Abstract
Objective
To assess the peripapillary retinal nerve fiber layer (RNFL) thickness, optic nerve head (ONH) morphologic parameters, and macular thickness and volume in patients affected by obstructive sleep apnea–hypopnea syndrome (OSAHS).
Methods
This prospective, observational case-control study consisted of 96 eyes of 50 OSAHS patients (mean age of 50.9 ± 12.4 years, best-corrected visual acuity ≥20/20, refractive error less than 3 spherocylindrical diopters, and intraocular pressure <21 mmHg) who were enrolled and compared with 64 eyes of 33 age-matched controls. Peripapillary RNFL thickness, ONH parameters, macular thickness and volume were measured by optical coherence tomography (OCT).
Results
OSAHS patients showed a significant reduction of the nasal quadrant RNFL thickness (74.7 ± 15.8 μm) compared with those values observed in control patients (81.1 ± 16.6 μm, p = 0.047, Student's t-test). No differences in peripapillary RNFL thickness were observed when dividing the OSAHS group in accordance with disease severity. Vertical integrated rim area (VIRA) (0.67 ± 0.41 mm3 in OSAHS vs 0.55 ± 0.29 mm3 in controls; p = 0.043, Student's t-test), horizontal integrated rim width (HIRW) (1.87 ± 0.31 mm2 in OSAHS vs 1.8 ± 0.25 mm2 in controls; p = 0.039, Student's t-test) and disc area (2.74 ± 0.62 mm2 in OSAHS vs 2.48 ± 0.42 mm2 in controls; p = 0.002, Student's t-test) showed significant differences, all of them being higher in the OSAHS group. Severe OSAHS had significant higher disc area (2.8 ± 0.7 mm2) than controls (2.5 ± 0.4 mm2; p = 0.016, ANOVA test). Temporal inner macular thickness was significantly higher in mild–moderate OSAHS patients (270 ± 12 μm) than in severe OSAHS patients (260 ± 19 μm; p = 0.021, ANOVA test).
Conclusions
OSAHS patients showed decreased peripapillary nasal RNFL thickness, and increased ONH area and volume parameters when they were evaluated by OCT. These findings suggest that neuronal degeneration might be present in the retina of OSAHS patients, as previously observed in some neurodegenerative disorders
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea–hypopnea syndrome (OSAHS) is part of a broad group of disorders known as “sleep-related breathing disorders”. OSAHS is characterized by brief episodes of complete or partial upper airway collapse during sleep, causing an increased thoraco-abdominal effort and a decreased arterial oxygen saturation, leading to an arousal response [1] which takes the form of apneas and periodic hypopneas during sleep. This produces an excessive daytime sleepiness. There are a great deal of evidence that local and systemic inflammation play an important role in the pathogenesis of OSAHS, contributing to anatomic narrowing of the upper airway, increased collapsibility of the airway tissues, and abnormalities in reflexes that affect upper respiratory tract caliber and pharyngeal inspiratory muscle function [2].
OSAHS has recently been associated with numerous ophthalmological disorders, such as floppy eyelid syndrome, visual field defects, retinal vein occlusion, central serous chorioretinopathy, and certain optic nerve dysfunctions [3–5]. Thus, papilledema and increased intracranial pressure have been reported in OSAHS patients [6, 7], improving after continuous positive airway pressure (CPAP) treatment [8, 9]. Non-arteritic anterior ischemic optic neuropathy has also been reported to be associated with OSAHS [10]. Likewise, an increased incidence of glaucoma in OSAHS patients is assumed [11–14]. Nevertheless, the pathogenesis of optic disc damage in OSAHS is complex and remains unknown. Frequent episodes of nocturnal hypoxemia would compromise optic nerve perfusion and oxygenation, leading to optic neuropathy [15]. Clinical features of OSAHS have inspired studies about brain structural abnormalities in this disease. Magnetic resonance imaging studies have reported a loss of gray and white matter in certain brain areas, suggesting a premature degeneration of the central nervous system in patients suffering from OSAHS [16, 17].
