Abstract
Background
Diabetic retinopathy is severe damage to the retina caused by complications of diabetes, and is the prevailing cause of blindness. Accumulating evidence from both animal models and humans suggests that the inflammatory process plays a key role in the development of diabetic retinopathy and is facilitated by innate immune response. The aim of this study was to examine whether the TLR4 signaling pathway was involved in the streptozotocin-induced diabetic rat retina.
Methods
Diabetes was induced by a single intraperitoneal injection of streptozotocin, and rat diabetic retinopathy was examined at 4 weeks of diabetes duration. Then the accumulated leukocytes were counted in vivo by acridine orange leukocyte fluorography, and the retinal vascular permeability was measured by the Evans blue assay. The expressions of TLR4 and its downstream signaling molecules were measured by RT-PCR or Western blot respectively. To evaluate the effect of blocking TLR4 on diabetic retinopathy, TAK-242, a selective TLR4 antagonist, was administered by intraperitoneal injection.
Results
Our results showed that the retina of diabetic rats demonstrated accumulated leukocytes and retinal vascular permeability. The mRNA and protein expressions of TLR4 were upregulated in streptozotocin-treated diabetic rat retina. Furthermore, the protein levels of TLR4 downstream signaling molecules were significantly increased in streptozotocin-treated animals. In addition, the protein levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and interferon (IFN)-β, three downstream proinflammatory cytokines of TLR4 signal transduction pathway, were also markedly increased in diabetic rats. Administration of TAK-242 attenuated leukocytes accumulated and retinal vascular permeability, and decreased TLR4 downstream signaling molecules and proinflammatory cytokines in streptozotocin-induced animals.
Conclusions
Together, these data have demonstrated that TLR4 has a critical role in streptozotocin-induced diabetic retinopathy at the level of inflammatory cytokine induction, in both the MyD88-dependent and MyD88-independent pathways. TLR4 may become a new potential pharmacological target for treating diabetic retinopathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic retinopathy (DR) is a highly specific vascular complication of both type 1 and type 2 diabetes, which is an mainly ocular manifestation of diabetes. The severity of retinopathy is closely related to the times for diabetes_ENREF_1. After about 20 years duration of diabetes, almost all type 1 diabetes patients and more than 60 % type 2 diabetes patients have some degree of retinopathy [1]. DR is mainly related with new cases of blindness among adults aged from 20 to 74 years [2]. In clinic, DR is characterized by early breakdown of the blood–retinal barrier (BRB), which gives rise to vision loss via macular edema or vitreoretinal neovascularization [3]. Recent researches showed many new intriguing pathways involved in pathogenesis of DR, e.g., protein kinase (PK)Cb/HuR/ vascular endothelial growth factor (VEGF) pathway [4]. However, the exact mechanism underlying DR still remains elusive. Therefore, the use of existing agents for treating DR often has limited efficacy and are associated with unwanted side-effects.
Accumulating evidence from both animal models and humans suggests that the inflammatory process plays a key role in the development of DR, and is facilitated by innate immune response [5–8]. Retinal vasculature in diabetes is accompanied by inflammatory cell adhesion [9], which causes vascular hyperpermeability [10] and pathologic retinal neovascularization [11]. Recently, it has been reported that the innate immune response is mediated by a number of receptors known as Toll-like receptors (TLRs), which are stimulated by different microbial stimuli [12]. On binding to the relevant ligands, these receptors could activate different signal transduction pathways that produce the induction of proinflammatory cytokines and up-regulation of costimulatory molecules [13]. TLRs also play a important role in connecting between the innate and adaptive immune responses [14]. TLR4, in particular, is gradually being considered to be the important modulator of neuronal survival in the brain during non-pathogen (sterile) injuries [15, 16]. Studies conducted in animal models of diabetes have showed that TLR4 pathways were implicated in mechanisms linked with both diabetes induction and prevention [17]. An up-regulation of TLR4 level has been accompanied by the increased inflammatory response in diabetic patients [18, 19]. Genetic deficiency of TLR4 significantly abrogates the pro-inflammatory state of diabetes [20]. Furthermore, TLR4 expression and localization in the retina has also been extensively studied and reported [21–23]. Therefore, it may be reasonable to postulate that TLR4 is involved in inflammation in diabetes and plays a putative role in the retinopathy of streptozotocin-induced diabetes.
