Abstract
Purpose
To report the 6-month results of a new method of refractive correction, femtosecond lenticule extraction (FLEx), and the preliminary outcomes in the first 108 eyes.
Methods
In this prospective study, a flap and a lenticule of intrastromal corneal tissue were cut simultaneously using a femtosecond laser. Thereafter, the lenticule was removed manually and the flap repositioned. One hundred and seven of 108 myopic eyes of 56 patients in the treatment group completed the final 6 months of follow-up. The patients’ mean age was 35 years. The preoperative mean spherical equivalent (SE) was −4.59 ± 1.3 diopters (D). The uncorrected visual acuity and the best spectacle-corrected visual acuity after 6 months, objective and manifest refractions, results of slit-lamp examination, the side effects, and the responses to a questionnaire are reported.
Results
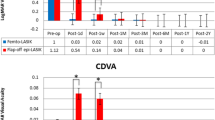
Six months postoperatively, the mean SE was −0.19 ± 0.47 D; 98.1% of treated eyes were within ±1.0 D, and 74.8% of eyes within ±0.5 D of the intended correction. Eight (7.4%) of 108 eyes lost one line of Snellen VA, one (0.9%) eye lost two Snellen lines, 46 eyes (43%) gained one line, ten eyes (9.3%) gained two Snellen lines, and the VA remained unchanged in 42 (39.3%) eyes. The patient responses to a standardized questionnaire indicated that 97.1% of patients were satisfied with the obtained results and would undergo the procedure again.
Conclusion
FLEx appears to be a safe and promising corneal refractive procedure for correcting myopia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Barraquer and Ruiz [1] were pioneers of corneal resectional refractive procedures for the correction of myopia. The removal of a layer of intrastromal tissue using an automated microkeratome, i.e., in situ keratomileusis, was not entirely satisfactory [2, 3]. Burrato and Pallikaris later developed a different approach that became the standard of care. While the first non-refractive cut was performed using a mechanical microkeratome, the second, so-called refractive cut was performed using excimer laser technology [4]. Today, several sophisticated excimer laser systems are available to perform the excimer laser-based laser in situ keratomileusis (LASIK) procedure with high accuracy.
Femtosecond laser technology recently has assumed an important role in refractive surgery. However, while these advanced laser systems replaced the mechanical microkeratome, the actual refractive procedure was performed using the 193 nm-ArF-excimer laser [5]. In the early 1990s, the idea of a full femtosecond laser system-based refractive correction already had been conceived. The clinical results in five blind or amblyopic eyes [6] and two highly myopic eyes [7] using laser extraction of a refractive lenticule were reported previously. These first studies lacked a large number of eyes and a detailed analysis of the refractive data. The studies have not been continued with a representative study cohort.
After research in the laboratory and with animals and blind patients (unpublished data) using a prototype of the VisuMax® femtosecond laser system (Carl Zeiss Meditec, Jena, Germany), this new refractive procedure became a reality. The procedure, which no longer requires an excimer laser, is referred to as femtosecond lenticule extraction (FLEx) to distinguish it from other known refractive procedures, especially femto-LASIK, which uses the excimer laser for the actual refraction. The first ten FLEx cases were reported by Sekundo et al. [8] in 2008. In the current manuscript, we report our 6-month results in the first 108 healthy eyes treated for myopia.
Participants and methods
This prospective study was approved by the Ethics Committee of the Chamber of Physicians of Thuringia, Germany, and by the Institutional Review Boards of Philipps University, Marburg, Germany. Each patient provided informed consent after the details of the study were explained fully. A total of 108 eyes were recruited and treated among 56 patients with spherical myopia between –2.0 and –8.5 diopters (D) and myopic astigmatism up to -6 D. Other inclusion criteria were a minimum age of 21 years, no other ocular conditions except myopia, a central corneal thickness measured using the AC-Master® (CZM, Jena, Germany) exceeding 500 µm, and a calculated residual stromal bed after treatment exceeding 290 µm. The preoperative simulated K readings ranged from 41.94 to 47.66 D, with a regular topographic pattern as verified by Atlas™ topography. The absence of asymmetric steepening was confirmed by a pachymetry map provided by Visante™-OCT (CZM). All eyes were followed for 6 months. To minimize overcorrection, the refractive target was set to –0.75 D for the first 50 patients and later to plano. According to the study protocol, the fellow eyes of the first ten patients were treated 1 month later at the earliest, based on measurements of the refractive outcome of the first treated eye.