Optical coherence tomography (OCT), a relatively new noninvasive imaging technique, provides reproducible, high-resolution cross-sectional imaging of the retinal nerve fiber layer (RNFL) and optic nerve head (ONH) topography, providing an objective tool to diagnose axonal damage. OCT is used in various ophthalmological disorders, including glaucoma [18] and macular diseases [19]. Likewise, a significant reduction in the peripapillary RNFL thickness has been reported in patients with various neurologic disorders [20], such as multiple sclerosis [21, 22], Alzheimer’s disease [23, 24], Parkinson’s disease [25, 26], and schizophrenia [27], suggesting that this technology might also prove useful in other neurodegenerative diseases. Recently, a decreased RNFL thickness measured with OCT has been found in patients with moderate/severe OSAHS [28].
The goals of our study were to determine, by using OCT, the differences in the ONH parameters, peripapillary RNFL thickness, macular thickness and volume, between OSAHS patients and control subjects, as well as to assess whether a correlation exists between the OCT measurements and the clinical severity of the disease.
Material and methods
Ninety-six eyes from 50 patients (41 males and nine females) with OSAHS were consecutively recruited in the Department of Otolaryngology at the Miguel Servet University Hospital in Zaragoza, Spain. The selected patients, whose age was 50.9 ± 12.4 years (14–75), have a newly discovered and previously untreated mild to severe OSAHS diagnosed according to clinical features and an apnea–hypopnea index (AHI) greater than 4. Before OSAHS was confirmed, the patients completed a questionnaire concerning epidemiological data (age, height, weight, co-morbidities, smoking, previous treatment, and past surgeries) and information about symptoms such as loud snoring, observed apnea, or excessive daytime sleepiness. The most common vascular risk factors, hypertension, diabetes, and hyperlipidaemia, were studied and treated if necessary. All smoker OSAHS patients were encouraged to stop the habit. After appropriate information, written informed consent of all subjects was obtained.
Every OSAHS patient was diagnosed with a full sleep study during an entire attended night. This investigation consisted of continuous polygraphic recording of two electroencephalographic leads, right and left electro-oculographic leads, and chin electromyography for sleep staging. Ribcage and abdominal motion were monitored by inductive plethysmography (Alice 4, Philips, Eindhoven, Holland), airflow by thermistor (Ambulatory Monitoring, Inc., Ardsley, NY, USA), and arterial oxyhaemoglobin saturation by finger pulse oximetry (OhmedaBiox 3700, Ohmeda, Boulder, CO, USA). Sleep stage scoring was done for 30-s intervals by trained technicians according to standard criteria [29]. Apnea was defined as the complete cessation of airflow, and hypopnea as a discernible reduction in airflow or thoraco-abdominal excursion lasting for 10 s or more, accompanied by a decrease in oxygen saturation of at least 4 %. AHI was defined as the total number of apneas and hypopneas per hour during sleep. In patients with a confirmed diagnosis, an individualized multidisciplinary treatment was initiated according to the sleep study, the severity of clinical symptoms and signs, the exploration of the superior airway, and the wishes of the patient [30].
The control group was formed by 64 eyes of 33 age-matched healthy control subjects (19 males and 14 females), with a mean age of 49.1 ± 14.3 years (15–74), who were recruited from the Department of Ophthalmology at the Lozano Blesa University Clinic Hospital in Zaragoza, Spain. The same epidemiological data as in the OSAHS group were collected. Smoking habit and vascular risk factors were treated in the same way.
Patients and controls were subsequently referred for a comprehensive ophthalmological examination at the ophthalmologic department at “Lozano Blesa” University Clinic Hospital from December 2010 to December 2011. Patients who had a history of stroke with central apnea, chronic uveitis, antiglaucomatous drug usage, optic neuropathy, and previous ocular trauma or surgeries were excluded from this study. After appropriate information, written informed consent of all subjects was obtained. The research followed the tenets of the Declaration of Helsinki, and the protocol was approved by the local ethics committee.
