Abstract
Background
The mouse retina contains three kinds of basement membrane (BM) structures; the inner limiting membrane (ILM), Bruch’s membrane (BrM), and the BM surrounding the capillaries. We aimed to investigate possible variations of individual BM components and to detect effects caused by diabetes in three different diabetic mouse models.
Methods
After 4 and 6 months of diabetes (defined by blood glucose > 250 mg/dl), we analyzed by immunohistochemistry the laminin, collagen IV, and nidogen-1 and nidogen-2 protein composition of the BMs obtained from diabetic and non-diabetic Leptin-receptor deficient (db/db) mice and insulin receptor (IR)/insulin receptor substrate-1 (IRS-1) double heterozygous knockout mice. In addition, C57BL/6 J mice were rendered diabetic by intraperitoneal injections of streptozotocin (STZ).
Results
All analyzed BM proteins were detected in all of the three BMs with the exception of collagen IV, which was not detectable in the ILM of db/db mice and IR/IRS-1 mice. We present the first analysis of nidogen expression in diabetic BM. The staining patterns did not differ between the type-1 diabetic model (STZ) or the type-2 diabetic models (db/db and IR/IRS-1) and the wild-type controls, with only one exception: both the db/db mice and the IR/IRS-1 mice but not the STZ mice showed a decreased nidogen-1 immunoreactivity in the BrM after 4 months of diabetes, but not after 6 months.
Conclusions
The BMs in the three mouse strains differ with regard to protein immunoreactivity in the inner limiting membrane. Changes in BM composition may affect both the assembly and the function of the retinal BM. However, there are no marked differences in the BM composition between type-1 and type-2 diabetes. These results provide evidence for BM remodelling during diabetic retinopathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic retinopathy is a major cause of blindness in developed countries [1]. Vascular changes, including breakdown of the blood–retina barrier, thickening of the capillary basement membrane (BM), loss of pericytes, microaneurysms and neovascularization have been widely documented in diabetic rats, dogs, and cats [2, 3]. Mice have been less extensively studied as a model for diabetic retinopathy [4]. Capillary BM thickening has been shown to result from the increased production and decreased degradation of BM proteins [5, 6]. In rat, STZ-induced diabetes results in elevated serum levels of the BM components laminin [7], collagen IV [8], and perlecan [9], indicating that some of the newly synthesized proteins are not assembled into BMs, but released to the blood stream. The turnover of BM proteins in the eye is not known, and in general, information on turnover is sparse. The best studied example is the BM-associated collagen VII at the epidermal dermal junction. By use of fibroblast injection into a mouse strain hypomorphic for collagen VII, it was shown that collagen VII laid down by these fibroblasts remains in the tissue for over 100 days [10]. Half-lives may, however, vary between BM proteins. For example, nidogen-1 is very prone to proteolytic degradation [11] and may therefore have a rapid turnover.
Other studies have shown that retinal BMs of diabetic FVB mice contain increased amounts of collagen IV, laminin ß1 and γ1, and fibronectin mRNA, and that these alterations occur as early as 8 weeks following the onset of diabetes [12]. All BMs contain members of the laminin, nidogen, and collagen IV protein families, as well as the proteoglycan perlecan [13]. Bruch’s membrane is made up of heparan sulfate and chondroitin sulfate proteoglycans, collagens I, III, IV, V, and VI, and glycoproteins such as laminin and fibronectin [14–16].
The retinal expression and localization of laminin isoforms has been described in great detail both for rats [17] and humans [18]. Laminins are vital for the assembly of BMs, and interact with the collagen IV network via nidogen and other extracellcular matrix molecules. The expression of laminin chains is regulated both spatially and temporally, suggesting that the different laminin isoforms have distinct roles [18]. They influence cell differentiation, migration, and adhesion, as well as cellular phenotype and survival. In a diabetic condition, extracellular matrix proteins such as laminin and collagen IV can be modified by glycation, which may have a severe impact on cellular function. Changes in the composition and structure of the ECM upon the development of diabetes have already been demonstrated in peripheral nerves. [19].
Collagen IV is the major collagenous components of BMs, and serves as a part of the filtration system in capillaries and the glomeruli in the kidney. Six genetically distinct type collagen IV chains, α1 (IV) to α6 (IV), have been identified [20]. The different collagen molecules have distinct topological locations and functions. In recent years, many studies have determined the distribution of collagen IV isoforms in the neurosensory retina and in Bruch’s membrane [21] under physiological conditions.
Nidogen, also referred to as entactin, is another component of BMs. In mammals, two nidogen proteins have been identified and named nidogen-1 and nidogen-2 (or entactin-1 and entactin-2). Both are elongated molecules composed of three globular domains (G1, G2, and G3), with G1 and G2 connected by a flexible, protease-sensitive link, and G2 and G3 connected by a rod-like domain [22, 23]. Nidogen-2 is present in most BMs, but is not an as ubiquitous component of BMs as nidogen-1 [24]. Nidogen-1 has been shown to bind to collagen IV [25], and is crucial in linking the laminin and the collagen IV networks. All the domains of nidogen-1 are conserved in nidogen-2, which interacts with collagen I and IV as well as with perlecan [26]. The loss of nidogens does not cause basement membrane disruption [27, 28], but inactivating the genes in mouse causes disturbances in the assembly of many BMs, multiple organ malformations, and death of the animals shortly after birth [29]. This indicates the overall importance of the nidogens for basement membrane maintenance. The composition of retinal BMs of the aging mice has only been analyzed in more detail recently [30].
