Abstract
Background
To report one year results of the first cohort of routine refractive lenticule extraction through a small incision (ReLEx SMILE) for correction of myopia and myopic astigmatism.
Methods
Fifty-four eyes of 27 patients with spherical equivalent of −4.68 ± 1.29D who underwent routine ReLEx SMILE by a single surgeon were prospectively followed-up for 1 year. We used the VisuMax® femtosecond laser system (Carl Zeiss Meditec AG, Germany) with a 500 kHz repetition rate. Folow-up intervals were at 1 day, 1 week, 1, 3, 6, and 12 months after surgery. We obtained following parameters: uncorrected (UDVA) and distance-corrected visual acuity (CDVA), contrast sensitivity, and wave front measurements. We also recorded all complications.
Results
Because of suction loss in one eye, 12-month results were obtained in 53 eyes as follows. After 1 year, 88 % of eyes with plano target had an UDVA of 20/20 or better. Twelve percent of eyes lost 1 line of CDVA, while 31 % gained 1 line and 3 % gained 2 lines. The mean SE after 1 year was −0.19 ± 0.19. The mean refraction change between month 1 and 12 was 0.08 D. Neither mesopic nor photopic contrast sensitivity showed any significant changes. The high-order aberrations (HOA) increased from 0.17 to 0.27 μm (Malacara notation). No visually threatening complications were observed.
Conclusion
In this first cohort, ReLEx® SMILE produced satisfactory refractive outcomes with moderate induction of HOA and unaffected contrast sensitivity after 1 year.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In autumn of 2006, during the American Academy of Ophthalmology (AAO) Meeting in Las Vegas/USA, Sekundo and Blum presented the very first cases of corneal refractive correction using a prototype of the VisuMax® femtosecond laser (Carl Zeiss Meditec AG, Jena/Germany). This new procedure, which did not require an excimer laser, was named femtosecond lenticule extraction (FLEx). The 6-month results of the first ten eyes appeared in the first peer-reviewed publication [7] in 2008, and were followed by other reports [2]. A further refinement of the technique through a small incision, called small-incision lenticule extraction (SMILE) was elaborated by Sekundo and Blum between 2008 and 2009, and published by their group in 2011 almost simultaneously with the publication by Shah et al. [8, 9]. In order to avoid confusion, the femtosecond laser procedure was renamed by the manufacturer as refractive lenticule extraction (ReLEx) with two possible techniques: the ReLEx® FLEx and ReLEx® SMILE. Since 2008, the VisuMax® Laser has become commercially available, initially as a 200 kHz laser followed by a 500 kHz upgrade. Particularly the introduction of the flapless small-incision technique (SMILE) raised interest in this new procedure. This led to an increasing number of peer-reviewed articles addressing all aspects of SMILE surgery, such as stability [5], corneal sensitivity [10], and others. However, there are only a few 6-month studies addressing the key issues of laser refractive surgery such as visual acuity, predictability, or safety, and virtually no studies with 1-year follow-up. Moreover, 1-year contrast sensitivity as a key parameter of the subjective quality of vision was published for ReLEx® FLEx surgery only during the preparation of the current manuscript [3].
Participants and methods
The herein-reported investigation followed up 27 patients, who were the first commercially treated ReLEx SMILE patients at Prof. Solomatin's Eye Clinic between January and July of 2012. This prospective interventional non-controlled study was performed over the first 54 eyes, which were consecutively operated by the same experienced surgeon (J.G). This study followed the tenets of the Declaration of Helsinki, and was approved by the institutional review board of Prof. Solomatin's Eye Clinic. An informed consent was obtained from each patient. The eligibility criteria did not differ from those patients treated either by the wavefront optimized Femto-LASIK or ReLEx® FLEx at the study site: spherical myopia up to – 10 dioptres (D) and myopic astigmatism up to −5 D cyl. Other criteria were a minimum age of 21 years, CDVA ≥ 0/25 ,and no other ocular conditions except myopia and astigmatism. The central corneal thickness had to be more than 500 μm and the calculated residual stromal bed after treatment >250 μm. A regular topographic pattern was verified by Atlas™ topography. The follow-up appointments were 1 day, 1 week, 1 month, 3, 6, and 12 months postoperatively. All patients had a bilateral simultaneous procedure. Patients’ average age at the time of surgery was 29 years (range 20–38 years), and all were white Caucasians. Preoperatively and starting from month 1 we measured the uncorrected (UDVA) and best-corrected (CDVA) Snellen visual acuity at 6 m distance with full manifest refraction. We also measured contrast sensitivity using Vector Vision CSV 1000E test (Vector Vision/USA) at spatial frequency of 3,6,12, and 18 cycles per degree (cpd) and carried out total wavefront measurements (Malacara notation) at 5 mm pupil diameter using a Hartmann–Shack WASCA Aberrometer (Carl Zeiss Meditec AG/Germany). All measured data was collected on standardized electronic patients’ charts and entered into Datagraph 3.5b software (Datagraph, Pieger GmbH, Germany) for analysis. All intra- and postoperative complications were meticulously collected and documented in our own database.
