cell isolation
Heparinized venous blood samples were collected aseptically, placed into tubes, and used to isolate peripheral blood mononuclear cells (PBMCs). PBMCs were separated by density gradient preparation over Ficoll-Uropoline. Mononuclear cells at the interface were carefully transferred into a centrifuge tube, then treated with RBC Lysis Buffer (BioLegend, San Diego, CA, USA) and washed twice in a phosphate buffer solution (PBS, 0.01 mol/L, pH 7.2). For Th17 analysis, cells were suspended at a density of 2 × 106 cells/mL. The cells were then stained for flow cytometric analysis.
Cell staining and flow cytometric analysis
Before staining, cells were washed with Flow Cytometry Staining Buffer (eBioscience, San Diego, CA, USA). First, cells were stained with a membranal anti-CD4 antibody (IgG2a, κ rat FITC, Clone OX35, eBioscience, USA). After 30-minute incubation at room temperature, cells were washed and stained for intracellular anti-IL-17A (IgG2a, κ rat PE, Clone eBio17B7; eBioscience). The monoclonal antibodies were used for Th17 expression of cell surface and intracellular markers, were gated on live lymphocytes according to forward and side scatter, and were assessed using flow cytometry (BD FACSVantage SE; BD Biosciences, USA). The FACSCalibur flow cytometer equipped with CellQuest software (BD Biosciences) was used.
Real-time polymerase chain reaction
Retinas were lysed in TRIzol reagent (Invitrogen; Life Technologies, Carlsbad, California, USA). Total RNA was extracted from the collected cell lysate and then reverse-transcribed into complementary DNA (cDNA) using the RTase kit (TaKaRa Bio, Otsu, Japan), according to manufacturer instructions. The polymerase chain reaction (PCR) was amplified using the Applied Biosystems 7500 Fast Real-Time PCR System (Foster City, CA, USA). Briefly, total cDNA (1 μg) was added per 20 μl of reaction buffer with SYBR® Premix Ex Taq™ II (Tli RNaseH Plus) (2×) and ROX Reference Dye (50×). The PCR primers employed were as follows: IL-17A, 5′-GCCGAGGCCAATAACTTTCT-3′ (forward) and 5′-AGAGTCCAGGGTGAAGTGGA-3′ (reverse); β-actin, 5′-CACCCGAGTACAACCCGTTC-3′ (forward) and 5′-CCCATACCCACCATCACACC-3′ (reverse). Cycling conditions were 30 seconds of polymerase activation at 95 °C, followed by 40 cycles at 95 °C for 5 seconds and at 60 °C for 34 seconds. The 2-ΔΔCT method was applied to estimate relative transcript levels. All experiments were carried out in duplicate, with three independent samples per group.
Cytokine detection
The retinas were homogenized in 150 μL of RIPA lysis buffer supplemented with 1 % protease inhibitors. The samples were ultracentrifuged at 12,000 rpm for 25 minutes at 4 °C, and the supernatant was extracted. IL-17A protein in the supernatant was measured using an ELISA kit (R&D Systems, Minneapolis, MN, USA), according to manufacturer instructions. The reaction was stopped, and absorbance was read immediately at 450 nm on a microplate reader (Model 3550, Bio-Rad Laboratories, Hercules, CA, USA).
Statistical analysis
The results were expressed as mean ± SD. Multiple comparisons among groups were performed using one-way analysis of variance (ANOVA) with GraphPad Prism 5.0. A two-tailed p < 0.05 was considered statistically significant.
Results
IL-17A+CD4+ T cells were increased in peripheral blood of STZ-treated rats
First, we investigated whether Th17 cells were involved in the pathogenesis of STZ-induced retinopathy. We measured the expression of Th17 cells in the peripheral blood mononuclear cells (PBMCs), with results reflecting a markedly higher ratio of IL-17A+CD4+ T cells in PBMCs from the STZ-treated group (42.78 %) compared to those of the WT group (1.12 %) (Fig. 1). In addition, there was significantly elevated IL-17A protein expression in the peripheral blood of the STZ-treated group compared to that of the WT group (218.19 ± 23.76 ng/L, 37.40 ± 0.96, ng/L respectively), which was consistent with the results for IL-17A+CD4+ T cells in PBMCs (p < 0.001). We also investigated the expression of IL-17A in the retinas of the STZ-treated rats. The results (Figs. 2 and 3) reflected significantly higher mRNA IL-17A levels in the retinas of the STZ-treated group compared to those of the WT group (p < 0.001), and the protein expression of IL-17A was also significantly elevated in the retinas of the STZ-treated rats compared to those of the WT group (3.13 ± 0.17 ng/mg, 0.13 ± 0.11 ng/mg, respectively, p < 0.01).