The average patient age at the time of surgery was 35 years (range, 21–62 years). There were 55 (51%) left and 53 (49%) right eyes. The mean preoperative spherical equivalent (SE) was −4.59 D ± 1.3 D (range, −8.5 to −2.5) with a mean preoperative sphere of −4.26 D ± 1.35 D (range, 0 to −8.50) and a mean myopic astigmatism of −0.66 D ± 0.85 D (range, 0 to −6.0).
The FLEx procedure
FLEx was performed under topical anesthesia using three drops of preservative-free oxybuprocaine tetrachloride (Conjucain EDO™, Bausch & Lomb, Berlin, Germany) applied 2 to 3 minutes before surgery. After standard sterile draping and insertion of the aspirating speculum, the patient’s eye was positioned under the VisuMax® integrated surgical microscope. The eye then was positioned for laser treatment under an illuminated and curved suction contact glass (so-called treatment pack). While the patient fixated on an internal target light in the microscope for centration, the cornea was applanated by moving the table upward toward the contact glass. The surgeon observed this motion through the operating microscope and controlled the movement with a joystick. When appropriate centration was achieved, the surgeon initiated the automatic suction. The patient continued to fixate on the blinking target even as the suction was applied. The VisuMax femtosecond laser produced ultrashort pulses of light at a repetition rate of 200 kHz with a typical pulse energy of 300 nJ or less, which was focused at a precise depth in the corneal tissue. A plasma state developed with optical breakdown, and a small gas bubble formed from the vaporization of tissue. A series of bubbles was created in a spiral fashion with the typical spot distance of 3 to 5 µm resulting in cleavage of the tissue planes.
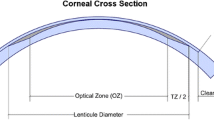
A schematic drawing of the procedure is shown in Fig. 1. Four subsequent femtosecond incisions were created, the first three on the posterior surface of the refractive lenticule, the lenticule border, and the anterior surface of the refractive lenticule; the last incision was extended centripetally as a flap, followed by a side cut for flap opening. After the suction was released, the patient was moved toward the observation position under the surgical microscope. A thin spatula was inserted under the flap near the hinge, and after dissection of the remaining tissue bridging, the flap was lifted. A thin layer of corneal tissue (lenticule) was grasped with a forceps and removed (Fig. 2). The flap was repositioned and the interface flushed as in an excimer-based LASIK procedure.
A schematic drawing of the femtosecond lenticule extraction procedure. The VisuMax femtosecond laser system is used to cut the back of the refractive lenticul, followed by the front surface incision. In the third step, a vertical incision is created, leaving an arc of 50° untouched (hinge). When the patient is returned to the observation position, the final fourth step is performed manually; the flap is lifted with a spatula, and the lenticule removed manually using forceps. The flap is then repositioned
All surgeries were performed by two experienced surgeons (WS and MB). The intended flap thickness varied between 110 and 160 µm; the flap diameter was between 7.0 and 8.5 mm. In all 108 cases, a superior hinge, 50° in chord length, was left. The lenticular diameter varied between 6.0 and 7.3 mm, according to the scotopic pupil diameter. A special equation (patent No. DE 102006053120 A1) was used to calculate the geometry and the thickness of the refractive lenticule. The minimum thickness of the lenticule edge was set at 15 µm. There is no transition zone.
The postoperative regimen included preservative-free ofloxacin (Floxal EDO, Bausch & Lomb), dexamethasone (Dexa EDO™, Bausch & Lomb), and hyaluronic acid lubricating drops (VisLube™, Chemedica, Switzerland), each instilled 4 times daily for 1 week. After this, only lubricating drops were used for up to 3 months as needed. Follow-up visits were scheduled for 1 day, 1 week, and 1, 3, and 6 months postoperatively.
The following parameters were obtained at each visit: the best spectacle-corrected (BSCVA) and uncorrected visual acuity (UCVA) using different ETDRS charts at each visit, the objective and manifest refractions, corneal topography (Atlas™, Carl Zeiss Meditec), pachymetry and pachymetry map (AC Master and Visante-OCT), flap thickness and flap diameter (Visante-OCT), and Goldmann applanation tonometry (not on day 1 and week 1). Because the FLEx procedure is new, the change in the BSCVA is the most important value. The side-effects were recorded.