Surgical details
The ReLEx® SMILE procedure was carried out exactly as previously described and modified by Shah et al. [9]. The cap thickness was set at 120 μm. The entering incision varied between 3 and 4 mm depending on the depth of the orbit and accessibility of the cornea, as it was always placed at 90° position. The average lenticule size was 6.4 ± 0.2 mm (range 6.0 – 7.0 mm) adjusted to the mesopic pupil with no transition zone for spherical errors and an 0.1 mm transition zone for concurrent astigmatism correction. The minimal lenticule thickness at the edge was pre-set at 15 μm. The cap diameter exceeded the lenticule diameter by 0.5 to 1.0 mm. Only a S-size treatment pack (“contact glass”) was used. The expected residual stromal bed was >00 μm in all cases. No adjustments to the manufacturer’s nomograms were done.
Results
The mean preoperative spherical equivalent was −4.68 D ± 1.29 D (range −D to −9.0D). The mean preoperative sphere was −4.37 D ± 1.34 D (range −2.0D to −8.25 D) and the mean astigmatism −0.41D cyl ± 0.51D cyl (range 0.0 D cyl to −3.0 D cyl).
A suction loss occurred in one eye. This treatment was intra-operatively converted to ReLEx® FLEx, and was therefore excluded from the analysis. This FLEx-treated eye had a 20/16 UDVA at the last follow-up. Thus, in total 53 SMILE-treated eyes were followed up for the entire year. There were no dropouts.
Safety
As shown in Fig. 1, not a single eye lost 2 lines of CDVA. Eleven percent of eyes lost 1 line of CDVA, while 38 % gained 1 line and 4 % gained 2 lines. The safety index was 1.01 at one month and 1.08 at the last follow-up.
Predictability & efficacy
At 1 month, 3 months, 6 months, and 1 year, 86 %, 88 %, 94 % and 88 % respectively of eyes with plano target (51 eyes) had an UDVA of 20/20 or better. At the last follow-up, 98 % of eyes were at least 20/25 uncorrected, and 57 % of eyes could see 20/16 or better (Table 1). This translates into an efficacy index of 0.99 at 1 year. For all eyes treated, 92 % were within 0.5D of intended correction and all eyes were within 1.0D of target refraction (Fig. 2). While at the 1-month follow-up an absolute remaining astigmatism ≤0.5D cyl was measured in 98 % of eyes, this number decreased to 92 % at the very last visit.
Stability
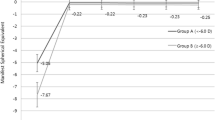
The preoperative mean SE of −4.68 D was reduced to −0.11 D after 1 month. It gradually regressed by 0.08 D to −0.19 ± 0.19 after 1 year. Four percent of eyes regressed >0.5 D (Fig. 3). These were the right eye of a 22-year-old female with a preoperative SE of −3.25 D and the left eye of a 29-year-old female with a preoperative SE of −4.25 D. The final SE was −1.0 D in both cases. As a matter of fact, the fellow eyes of these two patients did not experience any refractive change over 0.5D.
Contrast sensitivity
Figure 4 shows individual graphs for photopic (a) and scotopic (b) contrast sensitivity at four spatial frequencies. The individual numbers are displayed in the upper (photopic) and lower (scotopic) parts of Table 2. There is no significant difference at any measurement time point between the preoperative and the postoperative values for either photopic or scotopic ambient illumination.