IL-17A+CD4+ T cells were increased in the peripheral blood of the STZ-treated group after three months. a PBMCs from the WT group were unstained; n = 4 experiments. b PBMCs from the WT group were stained with antibodies against CD4 and IL-17A. The ratio of IL-17A+CD4+ T cells in PBMCs was determined by flow cytometry; n = 4 experiments. c PBMCs from the STZ-treated group were stained with antibodies as described above and analyzed using flow cytometry; n = 4 experiments. d The results were quantified. The upper right quadrant shows the ratio of IL-17A+CD4+ T cells in PBMCs. PBMCs peripheral blood mononuclear cells
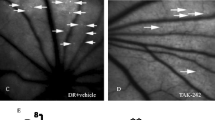
Administration of the anti-IL-23Rp19 antibody improved the structure of the blood–retinal barrier in the STZ-treated group. a and c show the normal retinal cell layers in the wild-type group after one and three months of treatment, respectively. b shows the disruption in the outer nuclear layer of the retina and loose retinal pigment epithelial cells in the STZ-treated group after one month. d shows the disruption of the inner and outer retinal nuclear layers in the STZ-treated group after three months, where retinal pigment epithelial cells became loose and micrangium endothelial cells increased. e shows the disordered structure of the retina at three months in STZ-treated rats injected with placebo. f illustrates that the inner and outer nuclear layers of the retina in the anti-IL-23Rp19-treated group became slightly more regular, retinal pigment epithelium cells became tight, and micrangium and endothelial cells decreased. Original magnification: ×400. Micrangium was made of endothelial cells. solid arrows: retinal pigment epithelium cells; small arrows: micrangium and endothelial cells; black line: inner blood–retinal barrier; irregular oval: micrangium
Disruption of the retinal structure in STZ-treated rats
The retinal tissue structure in the normal control group was characterized by orderly inner and outer nuclear layers, with a small number of micrangium cells in the retina (Figs. 2a and 2c). After one month of STZ treatment, the outer nuclear layer of the retina was looser in the treated group compared to the control group (Figs. 2a and 2b), although no difference was observed in the inner nuclear layer of the retina. There was a slight loosening in the retinal pigment epithelium, but the micrangium and endothelial cells showed no significant change. On the other hand, as can be seen in Table 1, the permeability of the blood–retinal barrier in the STZ-treated group after one month (0.806 ± 0.006 μL/g/h) was 1.32-fold higher than that of the control group after one month (0.612 ± 0.019 μL/g/h). Most notably, compared with the WT group at three months (Fig. 2c), rats that were treated with STZ for three months (Fig. 2d) showed not only considerable loosening of retinal pigment epithelium cells, but also higher numbers of micrangium and endothelial cells. In addition, greater disruption of the inner and outer nuclear layers of the retina was observed in the STZ-treated group after three months versus one month of treatment.
Anti-IL-23Rp19 antibody inhibited IL-17A expression in STZ-treated rats
The aforementioned results indicate that Th17 cells were involved in the pathogenesis of STZ-induced retinopathy. It has been reported that IL-23 plays a key role in the survival and maintenance of Th17 cells. Next, we investigated the effect of the anti-IL-23Rp19 antibody on Th17 cells in the retina. We found that expression of IL-17A mRNA in the retinas of anti-IL-23Rp19-treated rats was significantly reduced compared to that in the STZ-treated group (195.21 ± 26.72 vs. 96.49 ± 20.85, p < 0.01). Furthermore, IL-17A protein levels in the retinas of anti-IL-23R-treated rats (0.18 ± 0.05 ng/mg) were also markedly lower than those of the STZ-treated group (Table 1). There was no difference in the mRNA levels of IL-17F and IL-22 between the STZ-treated and control groups (data not shown).