In the present study, we employed streptozotocin-induced diabetic rats and normal control rats to identify key roles for TLR4 signaling in the retinopathy of streptozotocin-induced diabetes. To evaluate the effect of blocking TLR4 on diabetic retinopathy, TAK-242, a selective TLR4 antagonist, was also administered by intraperitoneal injection. The streptozotocin-induced DR animal model was confirmed by measuring the retinal leukocyte accumulation and retinal vascular permeability. Then, the expressions of TLR4 and its signal transduction factors were measured by RT-PCR or Western blot respectively. In addition, the protein levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and interferon (IFN)-β, three downstream proinflammatory cytokines of TLR4 signal transduction pathway, were also measured in diabetic rats.
Methods
Animals
Male Brown Norway rats (8–10 weeks of age, from Vital River Laboratories of Beijing) weighing approximately 200 to 250 g (n = 96) were each used in accordance with the ARVO Statement for the Use of Animals in Vision and Ophthalmic Research. Room temperature was maintained between 18 and 25 °C, average daily humidity between 50 and 70 %. All rats were given ad libitum access to water and food until they were used for the experiments.
Groups and treatment
Each rat was given a single intraperitoneal injection of streptozotocin (60 mg/kg in 0.1 mol/l of citrate buffer, pH 4.2–4.5) after an overnight fasting, finishing in 30 min [24]. Blood glucose levels were measured in tail veins 5 days after streptozotocin injection, and the diabetic condition was established with a glucose level of ≥250 mg/dl on 3 consecutive days. TAK-242 (InvivoGen, San Diego, CA, USA) was formulated with 1 % dimethyl sulfoxide and double-distilled water to a final concentration of 1 mg/ml, and it was intraperitoneally injected at a dose of 3 mg/kg once daily for 7 successive days beginning 3 weeks after streptozotocin injection. This dose was chosen on the basis of previous in-vivo studies [25, 26]. Animals injected with an equal volume of 1 % dimethyl sulphoxide were used as vehicle-diabetic group. Control rats received injections of citrate buffer alone. The physiological variables of each group were measured at 4 weeks after streptozotocin injection.
Evaluation of leukocyte accumulation in rat retina
At 4 weeks after the induction of diabetes, leukocyte accumulation in retinal microcirculation was evaluated with acridine orange (AO) digital fluorography, which has been described previously [24, 27]. Eight different rats were used in each group. This technique uses a scanning laser ophthalmoscope (Heidelberg Engineering), which makes high-resolution images of fundus stained by AO (Sigma Chemical). Before AO digital fluorography, rats were anesthetized with xylazine hydrochloride and ketamine hydrochloride (10 mg/kg) and their pupils were dilated with 0.5 % tropicamide and 2.5 % phenylephrine hydrochloride. Body temperature was maintained between 37 and 39 °C throughout the experiment. AO (0.1 % solution in saline) was injected continuously via the jugular vein at a rate of 1 ml/min. Thirty minutes after the injection, the fundus was detected with the scanning laser ophthalmoscope for evaluation of leukocyte accumulation in the retinal microcirculation. The video images in real time to 640 horizontal and 480 vertical pixels with an intensity resolution of 256 steps were captured individually with a frame-grabber for later processing. For evaluating retinal leukostasis, an observation area around the optic disc measuring 5 disc diameters in diameter was observed by drawing a polygon surrounded by the adjacent major retinal vessels. The area was measured in pixels, and the density of trapped leukocytes was calculated by dividing the number of trapped leukocytes, which were recognized as fluorescent dots, by the area of the observation region. The densities of leukocytes were calculated generally in eight peripapillary observation areas, and an average density was acquired by averaging the eight density values [10].
Measurement of blood-retinal barrier breakdown
Rats after 4-weeks-duration diabetes were anesthetized, and Evans blue dye (45 mg/kg; Sigma Chemical) was injected through the jugular vein as described previously [24, 28]. After 120 min, the chest cavity was opened and rats were perfused for 2 min at a physiological pressure through the left ventricle with 0.05 M, pH 3.5, citrate-buffered paraformaldehyde (1 % wt/vol; Sigma). The perfusion solution was warmed to 37 °C to prevent vasoconstriction. Both eyes were enucleated and bisected at the equator immediately after perfusion. Then retinas were carefully dissected and thoroughly dried in a Speed-Vac for 5 h. The dry weight was used to normalize the quantitation of Evans blue leakage. Evans blue was extracted by incubating each retina in 120 μl formamide (Sigma) for 18 h at 70 °C. The supernatant was filtered through Ultrafree-MC tubes (Millipore) at 3,000 rpm for 2 h, and filtrates (60 μl) were used for triplicate spectrophotometric measurements. Each measurement occurred over a 5-s interval, and all measurements were preceded by evaluation of known standards. The background-subtracted absorbance was acquired by detecting each sample at 620 and 740 nm. The dye level in the extracts was calculated from a standard curve of Evans blue in formamide. Blood-retinal barrier breakdown was calculated with the following equation, and results were expressed in μl plasma × g retina dry wt−1 × h−1.
