Abstract
Background
We aimed to study the inhibitory effects of topical cyclosporine A (CsA) 0.05 % on immune-mediated corneal neovascularization, and to compare its efficacy with those of dexamethasone 0.1 % and bevacizumab 0.5 %.
Methods
Immune-mediated corneal neovascularization was created in 36 right eyes of 36 rabbits. The rabbits were then randomized into four groups. Group I received CsA 0.05 %, Group II received dexamethasone 0.1 %, Group III received bevacizumab 0.5 %, and Group IV received isotonic saline twice a day for 14 days. The corneal surface covered with neovascular vessels was measured on the photographs. The rabbits were then sacrificed and the corneas excised. Paraffin-embedded sections were stained with hematoxylin-eosin and terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling assay.
Results
The means of percent area of corneal neovascularization in Group I, II, III, and IV were 24.4 %, 5.9 %, 37.1 %, and 44.1 %, respectively. The inhibitory effect of CsA 0.05 % was found to be better than the effect found in the bevacizumab 0.5 % and control groups (p = 0.03 and p = 0.02, respectively). CsA 0.05 % was found to have significantly lesser inhibitory effects on corneal neovascularization than dexamethasone 0.1 % (p < 0.001). Apoptotic cell density was higher in Group III and Group IV than in Group I and Group II. There was no difference between Group I and Group II in terms of apoptotic cell density (p = 0.7).
Conclusions
Topical CsA 0.05 % was shown to have an inhibitory effect on immune-mediated corneal neovascularization in rabbits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Corneal neovascularization (NV) is caused by the formation of new blood vessels from the limbal vasculature in the normally avascular cornea. Various factors can cause corneal NV, which includes infectious, inflammatory, toxic, immunologic, and traumatic diseases of the ocular surface. Although corneal NV might be helpful in combating infections and assisting corneal healing, it is usually undesirable because of its association with reduced corneal clarity and consequent reduction in vision.
Cyclosporine A (CsA) is a cyclic undecapeptide drug that inhibits the activity of transcription factors of the nuclear factor of the activated T-cell (NFAT) family, and has long been used successfully as a systemic immunomodulator. Topical ophthalmic emulsion of CsA at a dose of 0.05 % was approved by the Food and Drug Administration (FDA) to treat dry eye disease in 2003. Different concentrations of topical CsA have also been investigated for treatment of various ocular surface inflammatory disorders, including meibomian gland dysfunction [1], ocular rosacea [2], post-LASIK dry eye [3], atopic keratoconjunctivitis [4], acute corneal graft failure [5], graft-versus-host disease [6], and subepithelial infiltrates secondary to adenoviral keratoconjunctivitis [7].
Immunological graft rejection is the most common cause of corneal graft failure in the late postoperative period. Many corneal grafts in recipients with corneal NV undergo rejection [8]. Corneal NV appears to be a trigger for corneal graft rejection. Moreover, it has been shown that topical application of CsA prolongs corneal graft survival in an experimental study [9]. Hernández et al. showed that systemic CsA inhibits migration of primary endothelial cells and angiogenesis induced by vascular endothelial growth factor (VEGF) [10]. The authors speculated that this effect appears to be mediated through the inhibition of cyclooxygenase (Cox)-2, the transcription of which is activated by VEGF in primary endothelial cells. Benelli et al. previously showed that topical CsA 4 % inhibits corneal NV in a rat xenotransplantation model [11]. However, the therapeutic potential of topical CsA 0.05 % on immune-mediated corneal NV has not been studied so far.
Therefore, the aim of the present study is to assess the inhibitory effects of topical CsA 0.05 % on immune-mediated corneal NV in rabbits, and to compare its efficacy with those of topical dexamethasone 0.1 % and bevacizumab 0.5 %.
Materials and methods
A total of 36 eyes of 36 albino male New Zealand rabbits weighing 2–2.5 kg were included in the study. All of the procedures involving animals were conducted in accordance with the Association for Research in Vision and Ophthalmology resolution on the use of animals in research. The local ethics committee approved the study (2010/28). The experimental animals were maintained on food and water ad libitum and housed under conditions of controlled temperature (230 ± 2°C) and illumination (12-hour light–dark cycle) in Abant Izzet Baysal University, Application and Research Center for Experimental Animals during the study.