Patients completed a standardized questionnaire consisting of 16 items such as glare, night driving problems, dryness, and pain (sample questionaire see Appendix).
All measured data were collected on standardized study spread sheets and entered into Datagraph 3.5b software (Datagraph, Pieger GmbH, Germany) for analysis. Further statistical analysis was performed using Excel 2003 (Microsoft Inc., Redmond, WA, USA) and WINSTAT for Excel 2005.1 (R. Fitch Software Inc., Bad Krozingen, Germany). The Wilcoxon signed rank test was used to compare the mean outcomes.
Results
Safety
Two patients lost two or more lines of Snellen BSCVA at month 3 (Fig. 3). In one case, prolonged steroid treatment improved the vision, and the patient had no loss of BSCVA after 6 months. In the second patient, prolonged steroid therapy was discontinued due to pregnancy, and the loss of two Snellen lines persisted. Therefore, only one eye (0.9%) still lost two lines at the 6-month follow-up; eight eyes (7.4%) lost one Snellen line. No other eyes required prolonged steroid treatment. The VA in 42 eyes (38.8%) was unchanged. However, 46 eyes (42.6%) gained one line, and ten eyes (9.3%) gained two Snellen lines at the 6-month follow-up visit.
Predictability
At the 6-month follow-up visit, 74.8% of eyes treated were within ±0.5 D and 98.1% within ±1.0 D of the intended refractive target (Fig. 4). Six months postoperatively, the mean SE was −0.19 D ± 0.47 D. An UCVA of 20/40 or better was obtained in 97% of eyes treated (Fig. 5). The eyes showed a tendency for overcorrection in low myopia compared to the tendency for undercorrection in higher myopia (Fig. 6). The last group of 39 eyes treated with improved parameters did not show any overcorrection or undercorrection (Fig. 7).
Stability
The mean refraction was plano during the first several days postoperatively. The refraction stabilized at -0.12 D at the 1-week follow-up visit. After the 1-month follow-up, when the mean refraction was -0.17 D, there was no regression toward the final 6-month visit, at which time the refraction was -0.16 D (Fig. 8).
Induced astigmatism
The average induced astigmatism in all 108 eyes was measured to be -0.40 D (± 0.38 D), with a range of max. -1.75 D and min 0.0 D. A double angle plot shows the individual distribution 6 months postoperatively (Fig. 9).
Side effects
Transient enhanced visibility of the interface was apparent during the slit-lamp examination, which was similar to grade 1 haze that develops following photorefractive keratectomy (PRK) in 20.4% of patients during the first postoperative week; the finding later resolved. This finding differed from the typical appearance of diffuse lamellar keratitis (DLK). Peripheral, optically insignificant microstriae were observed during slit-lamp examinations in 15.7% of all patients 1 week postoperatively.
DLK grade 0.5 developed in one patient (0.9%). Transient light sensitivity syndrome did not develop; subconjunctival hemorrhages developed in 2.8% of eyes, and some debris-like findings reminescent of meibomian gland secretion were observed in the interface in 8.5% of eyes. No corneal ectasia developed during the 6-month period. Two patients lost three lines of Snellen acuity at the 3-month follow-up visit; only one patient still had a loss of two Snellen lines after 6 months.
Up to 3 months after the FLEx procedure, 42.9% of patients continued to use lubricants. However, none required lubricants at the last 6-month examination (Fig. 10).
Six eyes that were not included in the 108 eyes of 56 patients reported in the current study required a second procedure due to suction loss during the FLEx procedure. In these eyes, the new procedure was not completed. According to the tenets of the ethics committee, the enhancement treatments were not performed using the new procedure a second time, and either laser assisted subepithelial keratectomy, LASIK, or PRK were performed. All six eyes were treated successfully. All of the suction loss occurred during the first 50 eyes treated with the laser prototype. Once the VisuMax serial production device was used during the study, suction loss did not reoccur.
Questionnaire
The patients were asked to complete a questionnaire for each eye treated. Of the 108 eyes, we obtained completed questionnaires for 104 eyes at 6 months. Twenty-nine patients (27.9%) reported a marked improvement of their vision, and 72 patients (69.2%) reported an extreme improvement of their vision. In general, 97.1% of patients responded to the question “would you have the surgery again?” by answering “yes,” 2.9% of the patients answered “no.” The same 102 cases (97.1%) no longer wear spectacles or contact lenses for distant vision. However, three patients (2.9%) still wear glasses. Overall, on a scale of 0 indicating very unsatisfied to 100 indicating very satisfied, the quality of vision was rated 90.4.
