Abstract
Objective
To determine the safety and efficacy of topical 0.03 % tacrolimus ointment treatment for subepithelial corneal infiltrates (SEIs).
Methods
This prospective non-controlled interventional case series included patients with SEIs who had been previously treated with topical corticosteroids with either no improvement or the medication being withdrawn due to associated intraocular pressure (IOP) elevation. The patients were treated with 0.03 % tacrolimus ointment twice daily for 22 weeks (including a 1-month washout). The objective data were best-corrected Snellen visual acuity (BCVA), IOP, and full ocular examination results, including SEI severity and the Schirmer test. The subjective data were the patients’ responses to a questionnaire at all follow-up visits.
Results
The patients consisted of five males (45 %) and six females (55 %) (mean age 50 ± 11 years) who were followed up for an average of 22 weeks. The mean BCVA (logarithm of the minimum angle of resolution [logMAR]) before and after treatment was 0.34 ± 0.09 and 0.08 ± 0.04 respectively (p = 0.042). All the patients evidenced significant objective clinical improvement, and none had a severe degree of SEI at the end of the treatment. The patients reported considerable reduction in the severity of their symptoms (foreign body sensation, glare, etc.). Three patients were excluded due to side-effects (one had severe dizziness and discomfort), and their data were excluded from the study.
Conclusion
Topical tacrolimus 0.03 % is a safe and effective alternative treatment in patients with SEIs who do not respond to other treatment modalities or have untoward side-effects from topical steroids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epidemic keratoconjunctivitis (EKC) is most commonly caused by adenoviral serotypes 8, 19, and 37, and represents the most common of the external ocular viral infections [1, 2]. Keratitis that appears approximately 10 days after the onset of the follicular conjunctivitis may present with the formation of subepithelial corneal infiltrates (SEIs), which are usually bilateral and often asymmetrical. The SEIs have the potential to cause serious ocular morbidity in the form of reduced vision, photophobia, glare, halos, and foreign body sensation, and these problems can persist for months or even years after the initial infection [3, 4]. Histopathologic investigation of SEIs reveals lymphocytes, histiocytes, and fibroblasts that are accompanied by a disruption of the collagen fibers of Bowman’s layer [4], The hypothesis of a persisting viral replication in subepithelial keratocytes, which triggers an immunologic host reaction, is supported by the clinical observation that opacities usually resolve with topical steroid treatment but recur when steroids are discontinued [3].
The role of topical anti-inflammatory agents to control the SEI patient’s symptoms remains an important clinical goal in patient management. However, the use of topical steroids in this setting is controversial because of the complications of cataract formation, glaucoma, and superinfection associated with their long-term use [5–7]. In addition, the use of topical steroids may lead to steroid dependence in some patients [5–7].
Tacrolimus, also known as FK506, is a macrolide derived from the soil fungus Streptomyces tsukubaensis. Its mechanism of action is similar to that of cyclosporine, but it is described as being between 10 to 100 times more potent, despite differing chemical structure [8]. Both tacrolimus and cyclosporine inhibit B- and T-cell activity by decreasing the transcription of interleukin-2 and lymphokines. Systemic tacrolimus has been used successfully to prevent allograft rejection in liver, kidney, lung, and heart transplantation [8]. In ophthalmology, the systemic use of tacrolimus is already well-established in the treatment of immune-mediated diseases, uveitis, dry eyes related to graft-versus-host disease, corneal transplants, and ocular pemphigoid [9].
Topical tacrolimus has been successfully used "off label" as an ointment for treating ocular allergies, especially atopic blepharokeratoconjunctivitis, for high-risk penetrating keratoplasty, and for dry-eye syndrome [10]. The aim of this pilot study was to investigate both the subjective and objective efficacy of topical 0.03 % tacrolimus in patients with SEIs who had been treated with topical corticosteroids for a long period with no improvement or the medications being discontinued due to associated intraocular pressure (IOP) elevation.