Western blot analysis
Crude retinal extracts from healthy and 4-week diabetic rats (n = 8 for each experimental group) were homogenated and processed as previously described by Guo et al. [29]. Immunoblot analyses were performed using rabbit polyclonal antibodies for TLR4 and β-actin (1:2000, Santa Cruz Biotechnology), MyD88, interleukin-1 receptor-associated kinase (IRAK 1), tumor necrosis factor receptor-associated factor (TRAF 6), TRIF-related adaptor molecule (TRAM 1), nuclear factor kappa B (NF-κB) p-p65, NF-κB p65 (1:1000, Santa Cruz Biotechnology), and Toll/IL-1 receptor-domain-containing adaptor-inducing interferon-γ (TRIF) (1:1000, Cell Signal Technology). A secondary antibody conjugated with horseradish peroxidase (HRP, 1:5000, Bio-Rad) was used. Immunoblots were visualized on X-ray film by chemiluminescence reaction (Pierce), and image analysis was performed on optical densitycalibrated images by AlphaEase Stand Alone software (Alpha Innotech). Protein levels were expressed as the ratio of the values of the detected protein band to the β-actin band, except that NF-κB p-p65 level was relative to the p 65.
Real-time quantitative PCR
Retinas from healthy and 4-week diabetic rat retinas (n = 8 for each experimental group) were isolated, and the total RNA was obtained using TRIzol method. The concentration of RNA was measured by an absorbance at 260 nm. First-strand cDNA was done by using Superscript-II (Invitrogen, Burlington, ON, Canada) system and the resulting products were stored at −20 °C. Then, real-time quantitative PCR analyses for TLR4 and β-actin were performed in 96-well plates using the ABI PRISM 7700 Sequence Detection System instrument and software (PE Applied Biosystems). PCR was performed by using the SYBR Green PCR Master Mix (Applied Biosystems) according to the manufacturer’s protocol, with the following oligonucleotide primers: TLR4-forward 5′- TGCTCAGACATGGCAGTTTC -3′and reverse 5′- TCAAGGCTTTTCCATCCAAC-3′ (206 bp); β-actin-forward 5′- ACGTTGACATCCGTAAAGAC-3′ and reverse 5′- GGACTCATCGTACTCCTGCT -3′ (239 bp). The primers were designed to span an exon–exon junction, and there is also an intron between 5′ and 3′ primer at the genomic DNA. The basic protocol for real-time PCR was an initial incubation at 95 °C for 5 min, followed by 40 cycles of 94 °C for 30 s, 54 °C for 30 s, and 72 °C for 1 min. All samples were run in triplicate; the relative expression values were normalized with β-actin value. To quantify the results measured by real-time RT-PCR, plasmids containing cDNA were used as standard. The interest cDNA was amplified by RT-PCR using the same premiers as for RT-PCR. The PCR products were cloned into pGEM-T easy vector (Invitrogen) and confirmed by sequence information. The purified recombinant plasmid DNA was detected by UV spectrophotometer and then proportionally diluted (1:10) in double-distilled water as standard for numerical quantification. The standard curve was prepared for each gene, and β-actin was used as housekeeping gene in this study.
The TLR 4 mRNA copies relative to β-actin mRNA were calculated as TLR 4 mRNA expression (normalized value). The PCR products were run on 1.5 % agarose gels. Melting curve was also analyzed after PCR amplification. Table 1 shows the primer sequences.
Enzyme-linked immunosorbent assay (ELISA)
Retinas from healthy and 4-week diabetic rats (n = 8 for each experimental group) were isolated and homogenized followed by centrifugation. The concentrations of IL-1β, TNF-α, and IFN-β were measured by enzyme-linked immunosorbent assay (ELISA) kits (Invitrogen), and were normalized with a protein assay kit.
Statistical analysis
All the data are given as the mean ± SD for parametric data. The statistical analysis was performed using the SAS. Parametric data were analyzed with two-tailed t-tests, whereas non-parametric data were analyzed with Mann–Whitney U test. A value of P < 0.05 was considered statistically significant.
Results
Physiologic data
Table 2 indicates the physiological variables of each group. There was no significant difference among groups in peripheral leukocyte count (WBC). The blood glucose level was significantly higher than the control group in all three other groups, whereas the body weight was markedly decreased. No significant difference was found among those three groups in blood glucose levels and body weight.