All OSAHS patients and control subjects underwent a complete ophthalmologic examination, including assessment of best-corrected visual acuity (BCVA), ocular motility, pupillary reflexes, slit-lamp biomicroscopy, Goldmann applanation tonometry, gonioscopy, Humphrey automated visual field (HVF) examination, and dilated fundus examination. The examiners were masked to the diagnosis. All participants had a BCVA of 20/20 or better with a refractive error lower than 3 spherical diopters and 2 diopters of astigmatism. Eyes with HVF defects compatible with glaucoma (nasal step, paracentral or arcuate scotomas, or arcuate blind spot enlargement), applanation intraocular pressure (IOP) >21 mmHg, posterior pole pathology such as macular degeneration or diabetic retinopathy, or patients with media opacification such as cataract or vitreous hemorrhage which prevented ocular and OCT examination, were excluded.
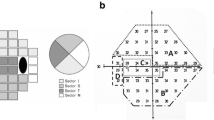
OCT was performed with the Stratus OCT (Carl Zeiss Meditec Inc., Dublin, CA, USA) following 1 % tropicamide instillation for dilation of the pupils. Only high-quality images (signal strength ≥7) were included. Each patient underwent scans to measure peripapillary RNFL thickness, ONH parameters, and macular thickness and volume at the same visit. Peripapillary RNFL thickness was automatically calculated by the fast RNFL algorithm. Three 360° circular scans with a diameter of 3.4 mm centered on the optic disc were performed. The software allows the mapping of the thickness data according to both quadrant-by-quadrant and clock hour analyses. We considered the average values of three different measurements per quadrant (superior, inferior, nasal and temporal): the overall data obtained in all quadrants were identified as overall RNFL thickness. ONH measurements were obtained by the fast optical disc scanning protocol, which consists of six radial scans centered on the ONH. ONH parameters were automatically calculated, including vertical integrated rim area (VIRA, measurement of neurorretinal rim volume, in mm3), horizontal integrated rim width (HIRW, measurement of neurorretinal rim area, in mm2), disc area, cup/disc area ratio, horizontal cup/ disc ratio, and vertical cup/disc ratio. Macular thickness measurements were obtained by the fast macular thickness protocol, which consists of six radial scans (each 6 mm) in a spoke-like pattern centered on the fovea, with each radial scan spaced 30° apart. To fill the gaps between scans, the OCT uses interpolation. Stratus OCT software calculates retinal thickness as the distance between the first signal from the vitreoretinal interface and the signal from the anterior boundary of the retinal pigment epithelium. The map is composed of nine sectorial thickness measurements in three concentric circles with diameters of 1 mm, 3 mm, and 6 mm. The area bounded by the outer (6-mm) and middle (3-mm) circles forms the outer ring, and the area bounded by the middle (3-mm) and inner circles (1-mm) forms the inner ring. The central 1-mm circular region represents the foveal area. Total average macular thickness, average macular thicknesses in the inner (1–3 mm) and outer (3–6 mm) rings, and the central 1-mm fovea thickness were analyzed in the study. Total macular volume was calculated automatically by the OCT software (Fig. 1).
a Optic nerve head examination. Vertical cross-section representation. b Retinal nerve fiber layer analysis report. Average values (microns) per quadrant (S superior. N nasal. I inferior. T temporal). c Macular thickness report; nine sectorial thickness measurements in three concentric circles with diameters of 1 mm, 3 mm, and 6 mm
Statistical analysis
Data analysis was conducted using SPSS software version 19.0 (SPSS, Inc, Chicago, IL, USA). Values were presented as mean ± standard deviation (SD) and expressed in microns (μm) for the peripapillary RNFL thickness and macular retinal thickness, and in mm3 for macular volume. Qualitative differences between the study variables were assessed using Pearson’s chi-squared test. Discriminant analysis, with Wilks’ lambda determination, was performed in order to evaluate parameters that better define the cases. The relationship between AHI and ophthalmologic significant variables was evaluated using Pearson’s correlation coefficient. A p value <0.05 was considered statistically significant.
We conducted two separate analyses. In the first one, mean values of the studied variables obtained in all OSAHS patients were compared with those obtained in the control group (only taking into account the presence of OSAHS, regardless of severity), the two-sample Student t-test was used for determining whether the values of a particular quantitative variable differ between OSAHS and the control eyes.
