Abstract
Background
This study was designed to objectively evaluate visual function and the causal relationship between sleep apnea and optic nerve dysfunction in patients with obstructive sleep apnea syndrome (OSAS) with or without diagnosis of normal-tension glaucoma (NTG) using multifocal visual evoked potentials (mfVEP).
Methods
This observational, cross-sectional study assessed 20 patients with recently diagnosed OSAS with or without NTG. Diagnosis of sleep apnea was based on overnight polysomnography (ApnoeScreen). All participants underwent a complete physical and ophthalmologic examination. MfVEP recordings obtained using VERIS software (Electro-Diagnostic Imaging, San Mateo, USA), SITA-standard 30-2 automated perimetry (Humphrey Visual Field Analyzer II) and optic coherence tomography (Topcon 3D OCT-1000) exams were performed to evaluate the changes.
Results
Abnormal mfVEP amplitudes defects (interocular and monocular probability analysis) were found in 40% of the eyes in the non-glaucoma group and in 90% of eyes of the NTG patients. As well, delayed mfVEP latencies (interocular and monocular probability analysis) were seen in 30 and 60% of the eyes of the non-glaucoma and NTG groups, respectively. The average RNFL (retinal nerve fiber layer) thickness was significantly reduced in the NTG eyes compared to the control database and the non-glaucoma eyes. On the Humphrey Visual Field total deviation analysis, all the NTG eyes showed significant clusters of abnormal points but none was detected in the non-glaucoma group eyes. However, the mfVEP amplitude and latency did not show any significant correlation with the standard perimetry and OCT variables, because the mfVEP technique was able to detect far more early visual defects in these patients. Systolic blood pressure, sleep efficiency, arousal index, mean and minimum arterial oxygen saturation (SaO2), time SaO2 < 90%, oxyhemoglobin desaturation index, number of central and mixed apneas and apnea-hipopneas index were shown to be significantly correlated with mfVEP amplitude and latency.
Conclusions
A significant incidence of subclinical optic nerve involvement, not detected with other structural and psychophysics diagnostic techniques was seen by means of the mfVEP. In this sense, the mfVEP may be a useful diagnostic tool in the clinic for early diagnosis and monitoring of optic nerve function abnormalities in patients with OSAS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea syndrome (OSAS) is characterized by recurrent partial or complete upper airway obstruction during sleep, which is generally terminated by arousal and increased muscle tone. Obstructive respiratory disturbances often lead to severe hypoxemia and consequent increases in vascular resistance which, in turn, may compromise optic nerve head perfusion and oxygenation [1–3], as well as vascular dysregulation [4].
Both mechanical and vascular factors have a function in optic nerve pathology reported with OSAS, such us glaucoma, anterior ischemic optic neuropathy (AION), and disc swelling. Vascular compromise can occur because of recurrent episodes of apnea with reductions in arterial oxygen saturations (SaO2), intermittent elevations in intracranial pressure, and systemic hypertension [5]. Mechanical factors may include the positive association between obesity, rather frequent in patients with OSAS and intraocular pressure by causing excessive intraorbital adipose tissue, increased blood viscosity, increased episcleral venous pressure, and decreased aqueous outflow [6].
OSAS has been associated with a variety of ocular diseases, including floppy eyelid syndrome, primary open-angle glaucoma (POAG) and normal-tension glaucoma (NTG), AION, and papilledema. It has been stressed that OSAS may be a predisposing condition for AION [7]. A high incidence (14.6–47.6%) of POAG has been reported in patients with sleep-disordered breathing [8, 9]. Similarly, the prevalence of NTG in patients with OSAS has also been found to be higher than in the general population [4, 10]. Mojon et al. [11] suggested that age may also influence the prevalence of OSAS in NTG, being more prevalent in older people, as well.
This first study was designed to objectively evaluate the visual function and the causal relationship between sleep apnea, hypoxia, and optic nerve dysfunction in patients with OSAS with or without diagnosis of NTG using multifocal visual evoked potentials (mfVEP). To our knowledge, this is the first study to evaluate the optic nerve function in patients with sleep apnea and NTG by using the mfVEP.