Higher-order aberrations
Figure 5 displays a graph showing an increase of HOA from 0.17 ± 0.08 μm to 0.26 μm and finally to 0.27 ± 0.1 μm at the last follow-up. The range is between 0.09 and 0.57 μm. In detail, the spherical aberration increased from −0.01 ± 0.23 μm (range −0.78 to 0.92) to −0.12 ± 0.2 μm (−0.49 to 0.39). For coma, the values were 0.28 ± 0.19 μm (range 0.04 to 0.93) preoperatively and 0.55 ± 0.3 μm {range 0.04 to 1.42) after 12 months.
Side-effects and complications
No visually threatening complications were observed. As mentioned above, there was one case of suction loss that was converted to ReLEx® FLEx. In two cases, the surgeon noted difficulties in lenticule separation, specifically at the bottom plane. In one case, a remnant of the peripheral lenticule (case no. 3) was detected at the slit lamp on the first day follow-up (Fig. 6). We also noted small erosions at the edge of the entering incision, particularly at the beginning of the learning curve. One of these eyes also developed a localized non-progressive epithelial ingrowth at the incision edge. Persistent haze-like interface opacity without visual sequelae was recorded in two eyes of the same patient.
Discussion
ReLEx® SMILE has basically replaced ReLEx® FLEx, which was a transitional step in the evolution of this procedure. The SMILE approach appears to have some distinct advantages over Femto-LASIK and FLEx, mainly because there is no flap creation. Since ReLEx® SMILE was developed by Sekundo and Blum [8], only 6-month results remain available in the peer-reviewed literature as on PubMed search of December 2013. The initial group of patients in this first 6-month approval study, however, was partially followed-up by Blum and Sekundo up to 12 months (Mele J (2013) 12-Monats-Ergebnisse der Small-Incision Lenticule Extraction (SmILE). Dissertation. Friedrich-Schiller University of Jena). It might be worthwhile to recall that 85 % of these very first eyes available at 1-year follow-up were within ± 0.5 D of intended correction. Fortunately ,we did not looe a single eye during the study period. This unusually good patients’ adherence is most likely related to the fact that the surgical site of the current investigation is the only eye clinic offering ReLEx® surgery in the Baltic States. Not unexpectedly, our results with 92 % of eyes within ± 0.5 D of target refraction are better than in the above-mentioned investigation by Mele, because we used a well-proven VisuMax™ commercial femtosecond laser, while the initial study was performed with a prototype machine and a 200 kHz laser head. Again, our results match the experience of Shah et al., with 91 % within ± 0.5 D in a 2011 6-month ReLEx SMILE paper where the current technology and the current laser scanning pattern was used [9].
In terms of stability, current results cannot fully confirm Mele’s previously mentioned retrospective follow-up study, in which virtually no regression was observed after 1 year. Despite the fact that 0.11D of regression after 12 months is an excellent result, we should keep in mind that this is a mean value, and that we had 4 % of eyes with refractive change more than 0.5D. In our experience, an under-correction of 0.75D or more becomes a symptomatic issue in fully accommodating patients. Fortunately, in both cases the fellow eye of the patient had a perfect result. Thus, the patients did not request enhancement, as they were happy with the achieved results. This observation does not necessarily go along with a 3-month paper by Hjørdtal et al. reporting a 0.1D of under-correction per decade of life in his cohort of highly myopic eyes [4].
In our opinion, the contrast sensitivity (CS) is a crucial parameter for patients’ satisfaction, and reflects the subjective quality of vision from the patient’s perspective better than a purely objective measurement of HOA. In this study, we used a proven technology of the Vector Vision CSV 1000E test (Vector Vision/USA) that served well in numerous FDA studies. Indeed, our setting did not differ from our previous comparative study in FLEx and Femto-LASIK treated eyes [3]. In that 2013 published paper, we observed a non-significant improvement of CS in FLEx-treated eyes at the highest level of 18 cpd from 1.03 to 1.06, while at lower levels almost identical numbers were obtained [3]. Since the lenticule resection algorithms are identical for both FLEx and SMILE, it came as no surprise to us that the current study on SMILE-treated eyes delivered a similar result, with no significant changes under either photopic or mesopic conditions. One can conclude that regardless of the type of ReLEx surgery (FLEx or SMILE), this procedure, when performed for moderate myopia, does not worsen subjective vision at normal and low illumination conditions.