In the second analysis, the OSAHS patients sample was divided according to the severity of OSAHS (measured by AHI) into two groups: those with mild–moderate OSAHS (group 1, AHI ≥5 and <30); and those with severe OSAHS (group 2, AHI ≥30). Quantitative differences between the studied variables in the three groups were compared using one-way ANOVA test. Post-hoc analyses with Bonferroni adjustments were performed. We joined mild and moderate OSAHS patients, assuming that severe OSAHS patients would show more differences when compared with the control group.
Results
All the patients screened from the otolaryngology department were informed in advance, and asked if they wanted to participate in the study. They were warned that to agree with the study did not necessarily imply participating in it, depending on the exclusive ophthalmologic findings. The reasons for not participating in the study were problems for medical visits (due to physical or mental condition) or disagreement with the study. Of 63 consecutive OSAHS patients who accepted to participate, 51 (80.9 %) were included in the study, whereas 12 (19.1 %) were excluded. Eight subjects were excluded because of refractive defect greater than 3 spherical diopters or 2 diopters of astigmatism, two with mature cataracts which prevented ocular and OCT examination, and two other patients because of diabetic macular edema and non-exudative age-related macular degeneration respectively. Three of the 86 people (3.5 %), including controls and cases, did not fulfill the quality criteria for OCT. Finally, 83 Caucasian individuals were included in the analysis, 50 OSAHS patients and 33 healthy controls, whose demographic data are summarized in Table 1. Both eyes of each patient were included, except when an exclusion criteria appeared in one eye, assuming that OSAHS influence could be asymmetric [31, 32]. Age showed no statistically significant difference between both groups (p = 0.401, Student's t-test). In both groups, more men than women were enrolled; although the difference was statistically significant (p < 0.05; chi-squared test), gender has no effect on RNFL evaluation as previously mentioned [33]. BCVA was similar in both groups (p = 0.577). Body mass index (BMI) was not matched. The mean BMI was higher in SAOS patients (29.6 ± 4.5) than that in controls (24.9 ± 4.3) (p < 0.05, Student's t-test). Nevertheless, no significant differences in vascular risk factors and prevalence of smoking habit between cases and controls were found (p = 0.09, chi-squared test). Table 2 summarizes polysomnographic data in OSAHS patients according to severity.
First analysis: OCT parameters in OSAHS patients versus non-OSAHS individuals
Table 3 shows the results of IOP, HVF indices, optic disc parameters, and macular and peripapillary RNFL thickness measurements in OSAHS patients vs controls. IOP values were higher in OSAHS group than those in controls (p < 0.001, Student's t-test). Humphrey visual field results, such as visual field index (VFI), mean deviation (MD), and pattern standard deviation (PSD), were significantly altered in patients with OSAHS patients as compared with the control group (p = 0.009, p = 0.002, p = 0.014 respectively by Student's t-test). VIRA, HIRW, and disc area values were higher in the OSAHS group than those in controls. These differences were statistically significant (p < 0.05, Student's t-test). Only the nasal quadrant of the peripapillary RNFL showed a decreased thickness in OSAHS patients (74.7 ± 15.8 μm) compared with that in control subjects (81.1 ± 16.6 μm) (p = 0.016, Student's t-test). No statistically significant difference was observed regarding the macular thickness and volume (p > 0.05; Student's t-test).
Discriminant analysis included disc area and peripapillary RNFL thickness of the nasal quadrant as differentiating variables, with a lambda value of 0.958 (p = 0.011) and 0.929 (p = 0.004) respectively. Standardized canonical discriminant function coefficients showed that disc area was the most important predictor variable (0.747). Thus, the larger the disc area in the discriminant function, the greater the tendency to classify the subject as OSAHS. Peripapillary RNFL thickness of the nasal quadrant had a negative value (−0.652), which implies that if patients with equal scores in the remaining variables have a thicker peripapillary RNFL in nasal quadrant, they will show a lower score in the discriminant function, and therefore they will be more easily classified as controls. AHI showed no correlation with any of the significant variables: VIRA (r = 0.045, p = 0.672, n = 96), HIRW (r = 0.082, p = 0.439, n = 96), disc area (r = 0.063, p = 0.554, n = 96), and peripapillary RNFL thickness in nasal quadrant (r = −0.061, p = 0.565, n = 96).