Discussion
Femtosecond laser keratomes are gaining in popularity in the refractive market for a variety of reasons as an alternative to mechanical microkeratomes. With regard to the quality of the surgical outcome and safety, femtosecond laser keratomes seem to have advantages over mechanical devices [9–11]. The use of two lasers during a refractive procedure increases the effort, the length, and the cost of the procedures. Complications, such as delayed-onset photophobia, increased suction time, corneal folds, and interface inflammation have been reported with mechanical microkeratomes [12, 13].
However, femtosecond laser systems can be important in other corneal surgeries, such as keratoplasty [14] and implantation of intracorneal ring segments [15]. The widely used flat applanation technology has some drawbacks, such as decentration [16] and the inability to perform a refractive cut in a highly deformed and compressed cornea.
To avoid these complications, the VisuMax femtosecond laser system with a curved treatment interface surface causes a lower intraocular pressure increase during suctioning, and has technical parameters that enable an extremely thin cavitation layer. Ideally, an advanced femtosecond laser system should perform a one-step, rapid refractive procedure accompanied by further reduction of possible complications.
In this prospective clinical study of 108 myopic eyes, the results of the FLEx surgery using VisuMax exceeded our expectations. Considering that no nomograms were available at the beginning, 98.1% of eyes within 1 D of the refractive target appeared to have a satisfactory result. The last generation of excimer lasers delivered refractive outcomes superior to the first results in the current study [17]. For example, using a combination of VisuMax (as a laser microkeratome) and the MEL 80™ excimer laser in another study, 95% of the eyes treated by our group were within 0.5 D of the intended target [18]. We know from previous suction experiments (unpublished data) that a low level of suctioning enables patients to see the blinking target, which is a positive experience for patients to actually see it working during surgery.
Analysis of the flaps using anterior segment OCT showed that they were of uniform thickness [18]. Similarly to the findings of Durrie and Kezirian and Kezirian and Stonecipher [9, 19], the femtosecond laser system used in the current study induced barely any astigmatism other than the normal measurement variation. Thus, the VisuMax also facilitates excellent flap cutting in wavefront-guided and topography-guided excimer laser ablations. Hypothetically, the gentle corneal interface concept with its low suction and a continuous fixation target may reduce potential risks to the posterior segment [20].
In summary, the first 108 eyes treated with FLEx, in which the femtosecond laser system performs the entire refractive procedure without the use of an excimer laser, had highly satisfactory results that exceeded our expectations in a prospective clinical study. Because FLEx seems to be a gentle and safe procedure, clinical studies are under way to further evaluate FLEx as a potential alternative to excimer laser-based LASIK.