Experimental procedures
Only the right eyes of each rabbit were included in the study. Bovine serum albumin (albumin from bovine serum, A7030, Sigma-Aldrich, St Louis) at a dose of 5 mg/ml was injected subcutaneously two times 1 week apart to all rabbits for sensitization. Fourteen days after the subcutaneous injection, the rabbits were anesthetized with intramuscular injections of ketamine hydrochloride (35 mg/kg of body weight) and xylazine hydrochloride (5 mg/kg of body weight). Topical 0.5 % proparacaine hydrochloride (Alcaine®, Alcon, Puurs, Belgium) was then instilled into the inferior fornix of the right eye of each rabbit. Bovine serum albumin at a dose of 1 mg/ml (a total of 50 μl) was injected into the central cornea intrastromally under binocular microscope (Olympus SZ61, Tokyo, Japan) using a 30-gauge needle.
Drugs
The experimental animals were divided into four equal groups in a randomized way after the intrastromal injection of bovine serum albumin. Group I (n = 9) received topical CsA 0.05 % (Restasis®, Allergan Inc., Irvine, CA), Group II (n = 9) received topical dexamethasone 0.1 % (Dexa-sine SE®, Alcon, Freiburg, Germany), Group III (n = 9) received topical bevacizumab 0.5 % (Altuzan®, Roche, San Francisco, USA), and Group IV (n = 9) received topical isotonic saline solution as a control. All drugs were instilled into the inferior fornix twice daily for 15 days.
Corneal NV
The rabbits were reanesthetized using the previously described methods. Photographs of the right eyes of the experimental animals were taken using a Nikon D90 digital camera on the 15th day when corneal NV in the control eyes reached its maximum. The following formula was used in order to calculate the percentage of neovascular corneal area over digital photographs; [12]
where C denotes corneal NV as clock hours; r denotes the radius of the rabbit cornea; and L denotes the length of the longest vein or the longest group of veins starting from the limbus and extending to the cornea center. Analysis of the photos was performed in a blinded manner.
Histopathologic evaluation
The rabbits were then sacrificed and their corneas excised 1 mm behind the limbus and fixed in 10 % buffered formalin for 24 h. The tissues were dehydrated in graded ethanol and set in paraffin. The paraffin-embedded tissues were cut into 5-μm-thick slices, mounted on poly-Lysine-coated slides, deparaffinized with xylene, and rehydrated through graded concentrations of ethanol. Tissue sections of all corneas were stained with hematoxylin and eosin (H&E). A scale varying from 0 to 3 was used to measure inflammation (0: No inflammation, 1: Minimal inflammation, 2: Moderate inflammation, 3: Severe inflammation) [13]. Examination of inflammation was conducted by scanning five different areas with an X40 objective zoom out of cornea incisions belonging to each of the experimental animals. In order to define and count apoptotic cells, immunohistochemical staining (In Situ Death Detection Kit, POD®, Roche, Mannheim, Germany) was conducted by applying the terminal deoxynucleotidyl transferase dUTP-biotin nick end labeling (TUNEL) method. Apoptosis evaluation was conducted by counting the apoptotic cells which were stained positive in five different fields with an X40 objective zoom in the corneal sections belonging to the each of the experimental animals. VEGF immunostaining was conducted in order to stain the vascular endothelium. VEGF staining intensity was determined semicantitatively with the previously described technique [14] as: no (0), weak (1), moderate (2), and intense (3), and photographed using a BH2 Olympus photomicroscope (Olympus Optical Company, Japan).
Statistical analysis
All statistical analyses were performed using SPSS 11.5 statistical software (SPSS Inc., Chicago, IL). Data were shown as mean ± standard deviation for continuous variables, median (minimum—maximum) for ordinal ones, and frequency with percent for categorical ones. The Shapiro–Wilk test was used to test for normality of continuous data. The significance of median values among the groups was analyzed by the Kruskal–Wallis test. If the Kruskal–Wallis test was significant, paired comparisons were performed using Conover’s nonparametric multiple comparison test. A probability value of p < 0.05 indicated a statistically significant difference.
Results
Macroscopic findings
The percent area of corneal NV was 24.4 ± 14.4 (5.7–50.5) in Group I, 5.9 ± 3.5 (2.1–13.6) in Group II, 37.1 ± 20.4 (8.0–56.4) in Group III, and 44.1 ± 25.8 (13.7–86.3) in Group IV. A statistically significant difference was found among the groups in terms of the percent area of corneal NV. The inhibitory effect of topical CsA 0.05 % treatment on the immune-mediated corneal NV was significantly higher than topical isotonic saline or bevacizumab 0.5 % (p = 0.02 and p = 0.03, respectively). However, the inhibitory effect of dexamethasone 0.1 % drops was better than the topical CsA 0.05 % (p < 0.001). Figure 1 shows digitally enhanced photographs of the corneas in each group at the end of the treatment protocol.