Anti-IL-23Rp19 antibody improved blood–retinal barrier function in STZ-treated rats
The blood–retinal barrier plays a critical role in normal retinal function. Because we observed disruption of the retinal structure in STZ-treated rats, we investigated the effect of the anti-IL-23Rp19 antibody on the function of the blood–retinal barrier in the retina. Treatment consisting of an anti-IL-23Rp19 antibody solution injected into the vitreous cavity in eyes of STZ-treated rats was conducted for one week, with results showing slightly improved regularity in the inner and outer retinal nuclear layers compared to the eyes treated with placebo (Fig. 2f). Significant tightening of retinal pigment epithelium cells was observed and the number of micrangium and endothelial cells significantly reduced compared to the placebo-treated group (Fig. 2e).
Discussion
Th17 cells play a central role in the development of inflammatory and autoimmune diseases, particularly in the case of retinopathy associated with type 1 diabetes [9]. The breakdown of the blood–retinal barrier is a contributory factor in the development of retinopathy, and can result in hemorrhage, edema, and retinal detachment. The aim of the present study was to clarify the function of the IL-23–Th17–IL-17A pathway in streptozotocin-induced diabetic retinopathy in rats. Our results reflected a marked increase in the ratio of IL-17A+CD4+ T cells in peripheral blood mononuclear cells (PBMCs) and in the expression of IL-17A protein in peripheral blood in rats treated with STZ for three months compared to those in the wild-type (WT) group. Increased mRNA and protein levels of IL-17A in the retinas of STZ-treated rats were also observed. Disruption of the blood–retinal barrier occurred in the early stage of STZ-treated retinopathy (one month of treatment), and the structure of the blood–retinal barrier showed marked changes in the late stage of STZ-treated retinopathy (three months of treatment). Interestingly, the pretreatment with anti-IL-23Rp19-neutralizing antibodies resulted in downregulation of IL-17A expression of and improved blood–retinal barrier structure in late-stage STZ-treated retinopathy. Previous research has described a strong correlation between increased levels of Th17 cells and the development of type 1 diabetes, which is consistent with our study [21]. The production of IL-17A, IL-17F, IL-22, and IL-17A has been described as the hallmark of Th17 cells [22]. Honkanen et al. [21] found that IL-17A and IL-22 mRNA expression was significantly elevated in circulating memory CD4 cells in patients with type 1 diabetes. Similarly, we observed markedly higher levels of IL-17A expression in STZ-treated rats compared to controls. Unexpectedly, we observed no difference between the STZ-treated and control groups with regard to the IL-17F and IL-22 mRNA levels present in the retina (data not shown). This discrepancy may be explained by the different experimental approaches and varied disease backgrounds among studies. Nevertheless, our results suggest that Th17 cells and IL-17A expression play important roles in the progression of STZ-treated retinopathy.
As we all know, the blood–retinal barrier consists of retinal vessels and retinal pigment epithelium cells. The retinal capillary endothelial cells form the blood–retinal inner barrier, and retinal pigment epithelium cells form the blood–retinal outer barrier [23]. Kaya et al. [24] found that disruption of the blood–retinal barrier began in the early stage of STZ-treated retinopathy. In our study, disruption in the permeability of the blood–retinal barrier was apparent after one month of STZ treatment. Looseness in the retinal pigment epithelium cells was also observed, although there was no marked change in the micrangium and endothelial cells. After three months of STZ treatment, in addition to looseness in retinal pigment epithelium cells, the number of micrangium and endothelial cells had increased significantly. Moreover, marked disruption of the inner and outer retinal nuclear layers was evident in the STZ-treated group. Importantly, the breakdown of the blood–retinal barrier was more severe after three months of STZ treatment than after one month. As such, attention has been focused on the group that was treated for three months.