The metabolic pathways (polyol pathway, hexosamine pathway, DAG-PKC pathway, advanced glycation end-products, and oxidative stress) triggered by hyperglycemia during diabetic retinopathy not only affect basement membrane proteins, but it has also been shown that retinal neurodegeneration is present before any microcirculatory abnormalities can be detected in ophthalmoscopic examination [31, 32].
Most animal studies on diabetic retinopathy have focused on insulin-deficient models (type 1 diabetes) instead of models for insulin-resistance (type 2 diabetes) (for review, see [33]). We used three mouse models representing both types of diabetes:
-
1. The streptozotocin (STZ)-induced diabetic mouse serves as a model for type 1 diabetes, and has been the primary model for research into the pathogenesis of the vascular lesions of diabetic retinopathy. This model allows short-term measurements of vascular leakage, leukocyte endothelial interactions, and retinal cell damage.
-
2. IR/IRS-1 deficient mice are heterozygous for the insulin receptor (IR) and insulin receptor substrate 1 (IRS-1) genes, and provide a unique model for studying changes in the diabetic retina during the course of type 2 diabetes.
-
3. Leptin-receptor deficient model (db/db): these mice carry a mutation in the leptin receptor gene (db), and provide a model for obesity-induced type 2 diabetes. The db/db mice develop hyperinsulinemia and hyperglycemia at an early age, and are therefore well-suited for studying the diabetic retina.
We aimed to investigate the immunoreactivity and distribution of individual BM components (laminin, collagen IV, and nidogen-1 and nidogen-2) in these three different diabetic mouse models.
Material and methods
Mouse models
The animal experiments were done according to the “Principles of laboratory animal care” (NIH publication No. 85–23, revised 1985), the OPRR Public Health Service Policy on the Humane Care and Use of Laboratory Animals (revised 1986) and the U.S. Animal Welfare Act, as amended, as well as to the current version of the German Law on the Protection of Animals (Tierschutzgesetz).
Leptin-receptor (BKS.Cg-Dock 7 m+/+Lepr db/J) deficient mice (type 2 diabetes-like model)
The db gene encodes a G-to-T point mutation of the leptin receptor, leading to abnormal splicing and defective signaling of the adipocyte-derived hormone leptin [34]. The diabetic allele (db) is transmitted as an autosomal recessive trait. Males exhibiting blood glucose concentrations of more than 250 mg/dl were used for the experiments. The mice were obtained from The Jackson Laboratory (Jax Stock Number: 000642).
Insulin receptor (IR)/insulin receptor substrate-1 (IRS-1) double heterozygous knockout mice (type 2 diabetes-like model)
Inactivation of one allele of the insulin receptor and IRS-1 genes on a mixed genetic background resulted in the development of a type 2 diabetes-like disease in 30 to 50 % of the animals. In the meantime, these animals have been backcrossed for more than nine generations onto C57BL/6 J background. Up to 90 % of the male double heterozygous animals on the C57BL/6 J background develop type 2 diabetes-like disease [35]. Males exhibiting blood glucose concentrations of more than 250 mg/dl were considered diabetic. For genotyping the insulin receptor, the following primers in two separated reaction sets were used: InsR-Ex4s1015 5′-CTGATCATCAACATCCGAGG-3′ and InsR-Ex5a1063 5′-CCTCCAGCTCAGCTGCCAGG-3′ to detect a 1,645-bp fragment for the wildtype and InsR-Ex4s894 5′-GATGTGCACCCCATGTCTGG-3′ and InsR-Ex4a940 5′-GGATTTGGCAGACCTTAGGG-3′ to detect a 1,650-bp fragment for the KO-mice. For detecting IRS-1, the primers IRS-Ex1s797 5′-AGGAACAGCGTGAATTTTGGG-3′ and IRS-Ex1a1234 5′-CTTGCTCAGCCTCGCTATCC-3′ were used to detect a 437-bp fragment in the wildtype and a 1,095-bp fragment in the KO-mice. The mice were a kind gift of Jens Brüning, Institute for Genetics, University of Cologne (Germany).
Streptozotocin-induced pancreatic β-cell ablation (type 1 diabetes-like model)
Following 5 h of fasting, C57BL/6 male mice at an age of 2–3 months received 50 mg/kg body weight of streptozotocin (STZ) as an intraperitoneal injection for 5 consecutive days [36]. Control mice were treated with carrier solution (citrate buffer) alone. Animals exhibiting blood glucose concentrations of more than 250 mg/dl 1 week after the last STZ injection were considered diabetic. All confirmed diabetic mice showed classical signs of diabetes, i.e., polydipsia, polyuria, and a loss in body weight (15 %). The mice were obtained from Charles River, Sulzfeld, Germany.
All mice were about 12 weeks old at the beginning of the experiments.
Antibodies
Polyclonal antibody against collagen IV was obtained from Cedarlane Laboratories (Burlington, Ont., Canada). Polyclonal antibodies against the laminin γ1-chain, nidogen-1 and nidogen-2 were kindly provided by Neil Smyth and Alexander Kunze (Southampton, UK, and Cologne, Germany). Secondary antibodies coupled to Cy3-fluorochrom were obtained from Dianova GmbH, Hamburg, Germany.