Microscopic findings
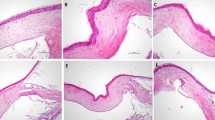
The median (min-max) inflammation score was 2 (1–2) in Group I, 0 (0–1) in Group II, 2 (2–3) in Group III, and 3 (2–3) in Group IV. There was no statistically significant difference between Group I and Group III in terms of inflammation score (p = 0.13). Group II showed the smallest inflammation score. Figure 2 shows the representative H&E staining samples in each group.
Hematoxylin-eosin staining samples in each group. Less new vessels and inflammatory cell infiltration was observed in the topical cyclosporine 0.05 % group (top left), compared to the topical bevacizumab 0.5 % group (bottom left). There were no new vessels or inflammatory cell infiltration in the topical dexamethasone 0.1 % group (top right). Increased new vessels and inflammatory cell infiltration in the control group is compared to the other groups (bottom right). (x20 magnification) (Scale bars indicate 100 μm)
The median (min-max) apoptotic cell density was 10 (9–14) cells/mm2 in Group I, 11 (6–18) cells/mm [2] in Group II, 23 (13–33) cells/mm2 in Group III, and 22 (17–30) cells/mm2 in Group IV. There was a statistically significant difference between the groups in terms of apoptotic cell density (p < 0.05). The apoptotic cell density difference was not significant between Group I and Group II (p = 0.7). There was also no significant difference between Group III and Group IV in terms of apoptotic cell density (p = 0.7). The apoptotic cell density was significantly lower in Group I and Group II compared to Group III and Group IV (p < 0.05). Figure 3 shows the TUNEL positive apoptotic cells in the groups. Table 1 shows the percent area of corneal NV, inflammation score, and TUNEL positive apoptotic cell intensity in the groups.
TUNEL positive apoptotic cells in the groups. The apoptotic cell count was less in the topical cyclosporine A 0.05 % group (top left) and topical dexamethasone 0.1 % group (top right), compared to the topical bevacizumab 0.5 % (bottom left) and control group (bottom right). (x20 magnification) (scale bars indicate 100 μm)
The VEGF staining intensity of the cornea was 1 (0–2) in Group I, 0 (0–1) in Group II, 1 (1–3) in Group III, and 2 (1–3) in Group IV. The VEGF staining intensity was significantly different among the groups (p < 0.05). There was no statistically significant difference between Group I and Group II in terms of VEGF immunostaining (p = 0.08) (Fig. 4).
VEGF immunostaining in the groups. VEGF staining was less in the topical cyclosporine A 0.05 % group (top left) and topical dexamethasone 0.1 % group (top right), compared to the topical bevacizumab 0.5 % (bottom left) and control group (bottom right). Increases VEGF staining was evident in the control group (x20 magnification) (scale bars indicate 100 μm)
Discussion
In the present study, we showed the inhibitory effects of topical CsA 0.05 % on experimental corneal NV established with the Arthus reaction mechanism in rabbits. Topical dexamethasone 0.1 % was found to be the most effective agent, and the efficacy of topical CsA 0.05 % was found to be superior to topical bevacizumab 0.5 % for reducing corneal NV. The density of apoptotic cells in topical dexamethasone and CsA groups was significantly lower than the other groups. The number of VEGF-stained endothelial cells was also detected to be significantly lower in dexamethasone and CsA groups compared to other groups.
Experimental and clinical studies on inhibiting corneal NV have gained popularity in recent years due to serious morbidity by reducing visual acuity [15–17]. The arachidonic acid metabolites play an active role in the pathogenesis of corneal neovascularization. E series prostaglandins, which are by-products of the cyclooxygenase pathway, strongly induce neovascular response. Leukotrienes, derived from the lipoxygenase pathway, also play a role in the formation of corneal neovascularization as they cause leukocyte chemotaxis. It is well known that nonsteroidal anti-inflammatory drugs and corticosteroids, inhibitors of arachidonic acid metabolism, are effective in reducing corneal neovascularization. To date, the inhibitory chemotherapeutic potentials of the selective cyclooxygenase-2 (COX-2) inhibitor, plasminogen kringle, triamcinolone, doxycycline, thymoquinone, and photodynamic therapy with heparin have been reported in the literature [18].