Second analysis: OCT parameters in mild–moderate OSAHS versus severe OSAHS patients
When dividing the OSAHS group according to its severity in mild/moderate and severe cases, age showed no statistically significant difference between the different pairs: controls/mild–moderate OSAHS (49.1 ± 14.3 years vs 50.7 ± 14.0 years respectively; p > 0.05, ANOVA test); controls/severe OSAHS (49.1 ± 14.3 years vs 51.0 ± 11.5 respectively; p > 0.05, ANOVA test); mild–moderate OSAHS/severe OSAHS (50.7 ± 14.0 years vs 51.0 ± 11.5 respectively; p > 0.05, ANOVA test).
HVF data are shown in Table 4. VFI and PSD were not significantly altered in patients with mild–moderate or severe OSAHS as compared with the control group. MD value was significantly altered in the mild–moderate OSAHS group as compared with controls (p = 0.005, ANOVA test).
Table 4 also contains mean values of IOP, optic disc measurements, and macular and peripapillary RNFL thickness measurements in OSAHS patients, classified according to IAH. Severe OSAHS eyes revealed higher disc area values than those in control group (p = 0.016, ANOVA test). No differences in IOP values were found among the three groups. Temporal inner macular thickness was significantly higher in mild–moderate OSAHS patients compared to the severe OSAHS group. No differences in peripapillary RNFL thickness among the three groups were observed. Only the nasal quadrant in the severe OSAHS group showed an almost statistically significant decrease when comparing with controls (p = 0.057).
Discriminant analysis just included temporal inner macular thickness as a differentiating variable between individuals with OSAHS and controls, with a lambda value of 0.532 (p < 0.001). The standardized canonical discriminant function coefficients showed a value of −0.400. Thus, patients with equal scores in the remaining variables, but thicker temporal inner macula, will be more easily classified as controls.
We found no correlation between IAH and the significant different parameters when the OSAHS group was divided according to its severity. Disc area in severe OSAHS (r = 0.071, p = 0.606, n = 58); temporal inner macular thickness in severe OSAHS (r = 0.11, p = 0.416, n = 58); temporal inner macular thickness in mild–moderate OSAHS (r = 0.325, p = 0.057, n = 38).
Discussion
During sleep, apneic episodes with consequent drop in oxygen saturation lead to the activation of the adrenergic system, proinflammatory mechanisms, endothelial dysfunction, oxidative stress, procoagulant mechanisms, and metabolic deregulation [34]. There is some evidence that OSAHS is a risk factor for neurovascular and cardiovascular diseases. Arterial hypertension, cardiac arrhythmia and/or ischemia, congestive heart failure, and cerebrovascular disease are events more likely in the presence of the obstructive sleep disturbance [35, 36]. This vascular phenomenon may compromise optic nerve perfusion and oxygenation, ultimately leading to optic neuropathy.
Several authors have reported a higher prevalence of glaucomatous neuropathy in OSAHS patients [7, 13], characterized by increased size of the optic disc cup and associated thinning of peripapillary RNFL. Moreover, some authors have reported a significant presence of visual field defects in patients with OSAHS, even with an improvement of this defects following treatment with CPAP in one OSAHS patient [37, 38]. This glaucomatous functional loss is thought to be preceded by thinning of RNFL in some years [39]. Thus, the first quantifiable sign of glaucomatous neuropathy in OSAHS patients is an axonal loss measured by OCT. In our study, we just included patients with normal IOP values (less than 22 mmHg), normal gonioscopy, and no perimetric evidence of glaucomatous neuropathy, in order to assess an hypothetical reduction of peripapillary RNFL thickness secondary to the respiratory disorder. Despite this, we found significantly higher IOP values as well as alterations in indices of VF in OSAHS patients compared to controls. In the present study, as in previous studies [15, 28, 40], and after excluding patients with glaucoma, nasal peripapillary RNFL thickness was found to be decreased in patients harboring OSAHS compared with controls. Moreover, several ONH parameters such as disc area, VIRA, and HIRW were increased in OSAHS patients compared to the controls. Therefore, patients with OSAHS not harboring glaucoma might already show some signs of a neuronal degeneration.