Patients and methods
This observational, cross-sectional study included 20 patients with OSAS recently diagnosed with (n = 10) or without NTG (n = 10), which were consecutively recruited from a total of 118 patients referred to the glaucoma department for further evaluation. A total of 14 patients were diagnosed with glaucoma, of which ten had NTG. Patients, who had a history suggestive of antiglaucomatous drug usage, chronic uveitis, family glaucoma, previous optic neuropathy or ocular trauma, and eye surgery or laser treatment, were excluded. The study was conducted in accordance with the Declaration of Helsinki for biomedical research and was approved by the Institutional Review Boards of University Hospital Doce de Octubre and University of Alcalá. All participants provided informed consent.
All patients had a medical history and complete physical examination, blood analysis, pulmonary function test, and gasometry. Diagnosis of OSAS was based on overnight polysomnography (ApnoeScreen®, Erich Jaeger GMBH & CoKg, Germany). OSAS severity was classified using the apnea-hipopneas index (AHI), defined as the number of apnea and hypopnea per hour of total sleep time. OSAS was considered mild if the AHI was 5–15 events/h, moderate if 15–30 events/h, and severe if ≥30 events/h. Other data investigated were: sleep efficiency, arousal index, mean and minimum SaO2, time with SaO2 under 90% (time SaO2 < 90%), oxyhemoglobin desaturation index (ODI), and number of central and mixed apneas. For this study, the normal control subjects were selected from those patients in our clinic that did not meet any OSAS diagnostic criteria.
All participants underwent a complete ophthalmologic examination that included: visual acuity (VA), pupillary reflexes, slit-lamp biomicroscopy, applanation tonometry, ultrasonic pachymetry, gonioscopy, and ophthalmoscopy. SITA-standard 30-2 automated perimetry was performed (Humphrey Visual Field Analyzer II; Carl Zeiss Meditec, Inc., Dublin, CA). All subjects had reliable visual fields with fewer than 15% fixation losses, false positives, and false negatives. Optic coherence tomography (OCT) was obtained with Topcon 3D OCT-1000 (Topcon Inc, Paramus, NJ, USA), employing retinal nerve fibber layer (RNFL) peripapillary map with an interpolated circle scan of 3.4 mm diameter around the optic disc. Patients were excluded if they had a refractive error exceeding 5 diopters equivalent sphere or 3 diopters of astigmatism, or a media opacity such as cataract that prevented OCT examination. We only used images with a quality score over 60 according to the manufacturer’s recommendations.
Diagnosis of NTG was established using standardized criteria: a cup-to-disc ratio over 0.5 or difference between two eyes >0.2 with thinning of the neuroretinal rim, glaucomatous visual field loss, open iridocorneal angle and intraocular pressure readings <21 mmHg without treatment [12]. The eye with worse mean deviation in the Humphrey visual field (HVF) was selected for analysis.
All control subjects had to meet the following ophthalmological criteria for inclusion in the study: no intraocular pressure elevation over 21 mmHg; no evidence of glaucomatous optic nerve appearance, no disc hemorrhages, and a cup-to-disc ratio less than 0.5; normal gonioscopic examination and, normal visual field test results.
Multifocal visual evoked potential recordings and analysis
MfVEP recordings were obtained using VERIS software (Electro-Diagnostic Imaging, San Mateo, CA, USA). The stimulus was a scaled dartboard with a diameter of 44.5°, contained 60 sectors, each with 16 checks alternating, eight white (200 cd/m2) and eight black (<3 cd/m2). The sectors were cortically scaled with eccentricity to stimulate approximately equal areas of the visual cortex [13]. The dartboard pattern reversed according to a pseudorandom m-sequence at a frame rate of 75 Hz [14].