The anti-angiogenic effects of topical corticosteroids were reported in 1950 by Jones and Meyer [19]. Since then, topical steroids remain the first choice in treating corneal NV. The action mechanisms of corticosteroids in corneal NV may occur in distinct ways, such as in the inhibition of chemotaxis, the inhibition of proinflammatory cytokines (e.g. TNF-α, IL-1 and IL-6) and the plasminogen activator, and by reducing the formation of inflammatory mediators such as prostaglandin and leukotrienes by suppressing arachidonic acid secretion out of membrane phospholipids. In spite of this strong effect, extended use of topical steroids to reduce corneal NV may cause intraocular pressure elevation, cataract formation or increased risk of infectious keratitis [20]. Weaker steroids such as fluorometholone bear these side effects at a lesser level. Although steroids are the mainstay of the treatment, they are not always effective or without side effects, which is the reason behind investigation into alternative agents in the treatment of corneal NV.
Recently, researchers found that VEGF acts as the key mediator during the neovascular processes. It regulates the growth of vascular endothelium, and controls the formation of new blood vessels. Binding to its receptors, VEGF triggers a signaling cascade that promotes endothelial cell growth, migration, and differentiation. One way to inhibit VEGF activity is by humanized monoclonal antibodies like bevacizumab, and it has been widely used in clinical and experimental corneal NV studies [21–23]. Two recent studies showed that topical bevacizumab was effective in decreasing neovascular area and vessel caliber in corneal NV secondary to a variety of causes [24,25]. Although anti-VEGF agents are effective in halting corneal NV, its resolution is usually incomplete [21,26]. In accordance with the previous studies, the present study showed that topical 0.1 % dexamethasone was found to be more effective in inhibiting corneal NV compared to topical 0.5 % bevacizumab. It is possible to explain this situation with two different hypotheses. First, it is likely that topical 0.1 % dexamethasone reduced corneal NV to a higher degree as it is a more potent antiangiogenic agent. Second, and more significantly, it is likely that having active intermediates other than VEGF due to the fact that the experimental model used in our study was dependent upon on an immune-based mechanism caused bevacizumab to demonstrate lesser effect.
Corneal NV is one of the most important risk factor that contributes to corneal graft rejection. The risk of an immune reaction is approximately 50 % in a vascularized cornea, compared with approximately 10 % in an avascular cornea [27–29]. Moreover, the risk of rejection rises incrementally as the number of stromal vessels increases [30]. Therefore, reducing corneal NV in patients with corneal allograft is critically important for graft survival and better vision. Topical steroids have remained the treatment of choice to control corneal NV and reduce the risk of graft rejection. More recently, preliminary clinical reports with equivocal results have been published on the efficacy and safety of CsA in the treatment of corneal NV associated with high-risk corneal grafts [31,32]. Topical CsA use instead of steroids in corneal NV related to corneal transplantation may be a reasonable option to protect the patient from the unwanted side effects of steroids, such as infectious recurrences, steroid induced glaucoma, and posterior subcapsular cataract formation. Topical CsA may be the second choice after steroid drops in the treatment of immune-based corneal neovascularization such as corneal graft vascularization especially when unwanted effects of the steroids were observed. Lipman et al. evaluated the effect of CsA in an experimental corneal NV stimulated with interleukin-2 [33]. The results showed that 25 mg/kg cyclosporine A in olive oil noticeably reduced corneal vascularization compared to the control group. The antiangiogenic effects of CsA may be associated with reduced expression of IL-2 and its receptor, whereas it may be demonstrated by diminishing proinflammatory cytokines charged with corneal neovascularization such as TNF-α. In a study conducted to determine the antiangiogenic effect of CsA in patients with rheumatoid arthritis, it was detected that CsA demonstrated inhibitory effects on activator protein-1-mediated VEGF expression [34]. It was observed in our study that topical CsA 0.05 % was more effective in inhibiting corneal NV than topical bevacizumab 0.5 %. The lower efficiency of topical bevacizumab compared to CsA in the present study might be related to the roles played by cytokines other than VEGF such as tumor necrosis factor-α, proinflammatory interleukins, angiopoietin and interferon-γ in the immunological-derived corneal NV model. In addition to this, topically applied bevacizumab has limited capacity to penetrate the corneas with intact epithelium which may delimitate its antiangiogenic effects [35].