Measurement of blood glucose
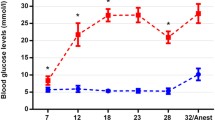
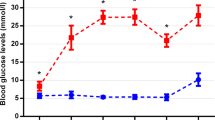
The blood glucose levels were measured in all three mouse strains at the beginning of the experiments, and 4 and 6 months later. In addition, the blood glucose levels of the STZ-treated mice were measured 1 week after injection, and non-responders were excluded. Blood glucose was measured using a blood glucometer (ascensia ELITE, Bayer, Leverkusen, Germany). Diabetes was defined by blood glucose >250 mg/dl.
Indirect immunofluorescence microscopy
To assess potential alterations in the distribution of BM proteins, immunofluorescent stainings were performed on retinal paraffin sections (diabetic and non-diabetic age-matched strain-specific wild-type controls) 4 and 6 months after the induction of diabetes.
Eyes were enucleated and immediately fixed in methacarn (60 % methanol, 30 % 1-1-1- trichoroethene, 10 % acetic acid) or in 4 % paraformaldehyde, dehydrated and embedded in paraffin (Richard-Allan Scientific, Kalamazoo, MI, USA). Sagittal sections were cut at 5 μm, deparaffinized, and hydrated. After treatment with 0.005 % trypsin in 0.1 % CaCl2 for 10 min at 37 °C, sections were blocked with 5 % BSA and 0.1 % Triton X-100 in 0.15 M NaCl, 50 mM Tris–HCl, pH 7.4 (TBS) for 1 h at RT. The primary antibody was incubated over night at 4 °C, followed by 1 h-incubation at RT with a fluorochrome-labeled secondary antibody. For negative controls, the primary antibodies were omitted. Tissue sections were examined with a Zeiss Axioplan2 fluorescence microscope and images taken by a Hammatsu ORCA ER camera with the Openlab program (Improvision Ltd.).
Statistics
At least three diabetic mice for each diabetic model and their respective controls were analyzed. Five randomly selected sections of the central retina were analyzed for each diabetic mouse model and controls. Quantification by scoring the fluorescence intensity between 0 and 3 was done blindly and independently by five persons not involved in the experiments.
Unpaired t-test or Mann–Whitney was used for the analysis of results (Prism v5.0, GraphPad Software, Inc., San Diego, CA, USA). Statistically significant difference was set at P < 0.05 or P < 0.005. Error bars represented the SEM of the data.
Results
In order to exclude possible strain-specific differences between the used diabetes models due to different genetic background, we included for each model the corresponding negative controls: non-diabetic BKS db/db mice and non-diabetic IR/IRS-1 mice as well as citrate-buffer-treated non-diabetic C57Bl/6 mice. The results of the scoring analysis after 4 months and 6 months of diabetes are displayed in Tables 1 and 2 respectively.
Laminin
The laminin γ1 chain is the most ubiquitous laminin chain in BMs, and so its distribution reflects the overall laminin expression. Therefore, the corresponding antibody gave a distinct staining of Bruch’s membrane (BrM), the capillary BMs (C) and the inner limiting membrane (ILM) when applied to 4- and 6-month-old diabetic and non-diabetic retinas of all three mouse strains (Tab. 1 and 2). The immunoreactivity of the capillary basement membranes and the BrM were similar in all three non-diabetic controls after 4 and 6 months, as was the immunoreactivity of the ILM after 6 months. However, after 4 months the immunoreactivity of the ILM in normal C57Bl/6-mice (non-diabetic control to STZ-mice) was three times higher than in the non-diabetic controls of the two other diabetes models (Table 1 and Fig. 1).
Laminin immunoreactivity in retinal cross-sections of diabetic mice after 4 months of diabetes. a, c, e Strain-specific wild-type controls. b, d, f Different diabetic mouse models. Between the cross-sections: quantification of laminin immunoreactivity in the inner limiting membrane (ILM), the retinal capillaries (C), and Bruch’s membrane (BrM) in wild-type control mice (open bars) and diabetic mice (grey bars). Only in diabetic IR/IRS-1 mice was a significantly stronger immunoreactivity in the ILM observed when compared with the wild-type controls (F) (** p < 0.005). The nuclei were counterstained with DAPI
After 4 months of diabetes, the immunoreactivity for laminin γ1 in the IR/IRS-1 mice was increased only in the ILM compared to their respective wild-type controls (Fig. 1c–d). The level of laminin 1 immunoreactivity in the membranes surrounding the capillaries and the ILM was similar between diabetic and non-diabetic retinal tissue. However, after 6 months of diabetes the immunoreactivity of laminin γ1 in the ILM was lower in the db/db and the STZ mice compared to wild-type controls (Table 2 and Fig. 2a–b and e–f). In diabetic IR/IRS-1 mice, the level of laminin 1 immunoreactivity in the BrM was increased after 6 months (Fig. 2c–d), but the immunoreactivity for laminin γ1 in the membranes surrounding the capillaries was still unchanged between diabetic and non-diabetic retinal tissue (Fig. 2c–d).