Three channels of continuous VEP recordings were obtained with gold cup electrodes. For the midline channel, the electrodes were placed 4 cm above the inion (active), at the inion (reference), and on the forehead (ground). For the other two channels, the same ground and reference electrodes were used, but the active electrodes were placed 1 cm above and 4 cm lateral to the inion on either side. The records were amplified with the high- and low-frequency cut-offs set at 3 and 100 Hz, respectively, (half amplitude preamplifier P511J; Grass Instruments, Rockland, MA), and sampled at 1,200 Hz (every 0.83 ms). The impedance was <5 K for all subjects. In a single session, two 7-minute recordings were obtained from monocular stimulation of each eye and were averaged for analysis. Second-order kernel best-channel responses were then extracted (VERIS 5.0 software; EDI) [15, 16]. This averaging, as well as all other analyses, was computed with custom-made programs written in commercial software (Matlab; MathWorks Inc., Natick, MA) [17].
Responses’ amplitudes were calculated by obtaining root mean square (RMS) of the amplitude for each mfVEP response over time intervals from 45 to 150 ms. Signal-to-noise ratios were calculated for each response by dividing the RMS of the signal window by the average of the 60 RMS values of the noise-only window. Each of these values was compared to values for normative group subjects [18] and monocular probability plot were derived. Interocular amplitude differences for each patient were also calculated by taking the logarithm of the interocular ratio at each location [17] and interocular probability plot were derived. The amplitude probability plot was color-coded with saturated red squares (left eye), saturated blue squares (right eye), with a difference significant being determined at p < 0.01 and for desaturated colors at p < 0.05. Black squares indicate no significant differences and grey squares indicate a signal too small to be compared.
Monocular and interocular latencies were measured as the temporal shift producing the best cross-correlation value between the corresponding responses of the patient’s eye and a template based on control eyes (monocular analysis) or between the corresponding responses from two eyes (interocular analysis).
To evaluate the mfVEP and HVF total deviation results, we analyzed cluster defects. A defective cluster had two or more contiguous points at p < 0.01, or three or more contiguous points at p < 0.05 with at least one point at p < 0.01 [19].
Statistical analysis
SPSS 15 (SPSS Inc, Chicago, IL, USA) was used for statistical analysis. Differences in proportions were evaluated by Chi-square test or Fisher’s exact test, as concerns. Kruskal–Wallis test was used to compare quantitative variables among three or more groups followed by the Mann–Whitney U test using the Bonferroni correction for multiple comparisons. The association between two quantitative variables was evaluated by Spearman’s correlation coefficient. P values below 0.05 or below 0.025, when Bonferroni correction was used, were considered statistically significant.
Results
In Tables 1 and 2 are summarized the systemic characteristics and sleep study data of the groups. According to the AHI, all our patients were suffering from severe sleep apnea syndrome. No significant differences between both groups were detected on the main clinical systemic and sleep parameters. The most relevant clinical ophthalmological and standard automated perimetry (SAP) data are shown in Table 3. VA was significantly lower in the NTG group compared to the control group, but not to the non-glaucoma group (Kruskal–Wallis test, p = 0.007). No significant relative afferent pupillary defects were detected in any group and there were no significant differences between groups in regard to the intraocular pressure. Mean deviation and pattern standard deviation HVF were significantly altered in the eyes of patients with NTG, compared to the non-glaucoma group eyes (Mann–Whitney U test, p < 0.001 and p = 0.001, respectively). On the HVF total deviation analysis, all the NTG eyes showed significant clusters of abnormal points (see Methods section); meanwhile, none was detected in the non-glaucoma group eyes.
In Table 4, OCT data are summarized. RNFL thickness measurements (total average and superior quadrant) were significantly reduced in the NTG eyes than in the control database and the non-glaucoma eyes (Kruskal–Wallis test, p = 0.001 and p = 0.002, respectively). The inferior and nasal RFNL thickness was significantly reduced in the NTG eyes compared to the control database (Mann–Whitney U test, p = 0.002 and p = 0.017, respectively). Abnormal visual field defects (cluster points) on the HVF total deviation plot were significantly associated with reduced average RNFL thickness (Mann–Whitney U test, p < 0.001), as well as in the superior and inferior RNFL quadrants (Mann–Whitney U test, p < 0.001) of NTG eyes.