Methods
Patient selection
This prospective non-controlled interventional case series study adhered to the tenets of the Declaration of Helsinki, and was approved by the Institutional Review Board/Ethics Committee of the Tel-Aviv Medical Center under protocol number 0359–09 (June 2012). Informed consent was obtained from all study participants after the nature of the study had been explained to them in detail. Our original study cohort had included 14 patients (14 eyes) who were clinically diagnosed as having unilateral SEI due to adenokeratoconjunctivitis and were treated with tacrolimus 0.03 % ointment (Protopic®; Fujisawa Healthcare Inc., Teva, Deerfield, IL, USA) twice daily for SEI secondary to adenoviral keratoconjunctivitis. Patients whom we suspected of having other possible reasons for SEIs (e.g., allergic conjunctivitis, herpetic conjunctivitis, bacterial conjunctivitis) were excluded from this study. All the patients had previously been treated with topical steroids for at least 13 months (dexamethasone sodium phosphate 0.1 %, Dr. Fischer, Brussels, Belgium, TID protocol). That treatment was discontinued either because of insufficient improvement in symptoms or because of IOP elevation in response to steroids. None of the patients had been treated with any other anti-inflammatory drugs before they started the treatment protocol that included cyclosporine drops. None of the study participants was willing to use medications to control IOP.
Methods
The treatment protocol included a 1-month period of washout after 6 weeks of tacrolimus 0.03 % ointment treatment, evaluation of the SEI status, and continuation of treatment for another 12 weeks. The overall course of treatment was 22 weeks. We used a recognized ointment (Protopic®) that is 100 times more potent than cyclosporine. Follow-up evaluations were carried out at 3, 6, 10, and 22 weeks after the initiation of treatment. The data recorded for each patient were as follows: best-corrected visual acuity (BCVA), IOP, functional acuity contrast sensitivity, and complete fundus examination. The objective parameters were evaluated by a clinical score for conjunctival injection (0 = none, 1 = mild, 2 = severe), conjunctival chemosis (0 = none, 1 = mild, 2 = severe), punctate epithelial keratitis (0 = none, 1 = mild, 2 = severe), corneal subepithelial infiltrates (0 = none, 1 = few ≤10, 2 = many > 10 ), and Schirmer’s test with topical anesthesia (0 = >15 mm, 1 = 5–15 mm, 2 = < 5 mm). All the study patients were examined by the same physician. For subjective evaluation of the treatment, the patients were asked to complete a non-validated questionnaire (consisting of seven items) before the initiation of the treatment and on every follow-up visit, grading their symptoms and overall satisfaction with treatment on a scale of 1 to 10 (Table 1).
Statistical methods
The patients' decimal BCVAs were converted to a logMAR scale for analysis. The data were analyzed using SPSS (Version 22 for Mac, IBM Inc.) employing the Wilcoxon signed-rank test and one-way analysis of variance as appropriate.
Results
Demographics and objective findings
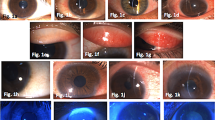
Table 1 lists the patients’ age, gender, and affected eye contrast sensitivity. Table 2 provides their visual acuity and ocular examination findings before and after treatment. There was a significantly improved decrease in LogMAR BCVA, from a mean value of 0.34 ± 0.09 to 0.08 ± 0.04, ∼2 Snellen lines, z = 2.03, p = 0.042). All other objective physical findings, including conjunctival hyperemia, conjunctival chemosis, punctate keratitis, and Schirmer test results showed a considerable reduction in SEIs. Figure 1 depicts slit-lamp photographs of one patient at the initiation and at the termination 22 weeks later of tacrolimus 0.03 % ointment treatment.
Figure 2 shows contrast sensitivity during treatment. The patients demonstrated a significant (p < 0.05) improvement in contrast sensitivity of the high spatial frequencies (greater than 18 cycles per degree) only when the final follow-up examination was compared to the baseline values. There were no other significant improvements during the follow-up period.