References
Barraquer JI (1996) The history and evolution of keratomileusis. Int Ophthalmol Clin 36:1–7
Ibrahim O, Waring GO, Salah T, el Maghraby A (1995) Automated in situ keratomileusis for myopia. J Refract Surg 11:431–441
Wiegand W, Krusenberg B, Kroll P (1995) Keratomileusis in situ bei hochgradiger Myopie. Erste Ergebnisse. Ophthalmologe 92:402–409
Sekundo W (2007) Refraktive Chirurgie. In: Augustin AJ (ed) Augenheilkunde. Springer, Heidelberg, New York, Tokyo, pp 823–845
Nordan LT, Slade SG, Baker RN (2003) Femtosecond laser flap creation for laser in situ keratomileusis: six-months follow-up of the initial US clinical series. J Refract Surg 19:8–14
Ratkay-Traub I, Ferincz IE, Juhasz T, Kurtz RM, Krueger RR (2003) First clinical results with the femtosecond neodymium–glass laser in refractive surgery. J Refract Surg 19:94–103
Krueger RR, Juhasz T, Gualano A, Marchi V (1998) The picosecond laser for nonmechanical laser in situ keratomileusis. J Refract Surg 14:467–469
Sekundo W, Kunert K, Russmann Ch et al (2008) First efficacy and safety study of femtosecond lenticule extraction for the correction of myopia. J Cataract Refract Surg 34:1513–1520
Durrie DS, Kezirian GM (2005) Femtosecond laser versus mechanical keratome flaps in wavefront-guided laser in situ keratomileusis: prospective contralateral eye study. J Cataract Refract Surg 31:120–126
Tran DB, Sarayba MA, Bor Z et al (2005) Randomized prospective clinical study comparing induced aberrations with IntraLase and Hansatome flap creation in fellow eyes: potential impact on wavefront-guided laser in situ keratomileusis. J Cataract Refract Surg 31:97–105
Binder PS (2004) Flap dimensions created with the IntraLase FS laser. J Cataract Refract Surg 30:804–811
Biser SA, Bloom AH, Donnenfeld ED et al (2003) Flap folds after femtosecond LASIK. Eye Contact Lens 29:252–254
Muňoz G, Albarran-Diego C, Sakla FH et al (2006) Transient light-sensitivity syndrome after laser in situ keratomileusis with the femtosecond laser. J Cataract Refract Surg 32:2075–2079
Steinert RF, Ignacio TS, Sarayba MA (2007) “Top hat”-shaped penetrating keratoplasty using the femtosecond laser. Am J Ophthalmol 143:689–691
Ertan A, Kamburoğlu G (2007) Analysis of centration of Intacs segments implanted with a femtosecond laser. J Cataract Refract Surg 33:484–487
Dupps WJ, Oberts C (2001) Effect of acute biomechanical changes on corneal curvature after photokeratectomy. J Refract Surg 17:658–669
Goes F (2005) LASIK for myopia with the Zeiss Meditec MEL 80. J Refract Surg 21:691–697
Blum M, Kunert K, Gille A, Sekundo W (2009) LASIK for myopia using the Zeiss VISUMAX® femtosecond laser and MEL 80 excimer laser. J Refract Surg 25:350–356
Kezirian GM, Stonecipher KG (2004) Comparison of the IntraLase femtosecond laser and mechanical microkeratome for laser in situ Keratomileusis. J Cataract Refract Surg 30:26–32
Reviglio VE, Kuo IC, Gramajo L et al (2007) Acute rhegmatogenous retinal detachment immediately following laser in situ keratomileusis. J Cataract Refract Surg 33:536–359