In Table 5, the mfVEP recordings summary is shown. When the mfVEP amplitude responses were analyzed combining the interocular and monocular probability analysis, abnormal cluster defects were found in 40% of the eyes in the non-glaucoma group and in 90% of eyes of the NTG patients (Fisher’s exact test, p = 0.019). MfVEP latency analysis (interocular and monocular) showed latency delays in 30% of the non-glaucoma group eyes and in 60% of eyes of the NTG group. In total, 60% of eyes in the non-glaucoma group and all NTG eyes showed amplitude and/or latency defects in the mfVEP. We were not able to demonstrate any significant correlation between the mfVEP amplitude and latency and the SAP and OCT variables, probably due that the mfVEP technique was able to detect far more early visual defects than those being detected by those psychophysic and structural diagnostic techniques.
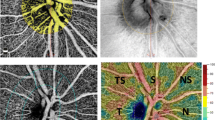
An example of mfVEP probability plots, OCT, and HVF total deviation results of a non-glaucoma patient are shown in Fig. 1: in the mfVEP temporal hemifield (monocular latency probability plot) a cluster of abnormal sectors are seen on the left eye correlating well with a RNFL thinning (OCT). Meanwhile, the HVF total deviation plot did not show any changes. On the other side, in Fig. 2, the recordings from a NTG patient are shown. Here, clusters of abnormal sectors are seen on both the mfVEP interocular amplitude and the monocular latency analysis probability plots of the left eye while the OCT shows a generalized RNFL thinning and a large scotoma is also depicted in SAP.
Multifocal visual evoked potentials (mfVEP) probability plots, optical coherence tomography (OCT) and Humphrey visual field (HVF) total deviation from a non-glaucoma sleep apnea patient. Clusters of abnormal sectors are seen in the temporal hemifield of the mfVEP monocular latency probability plot (left eye, LE). The OCT thickness map shows a retinal nerve fibber layer thinning in the temporal quadrant of the same eye, meanwhile the HVF total deviation plot is normal
Multifocal visual evoked potentials (mfVEP) probability plots, optical coherence tomography (OCT), and Humphrey visual field (HVF) total deviation plots from a normal-tension glaucoma sleep apnea patient. In the mfVEP, abnormal clusters are shown on both the interocular amplitude analysis probability plot and the monocular latency analysis probability plot from the left eye (LE). The OCT showed a thinning of retinal nerve fibber layer in the superior, nasal, and inferior quadrants of the left eye and the HVF total deviation plot shows a significant scotoma
Table 6 shows the relationships between the mfVEP variables and the systemic and sleep data. In the non-glaucoma group, the mfVEP amplitudes and latencies were statistically associated to systolic blood pressure (Mann–Whitney U test, p = 0.04), sleep efficiency (Mann–Whitney U test, p = 0.025), mean SaO2 (Mann–Whitney U test, p = 0.027), time SaO2 < 90% (Mann–Whitney U test, p = 0.039), and the number of mixed apneas (Mann–Whitney U test, p = 0.034). On the other side, in the NTG group, there were significant associations with a larger number of systemic/ sleep variables (see table) and both, mfVEP amplitudes and latencies, were significantly associated with AHI (Mann–Whitney U test, p = 0.001 and p = 0.037, respectively).
Additionally, in the non-glaucoma group, statistical correlations were found between the average RNFL thickness (OCT) and both the sleep efficiency (Spearman r = 0.766, p = 0.027) and the mean SaO2 (Spearman r = 0.683, p = 0.03). Meanwhile, in the NTG group, the average RNFL thickness statistically correlated with most systemic/sleep data, especially the body mass index (Spearman r = –0.503, p = 0.005), systolic blood pressure (Spearman r = –0.592, p = 0.001), AHI (Spearman r = –0.638, p < 0.001), arousal index (Spearman r = –0.498, p = 0.008), sleep efficiency (Spearman r = 0.634, p < 0.001), mean SaO2 (Spearman r = 0.646, p < 0.001), time SaO2 < 90% (Spearman r = –0.564, p = 0.002), number of mixed apneas (Spearman r = –0.436, p = 0.016) and central apneas (Spearman r = –0.518, p = 0.003).