As IL-23 is known to play a vital role in Th17 cell differentiation and proliferation during the late stage of STZ treatment [14], it would follow that the absence of IL-23 would reduce the mRNA and protein levels of IL-17A and relieve STZ-induced retinopathy. In the present study, in vivo development of Th17 cells was inhibited in the absence of IL-23. The effect of the anti-IL 23Rp19 antibody on retinopathy can be characterized by two aspects. First, when the anti-IL 23Rp19 antibodies were injected into the eyes of STZ-treated rats over a period of one week, the IL-17A mRNA and protein levels in the retina were significantly reduced compared to the control group. Indeed, the duration of the intervention was based on the pharmacodynamics and drug metabolism cycle in vivo. Secondly, our results showed that eyes treated with the anti-IL-23Rp19 antibody exhibited somewhat greater regularity in the inner and outer nuclear layers than the eyes treated with placebo. Remarkably, retinal pigment epithelium cells became tighter, and micrangium and endothelial cells were greatly reduced. Hence, in this regard, the blood–retinal barrier function was already improved. In short, the anti-IL-23Rp19 antibody not only reduced the expression of IL-17A mRNA and protein in the retina, but also alleviated the effects of retinopathy in STZ-treated rats. A previous study [25] demonstrated that TGF-β, IL-6, and IL-23 are responsible for promoting the differentiation and development of the Th17 subsets, whereas the effects of IL-27 are inhibitory. Most recent studies have shown that IL-23 plays a vital role in the development of Th17 cells in chronic inflammatory and autoimmune diseases [26–30]. Increased IL-23p19 in synovial fluid was has been associated with joint destruction in rheumatoid arthritis through interplay with other cytokines such as IL-17A, TNF-α, and IL-1β [31]. Ratsimandresy et al. [32] reported that targeting of IL-23p19 demonstrated protective effects in collagen-induced arthritis, which is consistent with our study. Although our study confirmed that Th17 cells and IL-17A expression promoted the development of STZ-induced retinopathy by disruption of the blood–retinal barrier, the precise mechanism by which the IL-23-Th17-IL-17A pathway influences the blood–retinal barrier is still unclear, and further work is needed in order to investigate the specific molecular mechanisms involved.
In conclusion, the IL-23–Th17–IL-17A pathway is involved in promoting the development of retinopathy in STZ-treated rats. The anti-IL-23Rp19 antibody reduces the inflammatory factor IL-17A and alleviates pathological changes in the retina. As such, it offers a new perspective on the pathogenesis of diabetic retinopathy, and may represent a novel approach for local treatment of this disease.
References
Ola MS, Nawaz MI, Siddiquei MM et al (2012) Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. J Diabetes Complicat 26:56–64. doi:10.1016/j.jdiacomp.2011.11.004
Yau JW, Rogers SL, Kawasaki R et al (2012) Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35:556–564. doi:10.2337/dc11-1909
Cheung CMG, Vania M, Ang M et al (2012) Comparison of aqueous humor cytokine and chemokine levels in diabetic patients with and without retinopathy. Mol Vis 18:830–837
Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circ Res 107:1058–1070. doi:10.1161/circresaha.110.223545
Milne R, Brownstein S (2013) Advanced glycation end products and diabetic retinopathy. Amino Acids 44:1397–1407. doi:10.1007/s00726-011-1071-3
Tomic M, Ljubic S, Kastelan S (2013) The role of inflammation and endothelial dysfunction in the pathogenesis of diabetic retinopathy. Coll Antropol 37(Suppl 1):51–57
Zipris D (2008) Innate immunity and its role in type 1 diabetes. Curr Opin Endocrinol Diabetes Obes 15:326–331. doi:10.1097/MED.0b013e3283073a46
Shao S, He F, Yang Y et al (2012) Th17 cells in type 1 diabetes. Cell Immunol 280:16–21. doi:10.1016/j.cellimm.2012.11.001
Maddur MS, Miossec P, Kaveri SV et al (2012) Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am J Pathol 181:8–18. doi:10.1016/j.ajpath.2012.03.044
Park H, Li Z, Yang XO et al (2005) A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 6:1133–1141. doi:10.1038/ni1261
Nadeem A, Javaid K, Sami W et al (2013) Inverse relationship of serum IL-17 with type-II diabetes retinopathy. Clin Lab 59:1311–1317