In conclusion, the present study showed that topical CsA 0.05 % was macroscopically and histologically effective in treating immune-mediated corneal NV in rabbits. Topical CsA 0.05 % eye drops may play a role in the treatment of immune-mediated cornea NV in humans. However, further investigations are necessary in order to provide additional evidence regarding the inhibitory effects of topical CsA on corneal NV.
References
Prabhasawat P, Tesavibul N, Mahawong W (2012) A randomized double-masked study of 0.05% cyclosporine ophthalmic emulsion in the treatment of meibomian gland dysfunction. Cornea 31:1386–9133
Schechter BA, Katz RS, Friedman LS (2009) Efficacy of topical cyclosporine for the treatment of ocular rosacea. Adv Ther 26:651–659
Salib GM, McDonald MB, Smolek M (2006) Safety and efficacy of cyclosporine 0.05% drops versus unpreserved artificial tears in dry-eye patients having laser in situ keratomileusis. J Cataract Refract Surg 32:772–728
Hingorani M, Moodaley L, Calder VL, Buckley RJ, Lightman S (1998) A randomized, placebo-controlled trial of topical cyclosporin A in steroid-dependent atopic keratoconjunctivitis. Ophthalmology 105:1715–1720
Poon A, Constantinou M, Lamoureux E, Taylor HR (2008) Topical Cyclosporin A in the treatment of acute graft rejection: a randomized controlled trial. Clin Exp Ophthalmol 36:415–421
Rao SN, Rao RD (2006) Efficacy of topical cyclosporine 0.05% in the treatment of dry eye associated with graft versus host disease. Cornea 25:674–678
Okumus S, Coskun E, Tatar MG, Kaydu E, Yayuspayi R, Comez A, Erbagci I, Gurler B (2012) Cyclosporine a 0.05% eye drops for the treatment of subepithelial infiltrates after epidemic keratoconjunctivitis. BMC Ophthalmol 12:42
Williams KA, Coster DJ (2007) The immunobiology of corneal transplantation. Transplantation 84:806–813
Liu Y, Jiang J, Xiao H, Wang X, Li Y, Gong Y, Wang D, Huang Y (2012) Topical application of FTY720 and cyclosporin A prolong corneal graft survival in mice. Mol Vis 18:624–633
Hernández GL, Volpert OV, Iñiguez MA, Lorenzo E, Martínez-Martínez S, Grau R, Fresno M, Redondo JM (2001) Selective inhibition of vascular endothelial growth factor-mediated angiogenesis by cyclosporin A: roles of the nuclear factor of activated T cells and cyclooxygenase 2. J Exp Med 193:607–620
Benelli U, Ross JR, Nardi M, Klintworth GK (1997) Corneal neovascularization induced by xenografts or chemical cautery. Inhibition by cyclosporin A. Invest Ophthalmol Vis Sci 38:274–282
Saita N, Fujiwara N, Yano I, Soejima K, Kobayashi K (2000) Trehalose 6,6′-dimycolate (cord factor) of Mycobacterium tuberculosis induces corneal angiogenesis in rats. Infect Immun 68:5991–5997
Peebo BB, Fagerholm P, Traneus-Röckert C, Lagali N (2011) Cellular level characterization of capillary regression in inflammatory angiogenesis using an in vivo corneal model. Angiogenesis 14:393–405
Philipp W, Speicher L, Humpel C (2000) Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas. Invest Ophthalmol Vis Sci 41:2514–2522
Chang JH, Gabison EE, Kato T, Azar DT (2001) Corneal neovascularization. Curr Opin Ophthalmol 12:242–249
Epstein RJ, Stulting RD, Hendricks RL, Harris DM (1987) Corneal neovascularization: pathogenesis and inhibition. Cornea 6:250–257
Lee P, Wang CC, Adamis AP (1998) Ocular neovascularization: an epidemiologic review. Surv Ophthalmol 43:245–269
Erdurmus M, Yagci R, Yilmaz B, Hepsen IF, Turkmen C, Aydin B, Karadag R (2007) Inhibitory effects of topical thymoquinone on corneal neovascularization. Cornea 26:715–719
Jones IS, Meyer K (1950) Inhibition of vascularization of the rabbit cornea by local application of cotisone. Proc Soc Exp Biol Med 74:102–104
Baratz KH, Hattenhauer MG (1999) Indiscriminate use of corticosteroid-containing eyedrops. Mayo Clin Proc 74:362–366