Discussion
To our knowledge, this is the first study looking at the optic nerve function in patients with sleep apnea with or without NTG by means of the mfVEP. Our results suggested that sleep disturbances may compromise the normal function of the retinal ganglion cells, due probably to the recurrent episodes of reduction in SaO2 and vascular dysregulation present in this condition.
Sixty percent of the eyes in the non-glaucoma group and 100% of eyes in the NTG group demonstrated significant changes in the mfVEPs, suggesting a significant incidence of subclinical optic nerve involvement, not detected with other structural and psychophysics diagnostic techniques. In this sense, several studies have reported that the mfVEP can detect visual field changes not seen with the SAP in different optic nerve pathologies [20–24]. When the interocular and monocular analyses were combined, mfVEP amplitude defects were shown to be present in 40% and 90% of the eyes in the non-glaucoma group and NTG patients group, respectively. Delayed mfVEP latencies were seen in 30% and 60% of the eyes of the non-glaucoma and NTG groups, respectively. It was interesting to point out that the latency delays seen in all these patients were in the same range as those described in eyes with AION. Eyes with AION had median monocular and interocular latencies of 2.2 and 2.1 ms; meanwhile in glaucoma eyes, latencies are shown to be in a normal range, with average monocular and interocular latencies of 3.1 and 1.3 ms, respectively [25].
MfVEP is an effective technique for objective assessment of the visual field in glaucoma and other optic nerve diseases [15, 20–22, 26, 27]. Although most studies have demonstrated close agreement between mfVEP and SAP, it has been shown that mfVEP abnormalities may appear in regions of the visual field that have normal sensitivity on SAP [15, 19, 23], and in glaucoma suspect eyes with completely normal SAP visual fields [20–24]. In this sense, it has been considered that such mfVEP defects may represent greater sensitivity of the mfVEP to detect early functional loss.
In the NTG group, systolic blood pressure, sleep efficiency, arousal index, mean and minimum SaO2, time SaO2 < 90%, ODI and AHI were shown to be significantly correlated with the mfVEP amplitude and latency changes and, the body mass index, the number of mixed and central apneas and FEV1_FCV had a significant association with either the amplitude or the latencies. On the other side, in the non-glaucoma group, significant associations were present between the mfVEP amplitude and the systolic blood pressure and mean SaO2 and between the mfVEP latency and sleep efficiency, time SaO2 < 90%, and number of mixed apneas. These mfVEP changes seen in non-glaucoma patients and the significant correlations found between the mfVEP and the systemic and sleep study data point to the presence of significant alterations in the perfusion pressure of the optic nerve in this condition. In this regard, it has been described that recurrent hypoxia during apnea may trigger either a direct anoxic damage, or an indirect effect on the optic nerve head blood flow [28]. Additionally, other studies have shown overnight repetitive hypoxemia and reoxygenation in these patients [29, 30]. All these clinical observations are supported by significant high serum inflammatory cytokine levels in these patients [31], a higher level of oxidative stress indicators [32], and adhesion factors, such the intercellular adhesion molecule 1 and vascular cell adhesion molecule [33]. All these studies point out that vascular dysregulation of the optic nerve combined with nocturnal systemic hypotension may trigger ganglion cell stress and neuronal death.
Preliminary studies have shown a significant reduction of the RNFL thickness in sleep apnea patients, correlating the RNFL thickness changes to OSAS severity, suggesting that the decreased ocular perfusion secondary to hypoxia and vasospasm may lead to RNFL thinning, which may precede clinically detectable glaucoma [34, 35]. Our results showed a significant loss of RNFL thickness in the eyes of sleep apnea patients, suggesting an early loss of ganglion cell axons, data that support the findings of the mfVEP. An important question in future studies is to elucidate if we are talking about two different stages or clinical manifestations of a similar etiopathogenic optic nerve disease in patients with OSAS.