Laminin immunoreactivity in retinal cross-sections of diabetic mice after 6 months of diabetes. a, c, e Strain-specific wild-type controls. b, d, f Different diabetic mouse models. Between the cross-sections: quantification of laminin immunoreactivity in the inner limiting membrane (ILM), the retinal capillaries (C), and Bruch’s membrane (BrM) in wild-type control mice (open bars) and diabetic mice (grey bars). In diabetic db/db mice, a significantly weaker immunoreactivity in the ILM was observed when compared with the wild-type controls (* p < 0.05), and in diabetic IR/IRS-1 mice a significantly stronger immunoreactivity in the BrM was observed compared to the controls (** p < 0.005). Also in diabetic STZ mice, a significantly weaker immunoreactivity in the ILM was observed when compared to their wild-type controls (** p < 0.005). The nuclei were counterstained with DAPI
Collagen IV
After 4 and 6 months of diabetes, virtually no immunoreactivity for collagen IV could be detected in the inner ILM of the db/db and IR/IRS-1 mouse strains in either diabetic or in non-diabetic retinas (Tables 1 and 2, Figs. 3a–b and c–d and 4a–b and c–d). Only the STZ retina showed a very limited immunoreactivity for collagen IV in the ILM (Figs. 3e–f and 4e–f). In the capillary BMs and in the BrM, the immunoreactivity was similar in all the controls of the three mouse models after 4 and after 6 months.
Collagen IV immunoreactivity in retinal cross-sections of diabetic mice after 4 months of diabetes. a, c, e Strain-specific wild-type controls. b, d, f Different diabetic mouse models. Between the cross-sections: quantification of collagen IV immunoreactivity in the inner limiting membrane (ILM), the retinal capillaries (C), and Bruch’s membrane (BrM) in wild-type control mice (open bars) and diabetic mice (grey bars). No immunoreactivity was detected in the ILM of diabetic db/db mice and diabetic IR/IRS-1 mice or in the controls, and only a faint immunoreactivity in both STZ mice. In diabetic IR/IRS-1 mice, a significantly stronger immunoreactivity of collagen IV in the capillaries and the BrM was observed when compared with the wild-type controls (* p < 0.05 and ** p < 0.005 respectively). The nuclei were counterstained with DAPI
Collagen IV immunoreactivity in retinal cross-sections of diabetic mice after 6 months of diabetes. a, c, e Strain-specific wild-type controls. b, d, f Different diabetic mouse models. Between the cross-sections: quantification of collagen IV immunoreactivity in the inner limiting membrane (ILM), the retinal capillaries (C), and Bruch’s membrane (BrM) in wild-type control mice (open bars) and diabetic mice (grey bars). No immunoreactivity was detected in the ILM of diabetic db/db mice and diabetic IR/IRS-1 mice or in controls, and only a faint immunoreactivity in both STZ mice. In diabetic db/db mice, a significantly stronger immunoreactivity in the capillaries was observed when compared with the wild-type controls (* p < 0.05). In diabetic IR/IRS-1 mice a significantly stronger immunoreactivity in the capillaries and the BrM was observed compared to controls (* p < 0.05 and ** p < 0.005 respectively). The nuclei were counterstained with DAPI
However, the collagen IV immunoreactivity in capillary BMs was increased in diabetic IR/IRS-1 mice after 4 and 6 months (Figs. 3c–d and 4c–d), and in diabetic db/db mice after 6 months of diabetes (Table 2 and Fig. 4a–b). In addition, there was increased collagen IV immunoreactivity in the BrM of both 4- and 6-month-old diabetic retinas in IR/IRS-1 mice (Figs. 3c–d and 4c–d).
Nidogen-1
The nidogen-1 immunoreactivity in all three non-diabetic controls and in all BMs was identical after 4 and after 6 months (Tables 1 and 2).
After 4 months, the nidogen-1 immunoreactivity was reduced in the BrM of diabetic db/db mice as well as in diabetic IR/IRS-1 mice (Fig. 5a–b and c–d). In addition, the nidogen-1 immunoreactivity was also reduced in diabetic db/db mice in the ILM (Fig. 5a–b). There was a constant immunoreactivity in all BMs in wild-type controls and diabetic STZ mice (Fig. 5e–f).
Nidogen 1 immunoreactivity in retinal cross-sections of diabetic mice after 4 months of diabetes. a, c, e Strain-specific wild-type controls. b, d, f Different diabetic mouse models. Between the cross-sections: quantification of nidogen 1 immunoreactivity in the inner limiting membrane (ILM), the retinal capillaries (C), and Bruch’s membrane (BrM) in wild-type control mice (open bars) and diabetic mice (grey bars). In diabetic db/db mice, a significantly weaker immunoreactivity in the ILM was observed when compared with the wild-type controls (* p < 0.05). In diabetic db/db mice and IR/IRS-1 mice a significantly weaker immunoreactivity in the BrM was observed compared to controls (** p < 0.005). The nuclei were counterstained with DAPI
After 6 months of diabetes, the immunoreactivity of nidogen-1 in the three BMs was similar in all diabetic mice models and did not differ when compared to their wild-type controls (Fig. 6a–f).