Evaluation of leukocyte accumulation in rat retina
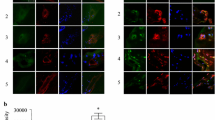
Figure 1 shows that the leukocyte accumulation in the retinal microcirculation of diabetic rats (6.2 ± 0.69 × 10−5 cells/pixel2) and diabetic + vehicle rats (6.4 ± 0.73 × 10−5 cells/pixel2) was significantly increased compared with the control group (2.2 ± 0.32 × 10−5 cells/pixel2, P < 0.01). After TAK-242 treatment, leukocytes reduced to 44.3 % compared with vehicle-treated diabetic group (P < 0.01).
Evaluation of leukocyte (white arrows) accumulation by AO fluorography. a Few leukocytes were found in the control retinas. b The number of accumulating leukocytes was significantly increased in the DM. c About the same number of leukocytes accumulated in the retina of the vehicle-treated DM rats as were seen in the untreated DM rats. d A significant reduction in the number of accumulated leukocytes was seen in the retina of the TAK 242-treated DM rats. e Evaluation of leukocyte lccumulation in rat retina. All the data are given as the mean ± SD (n = 8). **P < 0.01 indicates a significant difference compared with the control group, ## P < 0.01 indicates a significant difference compared with the vehicle-treated diabetic retinas group
Measurement of blood–retinal barrier breakdown
To determine the correlation between leukocyte accumulation and BRB breakdown, the Evans blue assay was performed to detect the retinal blood vessel leakage. As shown in Fig. 2, the data of retinal Evans blue leakage in diabetic retinopathy group (24.42 ± 1.84 ng/mg) and diabetic + vehicle group (26.37 ± 2.96 ng/mg) was markedly higher than that in control (13.23 ± 1.70 ng/mg, P < 0.01). Injection of TAK-242 decreased blood–retinal barrier breakdown in the diabetic retinas by 65.5 % compared with the vehicle-treated diabetic group (P < 0.01).
TLR4 gene and protein expression in the retinas
The length of amplification gene of TLR 4 and β-actin corresponds with design length (Fig. 3a) and RT-PCR-melting curve shows no miscellaneous peak and nonspecific amplification (Fig. 3b). As shown in Fig. 3c, compared with control group (1.181 ± 0.502), the TLR4 mRNA expression in the diabetic group (2.159 ± 0.872, P < 0.01) and vehicle-treated diabetic group (2.242 ± 0.779, P < 0.01) was significantly increased after streptozotocin treatment. TAK-242 treatment reduced TLR4 mRNA expression to 68.7 % compared with vehicle-treated diabetic group (P < 0.01). Meanwhile, the TLR4 protein level in the diabetic group (0.316 ± 0.071) and vehicle-treated diabetic group (0.335 ± 0.067) was also higher than that in the control group (0.138 ± 0.023, P < 0.01, Fig. 4). After TAK-242 treatment, TLR-4 protein level reduced to 63.6 % compared with vehicle-treated diabetic group (P < 0.01).
Real-time PCR analysis of TLR4 in the retinas. a Agarose gel electrophoresis to determine the length of amplification gene in RT-PCR. The length of PCR products is 239 bp (β-actin) and 206 bp (TLR4). b RT-PCR-melting curve. c Gene level of TLR4 in the retinas. Data are given as the mean ± SD (n = 8). **P < 0.01 indicates a significant difference compared with the control group, ## P < 0.01 indicates a significant difference compared with the vehicle-treated diabetic retinas group
Western blot analysis of TLR4 protein in the retinas. a Gel electrophoresis images of TLR4. b Protein levels of TLR4 in the retinas. Data are given as the mean ± SD (n = 8). **P < 0.01 indicates a significant difference compared with the control group, ## P < 0.01 indicates a significant difference compared with the vehicle-treated diabetic retinas group
Expression of TLR4 downstream signaling molecules
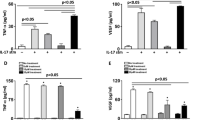
Compared with the control group (0.212 ± 0.047, 0.239 ± 0.044, 0.148 ± 0.025, 0.261 ± 0.084, 0.123 ± 0.037, and 0.230 ± 0.087 respectively), the protein expressions of TLR4 downstream signaling molecules MyD88, IRAK, TRAF, TRAM, TRIF, and NF-κB were significantly upregulated in the diabetic retinas (0.483 ± 0.110, 0.520 ± 0.094, 0.319 ± 0.047, 0.504 ± 0.125, 0.376 ± 0.088, and 0.497 ± 0.112, all P < 0.01,) and vehicle-treated diabetic group (0.494 ± 0.10, 0.523 ± 0.0949, 0.325 ± 0.054, 0.483 ± 0.085, 0.341 ± 0.12, and 0.469 ± 0.0881, all P < 0.01, Fig. 5). TAK-242 treatment significantly reduced these proteins levels compared with the vehicle-treated diabetic rats (0.303 ± 0.066, 0.291 ± 0.068, 0.206 ± 0.044, 0.290 ± 0.033, 0.181 ± 0.060, and 0.320 ± 0.062, P < 0.01).