Peripapillary RNFL thickness measurement reflects neuronal axons, and would allow quantification of ganglion cell axonal loss. Macular thickness and volume values would reflect neurons, including bodies of retinal ganglion cells, and allowing quantification of neuronal loss. Retinal ganglion cells have been reported to be particularly sensitive to mild systemic hypoxic stress [41]. Thus, in hypoxic conditions, cell death has been classified as apoptotic or necrotic. The last one presents swelling of cell body, disruption of plasma membrane, and alterations in nuclear DNA [42]. It is possible that this nuclear swelling could induce an increased macular thickness preceding atrophy secondary to neuron death (reflected as a decreased peripapillary RNFL thickness). In our results, temporal inner macular thickness was significantly higher in mild–moderate OSAHS patients compared to severe OSAHS patients and controls. This fact would support the attractive hypothesis that a previous inflammatory edema, with an increased macular thickness, might be an expression of VEGF, nitric oxide, and other proinflammatory mediators in early stages of OSAHS, preceding a final stage with loss of neuronal population and subsequent functional repercussion.
The significant increase in morphological ONH parameters, such as VIRA, HIRW, and disc area, in OSAHS patients compared with those in controls, especially disc area in severe OSAHS patients, might be justified by a hypothetical and slight optic nerve swelling caused by intracranial vascular dysfunction. Moreover, according to the discriminant analysis, disc area is the most distinguishing feature between patients and controls. Purvin et al. analyzed the relationship between intracranial pressure, papilledema, and OSAHS [6]. They suggested episodic hypoxia and hypercapnia as cause of papilledema in the OSAHS group, which would be secondary to a cerebral vasodilation phenomenon. It seems that frequent changes in the oxygenation of OSAHS patients would induce vascular problems in cerebral autoregulation. This fact might imply changes in intracranial volume due to vasodilation and increased brain water content [7]. Likewise, Lee et al. found an improvement of idiopathic intracranial hypertension when nocturnal oxygenation treatment was established [8]. O’Donoghue et al. [9] reported a 4 % decrease in total brain volume following treatment with positive pressure nocturnal oxygen in patients with OSAHS. The perpetuation of nerve fiber layer swelling could lead to a nerve fiber loss, with the consequent thinning of RNFL as the disease progresses. This would be consistent with the nasal peripapillary RNFL nasal thinning observed in severe OSAHS group. Future studies comparing the RNFL thickness before and after treatment of sleep apnea might add more information at this point.
A major limitation of the present study is the relatively small sample size, mainly when OSAHS patients were divided into two types according to their severity. Furthermore, a referral bias could be present because patients were referred for sleep disorder and, therefore, they might not be representative of the entire OSAHS population, mostly undiagnosed. In fact, we were not able to determine the exact duration of the respiratory disease. It is possible that the greater the severity and longer duration of the hypoxia, the greater the RNFL alterations.
In conclusion, these findings suggest that peripapillary RNFL thickness and optic disc area might be used as a biomarker to early diagnosis and classification of OSAHS patients. Further studies would be necessary to determine the usefulness of these OCT measurements in this disorder.