Nidogen 1 immunoreactivity in retinal cross-sections of diabetic mice after 6 months of diabetes. a, c, e Strain-specific wild-type controls. b, d, f Different diabetic mouse models. Between the cross-sections: quantification of nidogen 1 immunoreactivity in the inner limiting membrane (ILM), the retinal capillaries (C), and Bruch’s membrane (BrM) in wild-type control mice (open bars) and diabetic mice (grey bars). No significant difference in immunoreactivity in either diabetic mice was observed compared to controls. The nuclei were counterstained with DAPI
Nidogen-2
The immunoreactivity of the capillary BMs and the BrM were moderately high and similar in all three non-diabetic controls after 4 months. However, whereas in the non-diabetic IR/IRS-1 control the immunoreactivity in the ILM was high, it was low in both the non-diabetic db/db controls and normal C57Bl6 mice (non-diabetic STZ-controls). At 6 months, the immunoreactivity in the ILM of all used non-diabetic controls was hardly detectable, whereas in the capillary BMs and the BrM the immunoreactivity was high but different between the non-diabetic control mice.
After 4 months of diabetes, the nidogen-2 immunoreactivity in the ILM was low in the db/db-mouse retina and similar between wild-type controls and diabetic mice (Table 1 and Fig. 7a–b). In the BrM of the diabetic retina of IR/IRS-1 mice, the immunoreactivity was significantly higher than in the wild-type controls (Fig. 7c–d). In the STZ mouse retina, the immunoreactivity in the ILM was significantly increased in the diabetic mice (Fig. 7e–f). The immunoreactivity in the capillaries was similar between non-diabetic controls and the diabetic retinas. It was moderate in db/db mice (Fig. 7a–d), but higher in IR/IRS-1 and STZ mice. In the db/db mice and the STZ mice, the nidogen-2 immunoreactivity in the BrM between wild-type controls and diabetic mice also remained constant.
Nidogen 2 immunoreactivity in retinal cross-sections of diabetic mice after 4 months of diabetes. a, c, e Strain-specific wild-type controls. b, d, f Different diabetic mouse models. Between the cross-sections: quantification of nidogen 2 immunoreactivity in the inner limiting membrane (ILM), the retinal capillaries (C), and Bruch’s membrane (BrM) in wild-type control mice (open bars) and diabetic mice (grey bars). In diabetic IR/IRS-1 mice, a significantly stronger immunoreactivity in the BrM was observed compared to controls (** p < 0.005). In diabetic STZ mice a significantly stronger immunoreactivity in the ILM was observed compared to controls (** p < 0.005). The nuclei were counterstained with DAPI
After 6 months of diabetes, the overall immunoreactivity for nidogen-2 in the BMs of ILM, capillaries, and BrM was lower than after 4 months, but there was no difference between non-diabetic controls and diabetic mice (Talbe 2 and Fig. 8a–f). The lowest immunoreactivity was seen in the ILM in all mouse strains. In the db/db mice and the IR/IRs-1 mice, it was hardly detectable. The highest nidogen-2 immunoreactivity was detected in the capillaries of the diabetic STZ mice, with no difference to their non-diabetic controls (Fig. 8e–f).
Nidogen 2 immunoreactivity in retinal cross-sections of diabetic mice after 6 months of diabetes. a, c, e Strain-specific wild-type controls. b, d, f Different diabetic mouse models. Between the cross-sections: quantification of nidogen 2 immunoreactivity in the inner limiting membrane (ILM), the retinal capillaries (C), and Bruch’s membrane (BrM) in wild-type control mice (open bars) and diabetic mice (grey bars). No significant difference in immunoreactivity in either diabetic mice was observed compared to controls. The nuclei were counterstained with DAPI
In summary, all four analyzed basement membrane proteins were detected in all of the three BMs (ILM, capillaries, BrM), with the exception of collagen IV which was not detectable in the ILM of db/db mice and IR/IRS-1 mice, and was only faintly expressed in the ILM of STZ mice. Nidogen-1 showed the highest immunoreactivity in the wild-type controls of all mouse strains. There was no specific immunoreactivity pattern dependent for type-1 diabetic model (STZ) or the type-2 diabetic models (db/db and IR/IRS-1), with only one exception: both the db/db mice and the IR/IRS-1 mice, but not the STZ mice, showed a decreased nidogen-1-immunoreactivity in the BrM after 4 months of diabetes (Fig. 5), but this was not the case after 6 months of diabetes.
Discussion
We expected changes in the composition of retinal BMs in diabetic mice, as it is well-known that an increased synthesis of BM components, such as fibronectin, collagen IV, and laminin is closely associated with vascular BM thickening, a histological hallmark of diabetic retinopathy [37].
BMs are specialized extracellular matrix structures that play essential roles in tissue development and maintenance [38]. The distribution of collagen IV isoforms in human Bruch’s membrane is known [21], as well as the immunoreactivity and localization of laminin isoforms in rat [13] and human retinas [18]. However, the relative distribution and immunoreactivity of BM proteins in diabetic retinal tissue has not been studied in detail. We therefore analyzed the immunoreactivity of laminin γ1, collagen IV, nidogen-1, and nidogen-2 in the diabetic mouse retina.