This study was supported by Carl Zeiss Meditec, Germany.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have no financial interest in any technology mentioned.
Appendix
Appendix
Percentage [%]/share/total number of eyes | ||||
Problem | Pre-op | Post-op | ||
1 month | 3 months | 6 months | ||
1. Light sensitivity | ||||
None | 75.0%/81/108 | 50.5%/54/107 | 58.5%/62/106 | 67.5%/69/105 |
Mild | 17.6%/19/108 | 39.3%/42/107 | 33.0%/35/106 | 26.7%/28/105 |
Moderate | 5.6%/6/108 | 10.3%/11/107 | 6.6%/7/106 | 3.8%/4/105 |
Significant | 1.9%/2/108 | 0.0%/0/107 | 1.9%/2/106 | 3.8%/4/105 |
Severe | 0.0%/0/108 | 0.0%/0/107 | 0.0%/0/106 | 0.0%/0/105 |
2. Headache | ||||
None | 85.2%/92/108 | 94.4%/101/107 | 91.5%/97/106 | 88.6%/93/105 |
Mild | 11.1%/12/108 | 1.9%/2/107 | 4.7%/5/106 | 8.6%/9/105 |
Moderate | 1.9%/2/108 | 3.7%/4/107 | 3.8%/4/106 | 2.9%/3/105 |
Significant | 1.9%/2/108 | 0.0%/0/107 | 0.0%/0/106 | 0.0%/0/105 |
Severe | 0.0%/0/108 | 0.0%/0/107 | 0.0%/0/106 | 0.0%/0/105 |
3. Pains/burning | ||||
None | 92.6%/100/108 | 87.9%/94/107 | 84.9%/90/106 | 86.7%/91/105 |
Mild | 7.4%/8/108 | 10.3%/11/107 | 13.2%/14/106 | 11.4%/12/105 |
Moderate | 0.0%/0/108 | 0.0%/0/107 | 0.0%/0/106 | 0.0%/0/105 |
Significant | 0.0%/0/108 | 1.9%/2/107 | 1.9%/2/106 | 1.9%/2/105 |
Severe | 0.0%/0/108 | 0.0%/0/107 | 0.0%/0/106 | 0.0%/0/105 |
4. Dryness | ||||
None | 86.1%/93/108 | 41.1%/44/107 | 43.4%/46/106 | 56.2%/59/105 |
Mild | 13.9%/15/108 | 51.4%/55/107 | 44.3%/47/106 | 32.4%/34/105 |
Moderate | 0.0%/0/108 | 6.5%/7/107 | 10.4%/11/106 | 8.6%/9/105 |
Significant | 0.0%/0/108 | 0.9%/1/107 | 1.9%/2/106 | 1.9%/2/105 |
Severe | 0.0%/0/108 | 0.0%/0/107 | 0.0%/0/106 | 1.0%/1/105 |
5. Continuous tear production | ||||
None | 97.2%/105/108 | 98.1%/43/107 | 100%/106/106 | 98.1%/103/105 |
Mild | 2.8%/5/108 | 1.9%/1/107 | 0.0%/0/106 | 1.9%/2/105 |
Moderate | 0.0%/0/108 | 0.0%/0/107 | 0.0%/0/106 | 0.0%/0/105 |
Significant | 0.0%/0/108 | 0.0%/0/107 | 0.0%/0/106 | 0.0%/0/105 |
Severe | 0.0%/0/108 | 0.0%/0/107 | 0.0%/0/106 | 0.0%/0/105 |
6. Itching | ||||
None | 97.2%/105/108 | 83.2%/89/107 | 87.7%/93/106 | 87.6%/92/105 |
Mild | 2.8%/3/108 | 16.8%/18/107 | 12.3%/13/106 | 12.4%/13/105 |
Moderate | 0.0%/0/108 | 0.0%/0/107 | 0.0%/0/106 | 0.0%/0/105 |
Significant | 0.0%/0/108 | 0.0%/0/107 | 0.0%/0/106 | 0.0%/0/105 |
Severe | 0.0%/0/108 | 0.0%/0/107 | 0.0%/0/106 | 0.0%/0/105 |
7. Glare | ||||
None | 82.4%/89/108 | 59.8%/64/107 | 65.1%/69/106 | 70.5%/74/105 |
Mild | 12.0%/13/108 | 35.5%/38/107 | 29.2%/31/106 | 23.8%/25/105 |
Moderate | 5.6%/6/108 | 4.7%/5/107 | 3.8%/4/106 | 2.9%/3/105 |
Significant | 0.0%/0/108 | 0.0%/0/107 | 1.9%/2/106 | 2.9%/3/105 |
Severe | 0.0%/0/108 | 0.0%/0/107 | 0.0%/0/106 | 0.0%/0/105 |
8. Haloes around lights | ||||
None | 91.7%/99/108 | 53.3%/57/107 | 57.5%/61/106 | 57.1%/60/105 |
Mild | 4.6%/5/108 | 33.6%/36/107 | 29.2%/31/106 | 25.7%/27/105 |
Moderate | 1.9%/2/108 | 10.3%/11/107 | 7.5%/8/106 | 11.4%/12/105 |
Significant | 1.9%/2/108 | 2.8%/3/107 | 3.8%/4/106 | 5.7%/6/105 |
Severe | 0.0%/0/108 | 0.0%/0/107 | 1.9%/2/106 | 0.0%/0/105 |
9. Distorted vision | ||||