References
Guilleminault C (1994) Clinical features and evaluation of obstructive sleep apnea syndrome. In: Kryger MH, Roth T, Dement WC (eds) Principles and practice of sleep medicine. WB Saunders, Philadelphia, pp 667–677
Hatipoğlu U, Rubinstein I (2003) Inflammation and obstructive sleep apnea syndrome pathogenesis: a working hypothesis. Respiration 70:665–671
Leroux les Jardins G, Glacet-Bernard A, Lasry S, Housset B, Coscas G, Soubrane G (2009) Retinal vein occlusion and obstructive sleep apnea syndrome. J Fr Ophtalmol 32:420–424
Kloos P, Laube I, Thoelen A (2008) Obstructive sleep apnea in patients with central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol 246:1225–1228
Jain AK, Kaines A, Schwartz S (2010) Bilateral central serous chorioretinopathy resolving rapidly with treatment for obstructive sleep apnea. Graefes Arch Clin Exp Ophthalmol 248:1037–1039
Purvin VA, Kawasaki A, Yee RD (2000) Papilledema and obstructive sleep apnea syndrome. Arch Ophthalmol 118:1626–1630
Franklin KA (2002) Cerebral haemodynamics in obstructive sleep apnoea and Cheyne–Stokes respiration. Sleep Med Rev 6:429–441
Lee AG, Golnik K, Kardon R, Wall M, Eggenberger E, Yedavally S (2002) Sleep apnea and intracranial hypertension in men. Ophthalmology 109:482–485
O’Donoghue FJ, Briellmann RS, Rochford PD, Abbott DF, Pell GS, Chan CH, Tarquinio N, Jackson GD, Pierce RJ (2005) Cerebral structural changes in severe obstructive sleep apnea. Am J Respir Crit Care Med 171:1185–1190
Hayreh SS, Zimmerman MB, Podhajsky P, Alward WL (1994) Nocturnal arterial hypotension and its role in optic nerve head and ocular ischemic disorders. Am J Ophthalmol 117:603–624
Mojon DS, Hess CW, Goldblum D, Fleischhauer J, Koerner F, Bassetti C, Mathis J (1999) High prevalence of glaucoma in patients with sleep apnea syndrome. Ophthalmology 106:1009–1012
Mojon DS, Hess CW, Goldblum D, Böhnke M, Körner F, Mathis J (2000) Primary open-angle glaucoma is associated with sleep apnea syndrome. Ophthalmologica 214:115–118
Bendel RE, Kaplan J, Heckman M, Fredrickson PA, Lin SC (2008) Prevalence of glaucoma in patients with obstructive sleep apnoea—a cross-sectional case-series. Eye (Lond) 22:1105–1109
Faridi O, Park SC, Liebmann JM, Ritch R (2012) Glaucoma and obstructive sleep apnoea syndrome. Clin Exp Ophthalmol 40:408–419
Kargi SH, Altin R, Koksal M, Kart L, Cinar F, Ugurbas SH, Ayoglu F (2005) Retinal nerve fibre layer measurements are reduced in patients with obstructive sleep apnoea syndrome. Eye (Lond) 19:575–579
Macey PM, Henderson LA, Macey KE, Alger JR, Frysinger RC, Woo MA, Harper RK, Yan-Go FL, Harper RM (2002) Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med 166:1382–1387
Kumar R, Birrer BV, Macey PM, Woo MA, Gupta RK, Yan-Go FL, Harper RM (2008) Reduced mammillary body volume in patients with obstructive sleep apnea. Neurosci Lett 438:330–334
Jaffe G-J, Caprioli J (2004) Optical coherence tomography to detect and manage retinal disease and glaucoma. Am J Ophthalmol 137:156–169
Hassenstein A, Spital G, Scholz F, Henschel A, Richard G, Pauleikhoff D (2009) Optical coherence tomography for macula diagnostics. Review of methods and standardized application concentrating on diagnostic and therapy control of age-related macula degeneration. Ophthalmologe 106:116–126
Lamirel C, Newman N, Biousse V (2009) The use of optical coherence tomography in neurology. Rev Neurol Dis 6:E105–E120
Sergott RC, Frohman E, Glanzman R, Al-Sabbagh A (2007) The role of optical coherence tomography in multiple sclerosis: expert panel consensus. J Neurol Sci 263:3–14
Thrower BW (2007) Clinically isolated syndromes: predicting and delaying multiple sclerosis. Neurology 68:S12–S15
Parisi V, Restuccia R, Fattapposta F, Mina C, Bucci MG, Pierelli F (2001) Morphological and functional retinal impairment in Alzheimer’s disease patients. Clin Neurophysiol 112:1860–1867
Berisha F, Feke GT, Trempe CL, McMeel JW, Schepens CL (2007) Retinal abnormalities in early Alzheimer’s disease. Invest Ophthalmol Vis Sci 48:2285–2289