Most alterations which affected the composition of retinal BMs in experimental diabetes occurred during the embryogenesis. Deletions or mutations of BM proteins result in embryonic lethality [29, 39–42]. Postnatally and during early eye development, mutations and deletions of BM proteins cause vascular disruptions, such as a defective formation of the ILM leading to retinal dysplasia [43]. However, there are also investigations which indicate alterations in BMs proteins in aging animals. A previous study has described an increased immunoreactivity for extracellular matrix proteins in the retina of aging-related diseases such as age-related macular degeneration or diabetic retinopathy [44]. Recently, another study showed that the deposition of BM proteins within the normal retina is increased upon aging [30]. Our results in non-diabetic control mice show that signal intensity of laminin γ1, collagen IV, nidogen 1, and nidogen 2 in the retinal BMs was only changing slightly during the time frame of our experiments. The time points (4 and 6 months) were chosen to reveal changes in BM composition occurring after the onset of diabetes, whereas Kunze and co-workers investigated the deposition of basement membrane proteins in retinas from 1-, 12- and 18-month-old C57BL/6 mice. In agreement with their results on collagen IV and nidogen-2 immunoreactivity in the ILM, we also found no staining or only a weak staining respectively. This finding and our other results concerning the non-diabetic control mice are in agreement with the observations of Kunze et al. [30].
At the mRNA level, the BM components laminin β1 and collagen IV were upregulated after 5 months of diabetes in C57Bl/6 animals [45, 46], but surprisingly not in diabetic Rho−/− mice, which show a photoreceptor degeneration [45]. In rats, the increase of laminin and collagen IV protein expression after 2 months of STZ-induced diabetes were shown by Western blot analysis [47]. Contrary to those studies, we found an upregulated immunoreactivity of laminin γ1 and collagen IV in diabetic IR/IRS-1 mice, but not in the STZ or the db/db models. Although it is difficult to compare mRNA expression and protein expression in other species with protein immunoreactivity in our mouse models, this discrepancy supports our conclusion that changes in basement membrane composition is an effect of diabetic pathology rather than of the method of diabetes induction.
To our knowledge, no studies exist on the nidogen-1 and nidogen-2 expression in diabetic mice retinas, and only limited information is available on their expression on C57Bl/6 mouse retina [30]. The authors found that both nidogen-1 and -2 were present in vascular BMs and BrM throughout mice aged between 1 and 18 months. In the ILM, the nidogens were more strongly expressed at higher ages, with an earlier and higher deposition of nidogen-1. Our findings are in agreement, but we also detected a higher and variable immunoreactivity for nidogen-2 in the non-diabetic IR/IRS-1 mouse strain. For nidogen-1 and nidogen-2 in diabetic mouse retinas, our data are novel. We detected no significant differences in the immunoreactivity of both nidogens in the retinal BMs in any of the three diabetes models with the following exceptions. Firstly, after 4 months there was lower nidogen-1 immunoreactivity in the BrM of the diabetic db/db model and the diabetic IR/IRS-1 model, but not in the STZ model. However, these differences disappeared after 6 months of diabetes. Also, after 4 months of diabetes we detected higher nidogen-2 immunoreactivity in the BrM in the diabetic IR/IRS-1 mouse and in the ILM of the diabetic STZ mouse. Again, these differences disappeared after 6 months of diabetes. This variable nidogen immunoreactivity may be due to a higher turnover of nidogens in BMs, as nidogen-1 is known to be prone to proteolytic degradation [11].
Our finding of different but inconsistent immunoreactivity in the ILM may be important, due to their role in the survival of retinal ganglion cells during development [48]. We found a higher immunoreactivity in the ILM of diabetic IR/IRS-1 mice after 4 months, but a lower immunoreactivity in the diabetic retina of db/db mice and STZ mice; and also a lower immunoreactivity of nidogen-1 in diabetic db/db mice only, together with a higher immunoreactivity of nidogen-2 in the retina of diabetic STZ mice. If retinal ganglion cells are influenced by ILM abnormalities, their BM composition could cause retinal ganglion cell death in response to elevated intraocular pressure, which can lead to glaucoma, a complication of proliferative diabetic retinopathy [49]. A loss of cells in the ganglion cell layer in diabetic mice due to apoptosis was demonstrated [31, 50].
This study demonstrates that changes in BM composition reflected in altered immunoreactivity of key BM proteins are an effect of diabetic pathology rather than of the method of diabetes induction, as they occur in all analyzed diabetes models. No diabetes-model-specific alteration in basement membrane composition could be detected by our immune–histological examination. To detect small changes in BM organization, it may be necessary to use electron microscopic evaluation methods. It remains necessary to study both the STZ-model (type-1 diabetes model) — which allows short-term measurements of vascular leakage, leukocyte endothelial interactions, and retinal cell damage — and the type-2 diabetes models. In further studies, especially on the type-2 diabetes models, a longer time period should be chosen to reveal more detailed effects during and after the onset of diabetes.