None | 97.2%/105/108 | 85.0%/91/107 | 92.5%/98/106 | 91.4%/96/105 |
Mild | 2.8%/3/108 | 12.1%/13/107 | 6.5%/7/106 | 7.6%/8/105 |
Moderate | 0.0%/0/108 | 1.9%/2/107 | 0.9%/1/106 | 1.0%/1/105 |
Significant | 0.0%/0/108 | 0.9%/1/107 | 0.0%/0/106 | 0.0%/0/105 |
Severe | 0.0%/0/108 | 0.0%/0/107 | 0.0%/0/106 | 0.0%/0/105 |
10. Double vision | ||||
None | 100%/108/108 | 95.3%/102/107 | 94.3%/100/106 | 96.2%/101/105 |
Mild | 0.0%/0/108 | 4.7%/5/107 | 3.8%/4/106 | 2.9%/3/105 |
Moderate | 0.0%/0/108 | 0.0%/0/107 | 1.9%/2/106 | 0.0%/0/105 |
Significant | 0.0%/0/108 | 0.0%/0/107 | 0.0%/0/106 | 1.0%/1/105 |
Severe | 0.0%/0/108 | 0.0%/0/107 | 0.0%/0/106 | 0.0%/0/105 |
11. Vision fluctuations | ||||
None | 89.9%/97/108 | 49.5%/53/107 | 60.4%/64/106 | 63.8%/67/105 |
Mild | 8.3%/9/108 | 36.4%/39/107 | 29.2%/31/106 | 31.4%/33/105 |
Moderate | 1.9%/2/108 | 10.3%/11/107 | 9.4%/10/106 | 2.9%/3/105 |
Significant | 0.0%/0/108 | 3.7%/4/107 | 0.9%/1/106 | 0.0%/0/105 |
Severe | 0.0%/0/108 | 0.0%/0/107 | 0.0%/0/106 | 1.9%/2/105 |
12. Changed vision | ||||
In sunlight | ||||
None | 88.0%/95/108 | 71.0%/76/107 | 76.4%/81/106 | 83.8%/88/105 |
Mild | 10.2%/11/108 | 25.2%/27/107 | 20.8%/22/106 | 16.2%/17/105 |
Moderate | 1.9%/2/108 | 2.8%/3/107 | 1.9%/2/106 | 0.0%/0/105 |
Significant | 0.0%/0/108 | 0.0%/0/107 | 0.9%/1/106 | 0.0%/0/105 |
Severe | 0.0%/0/108 | 0.0%/0/107 | 0.0%/0/106 | 0.0%/0/105 |
In room lighting | ||||
None | 90.7%/98/108 | 76.6%/82/107 | 78.3%/83/106 | 84.8%/89/105 |
Mild | 9.3%/10/108 | 16.8%/18/107 | 18.9%/20/106 | 14.3%/15/105 |
Moderate | 0.0%/0/108 | 5.6%/6/107 | 2.8%/3/106 | 1.0%/1/105 |
Significant | 0.0%/0/108 | 0.9%/1/107 | 0.0%/0/106 | 0.0%/0/105 |
Severe | 0.0%/0/108 | 0.0%/0/107 | 0.0%/0/106 | 0.0%/0/105 |
In the dark | ||||
None | 75.9%/82/108 | 71.0%/76/107 | 67.9%/72/106 | 68.6%/72/105 |
Mild | 21.3%/23/108 | 17.8%/19/107 | 25.5%/27/106 | 26.7%/28/105 |
Moderate | 2.8%/3/108 | 8.4%/9/107 | 5.7%/6/106 | 2.9%/3/105 |
Significant | 0.0%/0/108 | 2.8%/3/107 | 0.9%/1/106 | 1.9%/2/105 |
Severe | 0.0%/0/108 | 0.0%/0/107 | 0.0%/0/106 | 0.0%/0/105 |
13. Problems when driving at night | ||||
None | 81.5%/88/108 | 71.0%/76/107 | 67.0%/71/106 | 74.3%/78/104 |
Mild | 13.9%/15/108 | 18.7%/20/107 | 23.6%/25/106 | 19.0%/20/104 |
Moderate | 4.6%/5/108 | 8.4%/9/107 | 5.7%/6/106 | 3.8%/4/104 |
Significant | 0.0%/0/108 | 1.9%/2/107 | 3.8%/4/106 | 2.9%/3/104 |
Severe | 0.0%/0/108 | 0.0%/0/107 | 0.0%/0/106 | 0.0%/0/104 |
14. Others | ||||
None | 100%/108/108 | 97.2%/104/108 | 100%/106/106 | 98.1%/103/104 |
Mild | 0.0%/0/108 | 1.9%/2/108 | 0.0%/0/106 | 1.9%/2/104 |
Moderate | 0.0%/0/108 | 0.0%/0/108 | 0.0%/0/106 | 0.0%/0/104 |
Significant | 0.0%/0/108 | 0.0%/0/108 | 0.0%/0/106 | 0.0%/0/104 |
Severe | 0.0%/0/108 | 0.0%/0/108 | 0.0%/0/106 | 0.0%/0/104 |
Rights and permissions
About this article
Cite this article
Blum, M., Kunert, K., Schröder, M. et al. Femtosecond lenticule extraction for the correction of myopia: preliminary 6-month results. Graefes Arch Clin Exp Ophthalmol 248, 1019–1027 (2010). https://doi.org/10.1007/s00417-009-1293-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-009-1293-1