Inzelberg R, Ramirez JA, Nisipeanu P, Ophir A (2004) Retinal nerve fiber layer thinning in Parkinson disease. Vision Res 44:2793–2797
Hajee ME, March WF, Lazzaro DR, Wolintz AH, Shrier EM, Glazman S, Bodis-Wollner IG (2009) Inner retinal layer thinning in Parkinson disease. Arch Ophthalmol 127:737–741
Ascaso FJ, Cabezón L, Quintanilla MA, Gutiérrez L, López-Antón R, Cristóbal JA, Lobo A (2010) Retinal nerve fiber layer thickness measured by optical coherence tomography in patients with schizophrenia: a short report. Eur J Psychiatr 24:227–235
Lin PW, Friedman M, Lin HC, Chang HW, Pulver TM, Chin CH (2011) Decreased retinal nerve fiber layer thickness in patients with obstructive sleep apnea/hypopnea syndrome. Graefes Arch Clin Exp Ophthalmol 249:585–593
Rechtschafen A, Kales A (1968) A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. UCLA Brain Information Service, Brain Research Institute, Los Angeles
Villar I, Izuel M, Carrizo S, Vicente E, Marin JM (2009) Medication adherence and persistence in severe obstructive sleep apnea. Sleep 32:1–6
Shrier EM, Adam CR, Spund B, Glazman S, Bodis-Wollner I (2012) Interocular asymmetry of foveal thickness in Parkinson disease. J Ophthalmol 2012:728457
Ederer F (1973) Shall we count numbers of eyes or numbers of subjects? Arch Ophthalmol 89:1–2
Bowd C, Zangwill LM, Blumenthal EZ, Vasile C, Boehm AG, Gokhale PA, Mohammadi K, Amini P, Sankary TM, Weinreb RN (2002) Imaging of the optic disc and retinal nerve fiber layer: effects of age, optic disc area, refractive error and gender. J Opt Soc Am A Opt Image Sci Vis 19:197–207
Marin JM, Carrizo SJ, Vicente E, Agusti AG (2005) Long-term cardiovascular outcomes in men with obstructive sleep apnoea–hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 365:1046–1053
Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, O’Connor GT, Boland LL, Schwartz JE, Samet JM (2001) Sleep-disordered breathing and cardiovascular disease: cross sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med 163:19–25
Rudnicka A, Pływaczewski R, Jończak L, Górecka D, Sliwiński P (2010) Prevalence of stroke in patients with obstructive sleep apnoea. Pneumonol Alergol Pol 78:121–125
Tsang CS, Chong SL, Ho CK, Li MF (2006) Moderate to severe obstructive sleep apnoea in patients is associated with a higher incidence of visual field defect. Eye (Lond) 20:38–42
Sebastian RT, Johns S, Gibson RA (2006) Treating obstructive sleep apnoea syndrome: does it improve visual field changes? Eye (Lond) 20:118–120
Sommer A, Katz J, Quigley HA, Miller NR, Robin AL, Richter RC, Witt KA (1991) Clinically detectable nerve fiber layer atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol 109:77–83
Gutiérrez-Díaz E, Pérez-Rico C, de Atauri MJ, Mencía-Gutiérrez E, Blanco R (2012) Evaluation of the visual function in obstructive sleep apnea syndrome patients and normal-tension glaucoma by means of the multifocal visual evoked potentials. Graefes Arch Clin Exp Ophthalmol 250:1681–1688
Kergoat H, Hérard ME, Lemay M (2006) RGC sensitivity to mild systemic hypoxia. Invest Ophthalmol Vis Sci 47:5423–5427
Sohn S, Kim EY, Gwag BJ (1998) Glutamate neurotoxicity in mouse cortical neurons: atypical necrosis with DNA ladders and chromatin condensation. Neurosci Lett 240:1–4
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors confirm that they were fully involved in the study and preparation of the manuscript, and that the material within has not been and will not be submitted for publication elsewhere. The authors have full control of all primary data, and they agree to allow Graefe's Archive for Clinical and Experimental Ophthalmology to review their data upon request. The authors have no financial interests in any aspect of this study.
Rights and permissions
About this article
Cite this article
Casas, P., Ascaso, F.J., Vicente, E. et al. Retinal and optic nerve evaluation by optical coherence tomography in adults with obstructive sleep apnea–hypopnea syndrome (OSAHS). Graefes Arch Clin Exp Ophthalmol 251, 1625–1634 (2013). https://doi.org/10.1007/s00417-013-2268-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-013-2268-9