Expression of the downstream signaling molecules in TLR4 signaling pathway. a Gel electrophoresis images of downstream signaling molecules in TLR4 signaling pathway. b Protein levels of TLR4 downstream signaling molecules in the retinas. All the data are given as the mean ± SD (n = 8). **P < 0.01 indicates a significant difference compared with the control group, ## P < 0.01 indicates a significant difference compared with the vehicle-treated diabetic retinas group
Pro-inflammatory cytokines in retina homogenate supernatant
The protein levels of TNF-α, IL-1β and IFN-β in the diabetic group (72.4 ± 9.8 pg/mg, 54.0 ± 8.9 pg/mg and 54.7 ± 9.9 pg/mg respectively) and vehicle-treated diabetic group (74.6 ± 12.9 pg/mg, 52.1 ± 10.4 pg/mg and 53.1 ± 13.3 pg/mg respectively) were significantly higher than those in the control group (40.8 ± 7.1 pg/mg, 24.2 ± 3.8 pg/mg and 11.5 ± 3.1 pg/mg, all P < 0.01 Fig. 6). Meanwhile, it was significantly suppressed in the TAK-242-treated group (57.2 ± 11.9 pg/mg, 30.8 ± 7.9 pg/mg and 20.8 ± 6.7 pg/mg, all P < 0.01).
Discussion
In the current study, we demonstrate that the expression of TLR4 is increased in a rat model of streptozotocin-induced diabetic retinopathy. Both TLR4 protein and mRNA expressions were significantly increased after experimental diabetic retinopathy. Furthermore, the protein levels of TLR4 downstream signaling molecules were significantly increased in streptozotocin-treated animals. In addition, the retina release of TNF-α, IL-1β, and IFN-β, three downstream proinflammatory cytokines of TLR4 signal transduction pathway, are also significantly increased in streptozotocin-induced diabetic rats. Administration of TAK-242 attenuated leukocytes accumulated and retinal vascular permeability, and decreased TLR4 downstream signaling molecules and proinflammatory cytokines in streptozotocin-induced animals. These results suggest that TAK-242, a specific inhibitor of TLR4 signaling, might be effective for the alleviation of streptozotocin-induced diabetic retinopathy animal model.
Autoimmune diabetes (Type I diabetes) results from immune-mediated beta cell destruction and is accompanied by islet-directed antibodies, while T-cell-mediated and cytokine-mediated cytotoxicity is thought to lead to beta cell destruction [30]. There is still not enough available data about the autoimmune process leading to diabetes, its complications, and the nature of the inflammatory response [31]. Streptozotocin is a small molecule that causes depletion of the pancreatic islet cells after systemic administration in rodents. In the present study, we have shown that the blood sugar concentration in streptozotocin-injected rats were over 250 mg/dl. Furthermore, the daily food and water consumptions were increased (data not shown), while the body weights were decreased significantly. DR caused by systemic changes in metabolic and cardiovascular control is a major complication of diabetes, and is also linked to leukostasis, capillary occlusion, and BRB breakdown. Adherent leukocytes lead to bleeding, elevated vascular permeability, and the formation of nonperfused areas [9, 32, 33]. Leukocytes accumulating in the retina could injure the tissue directly through inflammatory responses or via the formation of hard exudate that disrupt retinal transparency [10]. The increase in vascular permeability leads to clinical retinal edema and, when it involves the macula, can cause loss of visual acuity [31]. These are early clinical features of diabetic retinopathy, which is the important stage for preventing the progression of DR [33]. Here, both retinal leukocyte accumulation and vascular permeability were significantly increased in retina of streptozotocin-induced diabetic rats. Taken together, the above results indicated the DR animal model was successfully established.