References
Cai J, Boulton M (2002) The pathogenesis of diabetic retinopathy: old concepts and new questions. Eye (Lond) 16:242–260
Archer DB (1998) External beam radiotherapy in the management of subfoveal choroidal neovascular membranes of the eye: a new treatment for an old disease. Ulst Med J 67(Suppl 1):41–46
Engerman RL, Kern TS (1995) Retinopathy in animal models of diabetes. Diabetes Metab Rev 11:109–120
Feit-Leichman RA, Kinouchi R, Takeda M, Fan Z, Mohr S, Kern TS, Chen DF (2005) Vascular damage in a mouse model of diabetic retinopathy: relation to neuronal and glial changes. Invest Ophthalmol Vis Sci 46:4281–4287
Roy S, Maiello M, Lorenzi M (1994) Increased expression of basement membrane collagen in human diabetic retinopathy. J Clin Invest 93:438–442
Roy S, Sato T, Paryani G, Kao R (2003) Downregulation of fibronectin overexpression reduces basement membrane thickening and vascular lesions in retinas of galactose-fed rats. Diabetes 52:1229–1234
Risteli J, Draeger KE, Regitz G, Neubauer HP (1982) Increase in circulating basement membrane antigens in diabetic rats and effects of insulin treatment. Diabetologia 23:266–269
Brocks DG, Neubauer HP, Strecker H (1985) Type IV collagen antigens in serum of diabetic rats: a marker for basement membrane collagen biosynthesis. Diabetologia 28:928–932
Paulsson M, Timpl R, Brocks DG, Neubauer H (1988) Increased basement membrane heparan sulphate proteoglycan in serum of diabetic rats. Scand J Clin Lab Invest 48:379–380, Letter to Editor article
Kern JS, Loeckermann S, Fritsch A, Hausser I, Roth W, Magin TM, Mack C, Muller ML, Paul O, Ruther P, Bruckner-Tuderman L (2009) Mechanisms of fibroblast cell therapy for dystrophic epidermolysis bullosa: high stability of collagen VII favors long-term skin integrity. Mol Ther 17:1605–1615
Mayer U, Mann K, Timpl R, Murphy G (1993) Sites of nidogen cleavage by proteases involved in tissue homeostasis and remodelling. Eur J Biochem 217:877–884
Nishikawa T, Giardino I, Edelstein D, Brownlee M (2000) Changes in diabetic retinal matrix protein mRNA levels in a common transgenic mouse strain. Curr Eye Res 21:581–587
Libby RT, Champliaud MF, Claudepierre T, Xu Y, Gibbons EP, Koch M, Burgeson RE, Hunter DD, Brunken WJ (2000) Laminin expression in adult and developing retinae: evidence of two novel CNS laminins. J Neurosci 20:6517–6528
Lin WL, Essner E, McCarthy KJ, Couchman JR (1992) Ultrastructural immunocytochemical localization of chondroitin sulfate proteoglycan in Bruch’s membrane of the rat. Invest Ophthalmol Vis Sci 33:2072–2075
Marshall GE, Konstas AG, Reid GG, Edwards JG, Lee WR (1992) Type IV collagen and laminin in Bruch’s membrane and basal linear deposit in the human macula. Br J Ophthalmol 76:607–614
Grierson I, Hiscott P, Hogg P, Robey H, Mazure A, Larkin G (1994) Development, repair and regeneration of the retinal pigment epithelium. Eye 8(Pt 2):255–262
Libby RT, Brunken WJ, Hunter DD (2000) Roles of the extracellular matrix in retinal development and maintenance. Results Probl Cell Differ 31:115–140
Bystrom B, Virtanen I, Rousselle P, Gullberg D, Pedrosa-Domellof F (2006) Distribution of laminins in the developing human eye. Invest Ophthalmol Vis Sci 47:777–785
Duran-Jimenez B, Dobler D, Moffatt S, Rabbani N, Streuli CH, Thornalley PJ, Tomlinson DR, Gardiner NJ (2009) Advanced glycation end products in extracellular matrix proteins contribute to the failure of sensory nerve regeneration in diabetes. Diabetes 58:2893–2903
Sado Y, Kagawa M, Naito I, Ueki Y, Seki T, Momota R, Oohashi T, Ninomiya Y (1998) Organization and expression of basement membrane collagen IV genes and their roles in human disorders. J Biochem 123:767–776
Chen L, Miyamura N, Ninomiya Y, Handa JT (2003) Distribution of the collagen IV isoforms in human Bruch’s membrane. Br J Ophthalmol 87:212–215
Fox JW, Mayer U, Nischt R, Aumailley M, Reinhardt D, Wiedemann H, Mann K, Timpl R, Krieg T, Engel J et al (1991) Recombinant nidogen consists of three globular domains and mediates binding of laminin to collagen type IV. EMBO J 10:3137–3146
Kimura N, Toyoshima T, Kojima T, Shimane M (1998) Entactin-2: a new member of basement membrane protein with high homology to entactin/nidogen. Exp Cell Res 241:36–45
Miosge N, Holzhausen S, Zelent C, Sprysch P, Herken R (2001) Nidogen-1 and nidogen-2 are found in basement membranes during human embryonic development. Histochem J 33:523–530
Aumailley M, Battaglia C, Mayer U, Reinhardt D, Nischt R, Timpl R, Fox JW (1993) Nidogen mediates the formation of ternary complexes of basement membrane components. Kidney Int 43:7–12
Kohfeldt E, Sasaki T, Gohring W, Timpl R (1998) Nidogen-2: a new basement membrane protein with diverse binding properties. J Mol Biol 282:99–109
Murshed M, Smyth N, Miosge N, Karolat J, Krieg T, Paulsson M, Nischt R (2000) The absence of nidogen 1 does not affect murine basement membrane formation. Mol Cell Biol 20:7007–7012