Subjective findings
Figure 3 displays the data retrieved from the pre- and post-treatment questionnaires: the scores yielded a trend towards improvement in all the variables that were queried, including severity of symptoms, foreign body sensation, glare sensation, improvement in vision, and overall satisfaction. Interestingly, the patients’ scores deteriorated at the follow-up visit after washout and improved after treatment was resumed, although there were no comparable changes in the objective physical examination findings.
Side-effects
Tacrolimus ointment and drops can cause several side-effects, such as warmness in the eye, eye irritation, pain, conjunctival hyperemia, and foreign body sensation [10]. During the first 2 weeks into the study, five out of the 11 participants reported some eye irritation that eventually resolved spontaneously. There were no side-effects that required withdrawal from the study. However, three of the 14 patients who had been diagnosed with SEI secondary to adenoviral keratoconjunctivitis and had originally been recruited to the study were subsequently dropped from the study due to various side-effects. One of them was admitted to the neurology ward for severe dizziness: a complete work-up failed to find any neurological etiology. The symptoms were relieved after discontinuation of the ointment application, and the patient was asked to re-enter the study. Symptoms of dizziness recurred 48 hours after the second initiation of treatment, whereupon the tacrolimus treatment was stopped and the patient’s data were excluded from analysis. The two other patients declined to continue treatment due to warmness in the eye, eye irritation, and a sticky sensation in the eye.
Discussion
Subepithelial infiltrates caused by adenoviral infection are a common chronic ocular condition that typically presents with severe symptomatology and is considered to be a complication of adenoviral keratoconjunctivits [11]. Currently, in the absence of an effective antiviral for adenovirus in the acute phase, therapy is often supportive and includes conservative measures, such as antihistamine and non-steroidal anti-inflammatory agents (NSAID) [2, 12]. Until recently, community ophthalmologists routinely prescribed topical corticosteroid eye drops for symptomatic relief to their patients who had acute infection [13]. It is now known that topical steroids in the acute phase increases the replication rate and disease duration; therefore, its use is usually restricted to patients whose condition is complicated by SEI or pseudomembranes [14]. In an effort to avoid the appearance of SEIs, 0.5 % topical cyclosporine A in artificial tears has been used in rabbit studies and succeeded in reducing their incidence [15]. A subsequent randomized clinical trial on humans demonstrated that the use of cyclosporine A in combination with cidofovir (an antiviral drug) did not significantly diminish the incidence of SEI compared with cidofovir alone [1].
Tacrolimus (previously known as FK-506) is an immunosuppressive drug that is mainly used after allogeneic organ transplant [16]. Its topical ointment preparation, Protopic®, was approved for the treatment of moderate-to-severe atopic dermatitis by the US Food and Drug Administration in December, 2000. In addition to its action against T-cell proliferation, in-vitro tacrolimus demonstrated a direct inhibitory effect on mast-cell degranulation. It also seems to inhibit the production of the proinflammatory mediator, interleukin 8 (IL-8), and the IL-8 receptor, as well as to decrease the binding of IL-8 to its receptor on keratinocytes [17]. The results of in-vitro studies also suggested that tacrolimus enhances the action of the tumor suppressor gene, p53 [17].
Off-label use of tacrolimus has been described in the literature for the treatment of various conditions, such as pyoderma gangrenosum [16] and resistant chronic external otitis [18], and several studies have been published on its use for ocular indications. Kymionis et al. recently reported two cases of refractory phlyctenular keratoconjunctivitis treated with 0.03 % tacrolimus ointment: both patients showed improvement in symptoms and signs within 1 week of treatment initiation, and a complete resolution after 3 weeks of treatment [19]. Tacrolimus has also been used in penetrating keratoplasty among high-risk patients who showed signs of acute rejection: the median treatment time was 22.6 months (range 13–32), and there were no further episodes of graft rejection during the course of treatment [16]. Attas-Fox et al. reported an open-label study on 20 patients with intractable allergic conjunctivitis who were treated with tacrolimus 0.03 % for 8 weeks [20] Those authors observed significant improvement in all parameters that were checked (“conjunctivitis score”).