TLRs are transmembrane pattern-recognition receptors that play a key role in the initiation of innate immunity against invading microbial pathogens. These single membrane-spanning proteins bear a leucine-rich extracellular domain, through which they recognize pathogen-associated molecular patterns (PAMPs), leading to the transcriptional activation of genes encoding for pro-inflammatory cytokines, chemokines, and co-stimulatory molecules. Each TLR is now believed to recognize a discrete collection of molecules of microbial origin, and to signal the presence of infections. TLR4 in particular is gradually being considered to be as a modulator of neuronal survival in the brain during non-pathogen (sterile) injuries [16]. TLR4 is up-regulated in many neurodegenerative diseases and neuronal injuries, and has been found to steadily increase after neurons are exposed to toxic proteins and lipid peroxidation [16]. To date, however, no study has been performed to investigate the role of TLR4 in diabetic retinopathy. In the present study, we evaluated the mRNA and protein expressions of TLR4 in diabetic retinopathy. Our results showed that both mRNA and protein levels of TLR4 was significantly elevated after streptozotocin injection, and increased about 100 % at week 4 in the retina.
The signaling transduction pathways activated by TLRs are broadly divided into MyD88-dependent and MyD88-independent pathways [34]. MyD88 is an immediate and common downstream adaptor molecule recruited by activated TLRs through their Toll-IL-1 receptor domain. MyD88, in turn, recruits IRAK and induces phosphorylation. IRAK-1 associates with TRAF 6, leading to the activation of IKK complex, and resulting in the activation of NF-κB transcription factor [35]. The activation of MyD88-dependent signal transduction pathway causes the production of proinflammatory cytokines, including TNF-α and IL-1β [36]. Following TLR activation, a MyD88-independent pathway can be activated when TRIF is recruited in concert with TRAM. This culminates in MAPK signaling, activation of the transcription factors NF-κB and IRF3, and production of IFN-β production [37]. The significance of these two different downstream pathways and the role of distinct adapter molecules of TLR4 activation in diabetic retinopathy were also measured in the present study. Our findings showed that the protein expressions of MyD88, IRAK, TRAF, TRAM, TRIF, and NF-κB in DR group were all significantly increased. The release of inflammatory cytokines (TNF-α and IL-1β) was associated with a significant increase in NF-κB activity. The MyD88-independent pathway product IFN-β was also increased in the retina of diabetic rats. These results showed that TLR4 activated two types of downstream signaling pathways, both the MyD88-dependent and MyD88-independent pathways.
Two TLR4 antagonists, TAK-242 and eritoran tetrasodium (E5564), have been widely studied. E5564 has a large molecular weight and poor liposolubility, which make it difficult to cross the blood–brain barrier [38]. On the contrary, TAK-242 has a small molecular weight and high liposolubility [39]. In the present study, TAK-242 was administered by intraperitoneal injection. We demonstrated that TAK-242 significantly reduces MyD88, IRAK, TRAF, TRAM, and TRIF expression in diabetic retinopathy and inhibits NF-κB activation, thereby decreasing the release of inflammatory factors TNF-α, IL-1β, and FN-β, and reduces leukocytes accumulated and retinal vascular permeability, which ultimately improved diabetic retinopathy in streptozotocin rats. Therefore, TAK-242 protects diabetic retinopathy through the mechanism that TAK-242 downregulates the expression of TLR4 downstream signaling molecules and reduces the NF-κB activation, thereby alleviating the progression of diabetic retinopathy.
Taken together, our data demonstrate that TLR4 has a critical role in streptozotocin-induced DR at the level of inflammatory cytokine induction, in both the MyD88-dependent and MyD88-independent pathways. TLR4 inhibition exhibits a strong protective effect on diabetic retinopathy. These observations suggest that TLR4-induced pathways may play an important role in the pathogenesis of DR, and TLR4 may become a novel target in the treatment of diabetic retinopathy.