Schymeinsky J, Nedbal S, Miosge N, Poschl E, Rao C, Beier DR, Skarnes WC, Timpl R, Bader BL (2002) Gene structure and functional analysis of the mouse nidogen-2 gene: nidogen-2 is not essential for basement membrane formation in mice. Mol Cell Biol 22:6820–6830
Bader BL, Smyth N, Nedbal S, Miosge N, Baranowsky A, Mokkapati S, Murshed M, Nischt R (2005) Compound genetic ablation of nidogen 1 and 2 causes basement membrane defects and perinatal lethality in mice. Mol Cell Biol 25:6846–6856
Kunze A, Abari E, Semkova I, Paulsson M, Hartmann U (2010) Deposition of nidogens and other basement membrane proteins in the young and aging mouse retina. Ophthalmic Res 43:108–112
Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW (1998) Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest 102:783–791
Villarroel M, Ciudin A, Hernandez C, Simo R (2010) Neurodegeneration: An early event of diabetic retinopathy. World J Diabetes 1:57–64
King AJ (2012) The use of animal models in diabetes research. Br J Pharmacol 166:877–894
Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP (1996) Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84:491–495
Bruning JC, Winnay J, Bonner-Weir S, Taylor SI, Accili D, Kahn CR (1997) Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell 88:561–572
McEvoy RC, Andersson J, Sandler S, Hellerstrom C (1984) Multiple low-dose streptozotocin-induced diabetes in the mouse. Evidence for stimulation of a cytotoxic cellular immune response against an insulin-producing beta cell line. J Clin Invest 74:715–722
Stitt AW, Anderson HR, Gardiner TA, Archer DB (1994) Diabetic retinopathy: quantitative variation in capillary basement membrane thickening in arterial or venous environments. Br J Ophthalmol 78:133–137
Bai X, Dilworth DJ, Weng YC, Gould DB (2009) Developmental distribution of collagen IV isoforms and relevance to ocular diseases. Matrix Biol 28:194–201
Smyth N, Vatansever HS, Murray P, Meyer M, Frie C, Paulsson M, Edgar D (1999) Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J Cell Biol 144:151–160
Willem M, Miosge N, Halfter W, Smyth N, Jannetti I, Burghart E, Timpl R, Mayer U (2002) Specific ablation of the nidogen-binding site in the laminin gamma1 chain interferes with kidney and lung development. Development 129:2711–2722
Costell M, Carmona R, Gustafsson E, Gonzalez-Iriarte M, Fassler R, Munoz-Chapuli R (2002) Hyperplastic conotruncal endocardial cushions and transposition of great arteries in perlecan-null mice. Circ Res 91:158–164
Poschl E, Schlotzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U (2004) Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 131:1619–1628
Pinzon-Duarte G, Daly G, Li YN, Koch M, Brunken WJ (2010) Defective formation of the inner limiting membrane in laminin beta2- and gamma3-null mice produces retinal dysplasia. Invest Ophthalmol Vis Sci 51:1773–1782
Hiscott P, Sheridan C, Magee RM, Grierson I (1999) Matrix and the retinal pigment epithelium in proliferative retinal disease. Prog Retin Eye Res 18:167–190
de Gooyer TE, Stevenson KA, Humphries P, Simpson DA, Gardiner TA, Stitt AW (2006) Retinopathy is reduced during experimental diabetes in a mouse model of outer retinal degeneration. Invest Ophthalmol Vis Sci 47:5561–5568
Stitt A, Gardiner TA, Alderson NL, Canning P, Frizzell N, Duffy N, Boyle C, Januszewski AS, Chachich M, Baynes JW, Thorpe SR (2002) The AGE inhibitor pyridoxamine inhibits development of retinopathy in experimental diabetes. Diabetes 51:2826–2832
Oshitari T, Polewski P, Chadda M, Li AF, Sato T, Roy S (2006) Effect of combined antisense oligonucleotides against high-glucose- and diabetes-induced overexpression of extracellular matrix components and increased vascular permeability. Diabetes 55:86–92
Halfter W, Willem M, Mayer U (2005) Basement membrane-dependent survival of retinal ganglion cells. Invest Ophthalmol Vis Sci 46:1000–1009
Bai Y, Xu J, Brahimi F, Zhuo Y, Sarunic MV, Saragovi HU (2010) An agonistic TrkB mAb causes sustained TrkB activation, delays RGC death, and protects the retinal structure in optic nerve axotomy and in glaucoma. Invest Ophthalmol Vis Sci 51:4722–4731
Martin PM, Roon P, Van Ells TK, Ganapathy V, Smith SB (2004) Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci 45:3330–3336
Acknowledgments
We are grateful to Elzbieta Jaroslawska and Peter Knowlton for their help. The study was supported by Deutsche Forschungsgemeinschaft (DFG) PA660/10-1 and JO324/10-1 and by Dr. Werner Jackstädt-Stiftung
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abari, E., Kociok, N., Hartmann, U. et al. Alterations in basement membrane immunoreactivity of the diabetic retina in three diabetic mouse models. Graefes Arch Clin Exp Ophthalmol 251, 763–775 (2013). https://doi.org/10.1007/s00417-012-2237-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-012-2237-8