A major limitation of this first study was a relatively small sample size of the evaluated patients and lack of longitudinal follow-up. Males also predominated in our study group, probably due to the fact that OSAS is much more common among males than females. Further studies for age-matched and gender-matched subgroups with different severities of sleep apnea may be needed and the presence and progression of NTG should be investigated particularly in the long-term follow-up by means of the mfVEP and other structural diagnostic techniques.
References
Arimoto T, Toratani A, Ito K, Iwasaki Y, Nakamura T, Nakagawa M (1995) Relationship between sleep stage and blood pressure variability during apnea in patients with sleep apnea syndrome. Nihon Kyobu Shikkan Gakkai Zasshi 33:917–923
Bucci FA, Krohel GB (1988) Optic nerve swelling secondary to the obstructive sleep apnea syndrome. Am J Ophthalmol 105:428–430
Culbertson WW, Ostler HB (1981) The floppy eyelid syndrome. Am J Ophthalmol 92:568–575
Sergi M, Salerno DE, Rizzi M, Blini M, Andreoli A, Messenio D, Pecis M, Bertoni G (2007) Prevalence of normal tension glaucoma in obstructive sleep apnea syndrome patients. J Glaucoma 16:42–46
Stradling JR, Davies RJO (2004) Sleep 1: obstructive sleep apnoea/hypopnoea syndrome: definitions, epidemiology, and natural history. Thorax 59:73–78
Cheung N, Wong TY (2007) Obesity and eye diseases. Surv Ophthalmol 52:180–195
Hayreh SS, Zimmerman MB, Podhajsky P, Alward WL (1994) Nocturnal arterial hypotension and its role in optic nerve head and ocular ischemic disorders. Am J Ophthalmol 117:603–624
Onen SH, Mouriaux F, Berramdane L, Dascotte JC, Kulik JF, Rouland JF (2000) High prevalence of sleep-disordered breathing in patients with primary open-angle glaucoma. Acta Ophthalmol Scand 78:638–641
Geyer O, Cohen N, Segev E, Rath EZ, Melamud L, Peled R, Lavie P (2003) The prevalence of glaucoma in patients with sleep apnea syndrome: same as in the general population. Am J Ophthalmol 136:1093–1096
Marcus DM, Costarides AP, Gokhale P, Papastergiou G, Miller JJ, Johnson MH, Chaudhary BA (2001) Sleep disorders: a risk factor for normal-tension glaucoma? J Glaucoma 10:177–183
Mojon DS, Hess CW, Goldblum D, Boehnke M, Koerner F, Gugger M, Bassetti C, Mathis J (2002) Normal-tension glaucoma is associated with sleep apnea syndrome. Ophthalmologica 216:180–184
Karakucuk S, Goktas S, Aksu M, Erdogan N, Demirci S, Oner A, Arda H, Gumus K (2008) Ocular blood flow in patients with obstructive sleep apnea syndrome (OSAS). Graefes Arch Clin Exp Ophthalmol 246:129–134
Baeseler HA, Sutter EE, Klein SA, Carney T (1994) The topography of visual evoked response properties across the visual field. Electroencephalogr Clin Neurophysiol 90:65–68
Sutter EE (2001) Imaging visual function with the multifocal m-sequence technique. Vis Res 41:1241–1255
Hood DC, Greenstein VC (2003) Multifocal VEP and ganglion cell damage: applications and limitations for the study of glaucoma. Prog Retin Eye Res 22:201–251
Hood DC, Zhang X, Hong JE, Chen CS (2002) Quantifying the benefits of additional channels of multifocal VEP recording. Doc Ophthalmol 104:303–320
Hood DC, Greenstein VC, Odel JG, Zhang X, Ritch R, Liebmann JM, Hong JE, Chen CS, Thienprasiddhi P (2002) Visual field defects and multifocal visual evoked potentials: evidence of a linear relationship. Arch Ophthalmol 120:1672–1681
Fortune B, Zhang X, Hood DC, Demirel S, Johnson CA (2004) Normative ranges and specificity of the multifocal VEP. Doc Ophthalmol 109:87–100