One of our patients experienced severe dizziness that was attributed to the use of tacrolimus 0.03 % ointment. This is listed as a rare side-effect in the manufacturer’s drug insert. Systemic absorption of the agent is reportedly extremely low, and the treatment is considered as being safe [21]. Ebihara et al. reported low blood drug levels in the use of tacrolimus ophthalmic suspension in allergic conjunctivitis patients [22, 23]. In 2003, an FDA advisory committee recommended that the manufacturer, Fujisawa, revise the product insert to inform patients of cancer risks from this product. In 2005, an FDA black box warning of cancer risks was required for topical tacrolimus ointment (http://www.fda.gov). The relevance of this black box warning in ocular use is not known. Indeed, tacrolimus has been used successfully in Japan for the past few years to treat uveitis without any reported adverse effects, and it has recently been accepted worldwide for treating uveitis [9].
Our adenoviral keratitis patients experienced significant improvement in their objective eye examinations with the use of tacrolimus ointment, and overall patient satisfaction and subjective evaluation of vision improvement with tacrolimus were high. There was a significant improvement in our cohort’s mean logMAR BCVA (∼2 Snellen lines, p = 0.042) at the end of the 22-week course of treatment. None of our patients reported a foreign body sensation, glare or other ocular side-effects associated with topical tacrolimus treatment. We expected to see both subjective and objective deterioration during the scheduled washout period: the patients’ scores did deteriorate at the follow-up visit, but there was no significant reduction in BCVA or in any of the other objective ocular findings.
Our study has several limitations. One is the small size of our study population, which was due to the fact that tacrolimus ointment is an off-label drug for ocular use and to the difficulty in finding patients willing to participate in our study. Another limitation is our use of a non-validated questionnaire to assess patient satisfaction with the treatment.
In conclusion, we found that topical tacrolimus 0.03 % was safe and effective in treating a small number of patients with SEIs. Further prospective blinded randomized studies with larger patient populations are needed to evaluate the effects of topical tacrolimus in SEIs caused by adenoviral keratoconjunctivitis and other ocular conditions.
References
Hillenkamp J, Reinhard T, Ross RS, Böhringer D, Cartsburg O, Roggendorf M, De Clercq E, Godehardt E, Sundmacher R (2002) The effects of cidofovir 1% with and without cyclosporin A 1% as a topical treatment of acute adenoviral keratoconjunctivitis: a controlled clinical pilot study. Ophthalmology 109:845–850
Gordon YJ, Araullo-Cruz T, Romanowski EG (1998) The effects of topical nonsteroidal anti-inflammatory drugs on adenoviral replication. Arch Ophthalmol 116:900–905
Hillenkamp J, Reinhard T, Ross RS, Böhringer D, Cartsburg O, Roggendorf M, De Clercq E, Godehardt E, Sundmacher R (2001) Topical treatment of acute adenoviral keratoconjunctivitis with 0.2% cidofovir and 1% cyclosporine: a controlled clinical pilot study. Arch Ophthalmol 119:1487–1491
Lund OE, Stefani FH (1978) Corneal histology after epidemic keratoconjunctivitis. Arch Ophthalmol 96:2085–2088
Sahin A, Bozkurt B, Irkec M (2008) Topical cyclosporine a in the treatment of superior limbic keratoconjunctivitis: a long-term follow-up. Cornea 27:193–195
Ozcan AA, Ersoz TR, Dulger E (2007) Management of severe allergic conjunctivitis with topical cyclosporin a 0.05% eyedrops. Cornea 26:1035–1038
Doan S, Gabison E, Abitbol O, Gatinel D, Chast F, Hoang-Xuan T (2007) Efficacy of topical 2% cyclosporine A as a steroid-sparing agent in steroid-dependent vernal keratoconjunctivitis. J Fr Ophtalmol 30:697–701