In that regard, as well as in terms of predictability, our first experience with SMILE surgery produced similar results to those of a 6-month multicentre prospective study on low myopia using the latest generation excimer laser Amaris™ (Schwind/Germany) released at the time when we conducted the current study [1]. However, the percentage of eyes with UDVA of 1.0 or better was 98 % in the study by Arbelaez et al. [1], but 94 % in our study at the 6-month follow-up point. Longer follow-ups (1 year or more) can hardly be found for last-generation excimer lasers. The reasons for this small difference between our report and that of Arbelaez could be a much lower preoperative mean SE, lower preoperative cylinder, and younger age in their patients as compared to our study population. Also, for the new adaptors of this evolving procedure we should stress that ReLEx surgery, in particular the SMILE technique through a 2–3-mm incision, has a definitive learning curve and is more surgeon-dependent than Femto-LASIK. We had a higher loss of 1 line of visual acuity in this initial study, as compared to our decade-long experience in LASIK and Femto-LASIK surgery. Indeed, reviewing our results for submission, we realised that problems arose less and less as the principal surgeon of this study gained experience.
In summary, the present 1-year prospective study of fully commercial non-selected myopic population shows that an experienced (Femto-)-LASIK and FLEx surgeon can safely master the learning curve of the SMILE technique, producing refractive and visual results very close or similar to the last-generation excimer laser based corneal refractive surgery while offering the stable, flap-free, low-healing response advantages [5, 6] of intrastromal refractive surgery to his or her patients.
References
Arbelaez MC, Aslanides IM, Barraquer C, Carones F, Feuermannova A, Neuhann T, Rozsival P (2010) LASIK for myopia and astigmatism using the SCHWIND AMARIS excimer laser: an international multicenter trial. J Refract Surg 26:88–98
Blum M, Kunert K, Schröder M, Sekundo W (2010) Femtosecond lenticule extraction for the correction of myopia: preliminary 6-month results. Graefes Arch Clin Exp Ophthalmol 248:1019–1027
Gertnere J, Solomatin I, Sekundo W (2013) Refractive lenticule extraction (ReLEx flex) and wavefront-optimized Femto-LASIK: comparison of contrast sensitivity and high-order aberrations at 1 year. Graefes Arch Clin Exp Ophthalmol 251:1437–1442
Hjortdal JØ, Vestergaard AH, Ivarsen A, Ragunathan S, Asp S (2012) Predictors for the outcome of small-incision lenticule extraction for Myopia. J Refract Surg 28:865–871
Reinstein DZ, Archer TJ, Randleman JB (2013) Mathematical model to compare the relative tensile strength of the cornea after PRK, LASIK, and small incision lenticule extraction. J Refract Surg 29:454–460
Riau AK, Angunawela RI, Chaurasia SS, Lee WS, Tan DT, Mehta JS (2011) Early corneal wound healing and inflammatory responses following refractive lenticule extraction (ReLEx). Invest Ophthalmol Vis Sci 52:6213–6221
Sekundo W, Kunert K, Russmann C, Gille A, Bissmann W, Stobrawa G, Sticker M, Bischoff M, Blum M (2008) First efficacy and safety study of femtosecond lenticule extraction for the correction of myopia. J Cataract Refract Surg 34:1513
Sekundo W, Kunert KS, Blum M (2011) Small incision corneal refractive surgery using the small incision lenticule extraction (SMILE) procedure for the correction of myopia and myopic astigmatism: results of a 6 month prospective study. Br J Ophthalmol 95:335–339
Shah R, Shah S, Sengupta S (2011) Results of small incision lenticule extraction: All-in-one femtosecond laser refractive surgery. J Cataract Refract Surg 37:127–137
Wang Y, Wei S (2013) Comparison of corneal sensitivity between fs-lasik and femtosecond lenticule extraction (ReLEx flex) or small-incision lenticule extraction (ReLEx smile) for myopic eyes. Graefes Arch Clin Exp Ophthalmol 251:1645–1654
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented in parts at the annual ESCRS Meeting, Amsterdam, The Netherlands, September 2013.
Gertnere, Sekundo, and Solomatin are members of the Scientific Advisory Board of Carl Zeiss Meditec AG. They do not have any financial interests in any devices or methods mentioned in this publication.
Rights and permissions
About this article
Cite this article
Sekundo, W., Gertnere, J., Bertelmann, T. et al. One-year refractive results, contrast sensitivity, high-order aberrations and complications after myopic small-incision lenticule extraction (ReLEx SMILE). Graefes Arch Clin Exp Ophthalmol 252, 837–843 (2014). https://doi.org/10.1007/s00417-014-2608-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-014-2608-4