Erdurmus M, Totan Y (2007) Subconjunctival bevacizumab for corneal neovascularization. Graefes Arch Clin Exp Ophthalmol 245:1577–1579
Bock F, König Y, Kruse F, Baier M, Cursiefen C (2008) Bevacizumab (Avastin) eye drops inhibit corneal neovascularization. Graefes Arch Clin Exp Ophthalmol 246:281–284
Petsoglou C, Balaggan KS, Dart JK, Bunce C, Xing W, Ali RR, Tuft SJ (2013) Subconjunctival bevacizumab induces regression of corneal neovascularisation: a pilot randomised placebo-controlled double-masked trial. Br J Ophthalmol 97:28–32
Dastjerdi MH, Al-Arfaj KM, Nallasamy N, Hamrah P, Jurkunas UV, Pineda R 2nd, Pavan-Langston D, Dana R (2009) Topical bevacizumab in the treatment of corneal neovascularization: results of a prospective, open-label, noncomparative study. Arch Ophthalmol 127:381–389
Koenig Y, Bock F, Horn F, Kruse F, Straub K, Cursiefen C (2009) Short- and long-term safety profile and efficacy of topical bevacizumab (Avastin) eye drops against corneal neovascularization. Graefes Arch Clin Exp Ophthalmol 247:1375–1382
Joussen AM, Poulaki V, Mitsiades N, Stechschulte SU, Kirchhof B, Dartt DA, Fong GH, Rudge J (2003) VEGF-dependent conjunctivalization of the corneal surface. Invest Ophthalmol Vis Sci 44:117–123
Bock F, Konig Y, Dietrich T, Zimmermann P, Baier M, Cursiefen C (2007) Inhibition of angiogenesis in the anterior chamber of the eye [in German]. Ophthalmologe 104:336–344
Küchle M, Cursiefen C, Nguyen NX, Langenbucher A, Seitz B, Wenkel H, Martus P, Naumann GO (2002) Risk factors for corneal allograft rejection: intermediate results of a prospective normal-risk keratoplasty study. Graefes Arch Clin Exp Ophthalmol 240:580–584
Maier P, Bohringer D, Reinhard T (2007) Clear graft survival and immune reactions following emergency keratoplasty. Graefes Arch Clin Exp Ophthalmol 245:351–359
Maguire MG, Stark WJ, Gottsch JD, Stulting RD, Sugar A, Fink NE, Schwartz A (1994) Risk factors for corneal graft failure and rejection in the collaborative corneal transplantation studies. Collaborative corneal transplantation studies research group. Ophthalmology 101:1536–1547
Shimazaki J, Den S, Omoto M, Satake Y, Shimmura S, Tsubota K (2011) Prospective, randomized study of the efficacy of systemic cyclosporine in high-risk corneal transplantation. Am J Ophthalmol 152:33–39.e1
Gupta D, Illingworth C (2011) Treatments for corneal neovascularization: a review. Cornea 30:927–938
Lipman RM, Epstein RJ, Hendricks RL (1992) Suppression of corneal neovascularization with cyclosporine. Arch Ophthalmol 110:405–407
Cho ML, Cho CS, Min SY, Kim SH, Lee SS, Kim WU, Min DJ, Min JK, Youn J, Hwang SY, Park SH, Kim HY (2002) Cyclosporine inhibition of vascular endothelial growth factor production in rheumatoid synovial fibroblasts. Arthritis Rheum 46:1202–1209
Dastjerdi MH, Sadrai Z, Saban DR, Zhang Q, Dana R (2011) Corneal penetration of topical and subconjunctival bevacizumab. Invest Ophthalmol Vis Sci 52:8718–8723
Acknowledgments
Competing interests, Funding
None of the authors has any competing financial interests in this study. This study supported by Abant Izzet Baysal University Scientific Research Committee (Project number: 2011.08.02.388).
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was presented as a free paper in the XXX Congress of ESCRS, 8–12 September 2012, Milan, Italy.
Rights and permissions
About this article
Cite this article
Bucak, Y.Y., Erdurmus, M., Terzi, E.H. et al. Inhibitory effects of topical cyclosporine A 0.05 % on immune-mediated corneal neovascularization in rabbits. Graefes Arch Clin Exp Ophthalmol 251, 2555–2561 (2013). https://doi.org/10.1007/s00417-013-2467-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-013-2467-4