Hood DC, Zhang X, Rodarte C, Yang EB, Ohri N, Fortune B, Johnson CA (2004) Determining abnormal interocular latencies of multifocal visual evoked potentials. Doc Ophthalmol 109:177–187
Graham SL, Klistorner AI, Grigg JR, Billson FA (2000) Objective VEP perimetry in glaucoma: asymmetry analysis to identify early deficits. J Glaucoma 9:10–19
Graham SL, Klistorner AI, Goldberg I (2005) Clinical application of objective perimetry using multifocal visual evoked potentials in glaucoma practice. Arch Ophthalmol 123:729–739
Fortune B, Demirel S, Zhang X, Hood DC, Patterson E, Jamil A, Mansberger SL, Cioffi GA, Johnson CA (2007) Comparing multifocal VEP and standard automated perimetry in high-risk ocular hypertension and early glaucoma. Invest Ophthalmol Vis Sci 48:1173–1180
Thienprasiddhi P, Greenstein VC, Chu DH, Xu L, Liebmann JM, Ritch R, Hood DC (2006) Detecting early functional damage in glaucoma suspect and ocular hypertensive patients with the multifocal VEP technique. J Glaucoma 15:321–327
Goldberg I, Graham SL, Klistorner AI (2002) Multifocal objective perimetry in the detection of glaucomatous field loss. Am J Ophthalmol 133:29–39
Rodarte C, Hood DC, Yang EB, Grippo T, Greenstein VC, Liebmann JM, Ritch R (2006) The effects of glaucoma on the latency of the multifocal visual evoked potential. Br J Ophthalmol 90:1132–1136
Klistorner AI, Graham SL, Grigg JR, Billson FA (1998) Multifocal topographic visual evoked potential: improving objective detection of local visual field defects. Invest Ophthalmol Vis Sci 39:937–950
Hasegawa S, Abe H (2001) Mapping of glaucomatous visual field defects by multifocal VEPs. Invest Ophthalmol Vis Sci 42:3341–3348
Tsang CSL, Chong SL, Ho CK, Li MF (2006) Moderate to severe obstructive sleep apnoea patients is associated with a higher incidence of visual field defect. Eye 20:38–42
Palatini P, Penzo M, Racioppa A, Palatini P, Penzo M, Racioppa A (1992) Clinical relevance of nighttime blood pressure and of daytime blood pressure variability. Arch Intern Med 152:1855–1860
Shamsuzzaman AS, Gersh BJ, Somers VK (2003) Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA 290:1906–1914
Schulz R, Hummel C, Heinemann S, Seeger W, Grimminger F (2002) Serum levels of vascular endothelial growth factor are elevated in patients with obstructive sleep apnea and severe nighttime hypoxia. Am J Respir Crit Care Med 165:67–70
Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ (2003) 8-Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapy. Chest 124:1386–1392
Ohga E, Nagase T, Tomita T, Teramoto S, Matsuse T, Katayama H, Ouchi Y (1999) Increased levels of circulating ICAM-1, VCAM-1, and L-selectin in obstructive sleep apnea syndrome. J Appl Physiol 87:10–14
Kargi SH, Altin R, Koksal M, Kart L, Cinar F, Ugurbas SH, Ayoglu F (2005) Retinal nerve fibre layer measurements are reduced in patients with obstructive sleep apnoea syndrome. Eye 19:575–579
Lin PW, Friedman M, Lin HC, Chang HW, Wilson M, Lin MC (2011) Normal tension glaucoma in patients with obstructive sleep apnea/hypopnea syndrome. J Glaucoma 20:553–558
Acknowledgements
This study was supported by FIS PI11/00533, Fundación Lain Entralgo and FISCAM PI-2008/09.
Conflict of interest
No financial conflicts of interest exist.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gutiérrez-Díaz, E., Pérez-Rico, C., de Atauri, M.J. et al. Evaluation of the visual function in obstructive sleep apnea syndrome patients and normal-tension glaucoma by means of the multifocal visual evoked potentials. Graefes Arch Clin Exp Ophthalmol 250, 1681–1688 (2012). https://doi.org/10.1007/s00417-012-1982-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-012-1982-z