Chen Y, Yang P, Li F et al (2011) The effects of Th17 cytokines on the inflammatory mediator production and barrier function of ARPE-19 cells. PLoS One 6:e18139. doi:10.1371/journal.pone.0018139
Ryba-Stanislawowska M, Skrzypkowska M, Mysliwiec M et al (2013) Loss of the balance between CD4(+)Foxp3(+) regulatory T cells and CD4(+)IL17A(+) Th17 cells in patients with type 1 diabetes. Hum Immunol 74:701–707. doi:10.1016/j.humimm.2013.01.024
Zuniga LA, Jain R, Haines C et al (2013) Th17 cell development: from the cradle to the grave. Immunol Rev 252:78–88. doi:10.1111/imr.12036
Basso AS, Cheroutre H, Mucida D (2009) More stories on Th17 cells. Cell Res 19:399–411. doi:10.1038/cr.2009.26
Bi Y, Yang R (2012) Direct and indirect regulatory mechanisms in TH17 cell differentiation and functions. Scand J Immunol 75:543–552. doi:10.1111/j.1365-3083.2012.02686.x
Yang XO, Pappu BP, Nurieva R et al (2008) T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity 28:29–39. doi:10.1016/j.immuni.2007.11.016
Lee Y, Awasthi A, Yosef N et al (2012) Induction and molecular signature of pathogenic TH17 cells. Nat Immunol 13:991–999. doi:10.1038/ni.2416
Niimi N, Kohyama K, Matsumoto Y (2013) Therapeutic gene silencing with siRNA for IL-23 but not for IL-17 suppresses the development of experimental autoimmune encephalomyelitis in rats. J Neuroimmunol 254:39–45. doi:10.1016/j.jneuroim.2012.08.015
Toussirot E (2012) The IL23/Th17 pathway as a therapeutic target in chronic inflammatory diseases. Inflamm Allergy Drug Targets 11:159–168
Honkanen J, Nieminen JK, Gao R et al (2010) IL-17 immunity in human type 1 diabetes. J Immunol 185:1959–1967. doi:10.4049/jimmunol.1000788
Iwakura Y, Nakae S, Saijo S et al (2008) The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev 226:57–79. doi:10.1111/j.1600-065X.2008.00699.x
Runkle EA, Antonetti DA (2011) The blood-retinal barrier: structure and functional significance. Methods Mol Biol 686:133–148. doi:10.1007/978-1-60761-938-3_5
Kaya A, Kar T, Aksoy Y et al (2013) Insulin analogues may accelerate progression of diabetic retinopathy after impairment of inner blood-retinal barrier. Med Hypotheses 81:1012–1014. doi:10.1016/j.mehy.2013.09.018
Chen HT, Wang H, Zhao M et al (2012) Regulation of antigen specific Th17 cells differentiation in experimental autoimmune uveitis. Zhonghua Yan Ke Za Zhi 48:234–240
Brandon JA, Jennings CD, Kaplan AM et al (2013) Anti-IL-23p19 therapy inhibits the adoptive transfer of syngeneic graft-versus-host disease. Cytokine 61:732–735. doi:10.1016/j.cyto.2013.01.005
Wu SY, Yu JS, Liu FT et al (2013) Galectin-3 negatively regulates dendritic cell production of IL-23/IL-17-axis cytokines in infection by Histoplasma capsulatum. J Immunol 190:3427–3437. doi:10.4049/jimmunol.1202122
Sato K (2013) The IL-23/IL-17 axis as a therapeutic target. Nihon Rinsho Men’eki Gakkai Kaishi Jpn J Clin Immunol 36:203–208
Kwok SK, Cho ML, Her YM et al (2012) TLR2 ligation induces the production of IL-23/IL-17 via IL-6, STAT3 and NF-kB pathway in patients with primary Sjogren’s syndrome. Arthritis Res Ther 14:R64. doi:10.1186/ar3780
Korn T, Bettelli E, Oukka M et al (2009) IL-17 and Th17 cells. Annu Rev Immunol 27:485–517. doi:10.1146/annurev.immunol.021908.132710
Kim HR, Kim HS, Park MK et al (2007) The clinical role of IL-23p19 in patients with rheumatoid arthritis. Scand J Rheumatol 36:259–264. doi:10.1080/03009740701286813
Ratsimandresy RA, Duvallet E, Assier E et al (2011) Active immunization against IL-23p19 improves experimental arthritis. Vaccine 29:9329–9336. doi:10.1016/j.vaccine.2011.09.134
Acknowledgments
All authors have completed and submitted the ICMJE form for disclosure of potential conflicts of interest, and none were reported. This study was conducted with the approval of the Ethics Committee of Chongqing Medical University. The work was supported by the Health Bureau Foundation of Chongqing Project (2011-1-029) and a National Natural Science Foundation Project Grant (81371843). X.D Zhang and H.Y Xu were involved in the design and conduct of the study; H.Y Xu, M Cai, and C.K Wang were involved in the collection, management, analysis, and interpretation of the data.
Conflict of Interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, H., Cai, M. & Zhang, X. Effect of the blockade of the IL-23-Th17-IL-17A pathway on streptozotocin-induced diabetic retinopathy in rats. Graefes Arch Clin Exp Ophthalmol 253, 1485–1492 (2015). https://doi.org/10.1007/s00417-014-2842-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-014-2842-9