Kino T, Hatanaka H, Hashimoto M, Nishiyama M, Goto T, Okuhara M, Kohsaka M, Aoki H, Imanaka H (1987) FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. J Antibiot 40:1249–1255
Gallego-Pinazo R, Dolz-Marco R, Martínez-Castillo S, Arévalo JF, Díaz-Llopis M (2013) Update on the principles and novel local and systemic therapies for the treatment of non-infectious uveitis. Inflamm Allergy Drug Targets 12:38–45
Dhaliwal JS, Mason BF, Kaufman SC (2008) Long-term use of topical tacrolimus (FK506) in high-risk penetrating keratoplasty. Cornea 27:488–493
Meyer-Rüsenberg B, Loderstädt U, Richard G, Kaulfers P-M, Gesser C (2011) Epidemic keratoconjunctivitis: the current situation and recommendations for prevention and treatment. Dtsch Arztebl Int 108:475–480
Shiuey Y, Ambati BK, Adamis AP (2000) A randomized, double-masked trial of topical ketorolac versus artificial tears for treatment of viral conjunctivitis. Ophthalmology 107:1512–1517
Kowalski RP, Foulks GN, Gordon YJ (2000) Comparison of treatment regimens for ocular infections: community vs academic practice. Ann Ophthalmol 32:295–300
Romanowski EG, Yates KA, Gordon YJ (2002) Topical corticosteroids of limited potency promote adenovirus replication in the Ad5/NZW rabbit ocular model. Cornea 21:289–291
Romanowski EG, Pless P, Yates KA, Gordon YJ (2005) Topical cyclosporine A inhibits subepithelial immune infiltrates but also promotes viral shedding in experimental adenovirus models. Cornea 24:86–91
Lucchina S, Parvex SL, Biegger P, Fusetti C (2009) FK-506 ointment: an effective adjuvant therapy to treat a dramatic case of pyoderma gangrenosum of unilateral hand. Chin J Traumatol 12:181–183
Lauerma AI, Granlund H, Reitamo S (1997) Use of the newer immunosuppressive agents in dermatology. BioDrugs 8:96–106
Caffier PP, Harth W, Mayelzadeh B, Haupt H, Sedlmaier B (2007) Tacrolimus: a new option in therapy-resistant chronic external otitis. Laryngoscope 117:1046–1052
Kymionis GD, Kankariya VP, Kontadakis GA (2012) Tacrolimus ointment 0.03% for treatment of refractory childhood phlyctenular keratoconjunctivitis. Cornea 31:950–952
Attas-Fox L, Barkana Y, Iskhakov V, Rayvich S, Gerber Y, Morad Y, Avni I, Zadok D (2008) Topical tacrolimus 0.03% ointment for intractable allergic conjunctivitis: an open-label pilot study. Curr Eye Res 33:545–549
MedLine Plus (2013) Tacrolimus TM Drug Insert. National Institutes of Health (nlm.nih.gov)
Ebihara N, Ohashi Y, Fujishima H, Fukushima A, Nakagawa Y, Namba K, Okamoto S, Shoji J, Takamura E, Uchio E, Miyazaki D (2012) Blood level of tacrolimus in patients with severe allergic conjunctivitis treated by 0.1% tacrolimus ophthalmic suspension. Allergol Int 61:275–282
Ohashi Y, Ebihara N, Fujishima H, Fukushima A, Kumagai N, Nakagawa Y, Namba K, Okamoto S, Shoji J, Takamura E, Hayashi K (2010) A randomized, placebo-controlled clinical trial of tacrolimus ophthalmic suspension 0.1% in severe allergic conjunctivitis. J Ocul Pharmacol Ther 26:165–174
Conflict of interest
The authors did not receive any financial support from any public or private sources. The authors have no financial or proprietary interest in a product, method, or material described herein.
Author information
Authors and Affiliations
Corresponding author
Additional information
Drs. Levinger and Trivizki contributed equally to this work
Rights and permissions
About this article
Cite this article
Levinger, E., Trivizki, O., Shachar, Y. et al. Topical 0.03 % tacrolimus for subepithelial infiltrates secondary to adenoviral keratoconjunctivitis. Graefes Arch Clin Exp Ophthalmol 252, 811–816 (2014). https://doi.org/10.1007/s00417-014-2611-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-014-2611-9