References
Karadayi K, Top C, Gulecek O (2003) The relationship between soluble L-selectin and the development of diabetic retinopathy. Ocul Immunol Inflamm 11:123–129
Lu B, Wen J, Song XY, Dong XH, Yang YH, Zhang ZY, Zhao NQ, Ye HY, Mou B, Chen FL, Liu Y, Shen Y, Wang XC, Zhou LN, Li YM, Zhu XX, Hu RM (2007) High prevalence of albuminuria in population-based patients diagnosed with type 2 diabetes in the Shanghai downtown. Diabetes Res Clin Pract 75:184–192
El-Remessy AB, Al-Shabrawey M, Khalifa Y, Tsai NT, Caldwell RB, Liou GI (2006) Neuroprotective and blood–retinal barrier-preserving effects of cannabidiol in experimental diabetes. Am J Pathol 168:235–244
Amadio M, Bucolo C, Leggio GM, Drago F, Govoni S, Pascale A (2010) The PKCbeta/HuR/VEGF pathway in diabetic retinopathy. Biochem Pharmacol 80:1230–1237
Devaraj S, Glaser N, Griffen S, Wang-Polagruto J, Miguelino E, Jialal I (2006) Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes 55:774–779
Satofuka S, Ichihara A, Nagai N, Noda K, Ozawa Y, Fukamizu A, Tsubota K, Itoh H, Oike Y, Ishida S (2009) (Pro)renin receptor-mediated signal transduction and tissue renin–angiotensin system contribute to diabetes-induced retinal inflammation. Diabetes 58:1625–1633
Tang J, Kern TS (2011) Inflammation in diabetic retinopathy. Prog Retin Eye Res 30:343–358
Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof B, Kern TS, Adamis AP (2004) A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J 18:1450–1452
Miyamoto K, Hiroshiba N, Tsujikawa A, Ogura Y (1998) In vivo demonstration of increased leukocyte entrapment in retinal microcirculation of diabetic rats. Invest Ophthalmol Vis Sci 39:2190–2194
Miyamoto K, Khosrof S, Bursell SE, Rohan R, Murata T, Clermont AC, Aiello LP, Ogura Y, Adamis AP (1999) Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci U S A 96:10836–10841
Ishida S, Usui T, Yamashiro K, Kaji Y, Amano S, Ogura Y, Hida T, Oguchi Y, Ambati J, Miller JW, Gragoudas ES, Ng YS, D’Amore PA, Shima DT, Adamis AP (2003) VEGF164-mediated inflammation is required for pathological, but not physiological, ischemia-induced retinal neovascularization. J Exp Med 198:483–489
Wong FS, Hu C, Zhang L, Du W, Alexopoulou L, Flavell RA, Wen L (2008) The role of Toll-like receptors 3 and 9 in the development of autoimmune diabetes in NOD mice. Ann N Y Acad Sci 1150:146–148
Akira S, Takeda K, Kaisho T (2001) Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2:675–680
Lee MS, Kim YJ (2007) Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu Rev Biochem 76:447–480
Mollen KP, Anand RJ, Tsung A, Prince JM, Levy RM, Billiar TR (2006) Emerging paradigm: toll-like receptor 4-sentinel for the detection of tissue damage. Shock 26:430–437
Yi H, Patel AK, Sodhi CP, Hackam DJ, Hackam AS (2012) Novel role for the innate immune receptor Toll-like receptor 4 (TLR4) in the regulation of the Wnt signaling pathway and photoreceptor apoptosis. PLoS One 7:e36560
Dvoriantchikova G, Barakat DJ, Hernandez E, Shestopalov VI, Ivanov D (2010) Toll-like receptor 4 contributes to retinal ischemia/reperfusion injury. Mol Vis 16:1907–1912
Devaraj S, Dasu MR, Rockwood J, Winter W, Griffen SC, Jialal I (2008) Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a proinflammatory state. J Clin Endocrinol Metab 93:578–583
Devaraj S, Dasu MR, Park SH, Jialal I (2009) Increased levels of ligands of Toll-like receptors 2 and 4 in type 1 diabetes. Diabetologia 52:1665–1668
Devaraj S, Tobias P, Jialal I (2011) Knockout of toll-like receptor-4 attenuates the pro-inflammatory state of diabetes. Cytokine 55:441–445
Jiang G, Ke Y, Sun D, Wang Y, Kaplan HJ, Shao H (2009) Regulatory role of TLR ligands on the activation of autoreactive T cells by retinal astrocytes. Invest Ophthalmol Vis Sci 50:4769–4776
Luo C, Yang X, Kain AD, Powell DW, Kuehn MH, Tezel G (2010) Glaucomatous tissue stress and the regulation of immune response through glial Toll-like receptor signaling. Invest Ophthalmol Vis Sci 51:5697–5707
Gnana-Prakasam JP, Martin PM, Mysona BA, Roon P, Smith SB, Ganapathy V (2008) Hepcidin expression in mouse retina and its regulation via lipopolysaccharide/Toll-like receptor-4 pathway independent of Hfe. Biochem J 411:79–88
Wang K, Wang Y, Gao L, Li X, Li M, Guo J (2008) Dexamethasone inhibits leukocyte accumulation and vascular permeability in retina of streptozotocin-induced diabetic rats via reducing vascular endothelial growth factor and intercellular adhesion molecule-1 expression. Biol Pharm Bull 31:1541–1546
Wang YC, Wang PF, Fang H, Chen J, Xiong XY, Yang QW (2013) Toll-like receptor 4 antagonist attenuates intracerebral hemorrhage-induced brain injury. Stroke 44:2545–2552
Seki H, Tasaka S, Fukunaga K, Shiraishi Y, Moriyama K, Miyamoto K, Nakano Y, Matsunaga N, Takashima K, Matsumoto T, Ii M, Ishizaka A, Takeda J (2010) Effect of Toll-like receptor 4 inhibitor on LPS-induced lung injury. Inflamm Res 59:837–845
Bucolo C, Ward KW, Mazzon E, Cuzzocrea S, Drago F (2009) Protective effects of a coumarin derivative in diabetic rats. Invest Ophthalmol Vis Sci 50:3846–3852
Bucolo C, Leggio GM, Drago F, Salomone S (2012) Eriodictyol prevents early retinal and plasma abnormalities in streptozotocin-induced diabetic rats. Biochem Pharmacol 84:88–92
Guo JY, Li CY, Ruan YP, Sun M, Qi XL, Zhao BS, Luo F (2009) Chronic treatment with celecoxib reverses chronic unpredictable stress-induced depressive-like behavior via reducing cyclooxygenase-2 expression in rat brain. Eur J Pharmacol 612:54–60
Atkinson MA, Eisenbarth GS (2001) Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet 358:221–229
Yan JE, Yuan W, Lou X, Zhu T (2012) Streptozotocin-induced diabetic hyperalgesia in rats is associated with upregulation of Toll-like receptor 4 expression. Neurosci Lett 526:54–58
Miyamoto K, Ogura Y, Kenmochi S, Honda Y (1997) Role of leukocytes in diabetic microcirculatory disturbances. Microvasc Res 54:43–48
Miyahara S, Kiryu J, Yamashiro K, Miyamoto K, Hirose F, Tamura H, Katsuta H, Nishijima K, Tsujikawa A, Honda Y (2004) Simvastatin inhibits leukocyte accumulation and vascular permeability in the retinas of rats with streptozotocin-induced diabetes. Am J Pathol 164:1697–1706
Okun E, Griffioen KJ, Lathia JD, Tang SC, Mattson MP, Arumugam TV (2009) Toll-like receptors in neurodegeneration. Brain Res Rev 59:278–292
Dasu MR, Devaraj S, Zhao L, Hwang DH, Jialal I (2008) High glucose induces toll-like receptor expression in human monocytes: mechanism of activation. Diabetes 57:3090–3098
Takeuchi O, Kaufmann A, Grote K, Kawai T, Hoshino K, Morr M, Muhlradt PF, Akira S (2000) Cutting edge: preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a toll-like receptor 2- and MyD88-dependent signaling pathway. J Immunol 164:554–557
Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S (2003) Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301:640–643
Tidswell M, Tillis W, Larosa SP, Lynn M, Wittek AE, Kao R, Wheeler J, Gogate J, Opal SM, Eritoran Sepsis Study G (2010) Phase 2 trial of eritoran tetrasodium (E5564), a toll-like receptor 4 antagonist, in patients with severe sepsis. Crit Care Med 38:72–83
Ii M, Matsunaga N, Hazeki K, Nakamura K, Takashima K, Seya T, Hazeki O, Kitazaki T, Iizawa Y (2006) A novel cyclohexene derivative, ethyl (6R)-6-[N-(2-Chloro-4-fluorophenyl)sulfamoyl]cyclohex-1-ene-1-carboxylate (TAK-242), selectively inhibits toll-like receptor 4-mediated cytokine production through suppression of intracellular signaling. Mol Pharmacol 69:1288–1295
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (81141008, 31170992 and 31371038) and Natural Science Foundation of Beijing (No.7122046).
Authors’ contributions
Wang YL and Wang K carried out all of the studies in the Methods section. Lin PY and LI MM performed the analysis of collected data. Yu SJ, Li Q and Li N drafted the manuscript. Guo JY conceived the study, participated in its design and coordination, and helped in drafting of the final manuscript. All authors read and approved the final manuscript.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yan-Ling Wang and Kang Wang contributed equally to this work
Rights and permissions
About this article
Cite this article
Wang, YL., Wang, K., Yu, SJ. et al. Association of the TLR4 signaling pathway in the retina of streptozotocin-induced diabetic rats. Graefes Arch Clin Exp Ophthalmol 253, 389–398 (2015). https://doi.org/10.1007/s00417-014-2832-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-014-2832-y