Abstract
Purpose
To investigate early functional changes of local retinal defects in type II diabetic patients using the global flash multifocal electroretinogram (MOFO mfERG).
Methods
Thirty-eight diabetic patients and 14 age-matched controls were recruited. Nine of the diabetics were free from diabetic retinopathy (DR), while the remainder had mild to moderate non-proliferative diabetic retinopathy. The MOFO mfERG was performed at high (98 %) and low (46 %) contrast levels. MfERG responses were grouped into 35 regions for comparison with DR classification at those locations. Z-scores of the regional mfERG responses were compared across different types of DR defects.
Results
The mfERG waveform consisted of the direct component (DC) and the induced component (IC). Local reduction in DC and IC amplitudes were found in diabetic patients with and without DR. With increasing severity of retinopathy, there was a further deterioration in amplitude of both components. Under MOFO mfERG paradigm, amplitude was a useful screening parameter.
Conclusion
The MOFO mfERG can help in detecting early functional anomalies before the appearance of visible signs, and may assist in monitoring further functional deterioration in diabetic patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a group of metabolic diseases which lead to systemic hyperglycemia. Type I DM is caused by beta-cell destruction in the pancreas, which is often mediated by the immune system, and results in a loss of insulin secretion and absolute insulin deficiency. Type II DM is caused by a combination of genetic and non-genetic factors which result in insulin resistance and deficiency. Type II DM accounts for about 90 % of cases of diabetes [1]. It is estimated that the prevalence of diabetes will double to 366 million worldwide by 2030; many of these patients will be over 65 years of age [2]. DM can impair ocular capillary perfusion, and thus lead to the development of diabetic retinopathy (DR).
DR is the most frequent cause of new cases of blindness among the working population [3]. In the first two decades after diagnosis of the disease, over half of the patients with Type II DM have retinopathy [4, 5]. Diabetic patients are assessed using ophthalmoscopy and fundus photography [4, 6]. The main focus is to detect visible sign of vascular retinopathy in order to monitor progress of DR and to avoid its sight-threatening complications [5, 7]; however, the basis of functional changes in the retina, especially in the early stages, has not been determined.
The Ganzfeld full-field electroretinogram has been used to study retinal functional changes in diabetic patients [8–11]. The defects of DR are not distributed uniformly across the retina, and show a range of stages of development [12]. The full-field electroretinogram, which is a summated retinal response measurement, is not likely to reflect local or eccentric functional changes in diabetes. The multifocal electroretinogram (mfERG), however, provides objective topographical measurements of retinal responses across the visual field [13]. Palmowski et al. [14] and Shimada et al. [15] examined retinal function in diabetes using the mfERG; responses were either grouped into rings or quadrants, or summed across the retina. Such groupings lose the fine topographic details in studying various types of vascular defect [16, 17]. Bearse et al. applied slow flash mfERG to study local oscillatory potentials in the diabetic retina, and suggested that retinal adaptation was more likely to be abnormal at sites with early retinopathy [17]. Bronson-Castain et al. and Fortune et al. applied the conventional mfERG to the diabetic retina, and observed an implicit time delay increasing with the severity of retinopathy. However, local response amplitudes failed to show a consistent relationship with retinal abnormalities in diabetic eyes [16, 18].
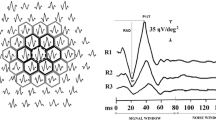
Sutter and Bearse have proposed a modified mfERG protocol, named the global flash mfERG, to study retinal adaptive effects. It has been suggested that the retinal adaptive response would be enhanced by inserting a periodic full-field (global) flash between successive m-sequence focal flashes [19–21]. In the global flash mfERG, there are two main components (Fig. 1): the direct component (DC) arises predominantly from bipolar and N-methyl-D-aspartic-acid (NMDA)-sensitive cells, the induced component (IC) is predominantly from NMDA-sensitive cells and ganglion cells from the inner retina [22]. This global flash mfERG allows separate examination of the response from the outer and inner retina. In addition, Hood and co-workers found that nonlinear retinal responses are saturated at high contrast levels, and they suggested that low contrast stimulus would enhance the inner retinal response [20].
Waveform of the MOFO response contains two main components: the direct component (DC) and the induced component (IC). Note that the implicit time of DC is measured from the onset of the multifocal stimulus to the response peak of DC, while the implicit time of IC is measured from the onset of the global flash (26.6 ms) to the response peak of IC
The aim of this study was to investigate the early local functional changes in diabetic retina at both high (98 %) and low (46 %) contrast levels. The use of the periodic global flash multifocal electroretinogram (MOFO mfERG) in diabetic patients assisted in correlating the local functional changes with retinopathy, and in investigating the depth of retinal dysfunction in diabetic patients.
Methods
Subjects
Thirty-eight type II diabetic patients were examined: 9 (aged 49.7 ± 6.4 years) did not have diabetic retinopathy (DR) while 29 (aged 49.8 ± 6.4 years) had non-proliferative diabetic retinopathy (NPDR). Fourteen control subjects (aged 49.4 ± 7.0 years) were also examined. All subjects had visual acuities better than 6/9. Their refractive errors were between +3.00 and −6.00 D, and astigmatism was less than −1.25 D. None had any clinically significant ocular or systemic disorders other than DR or DM. The plasma glucose level of the subjects was measured during the visit using a blood glucose meter (Accu-Chek Compact Plus, F. Hoffmann-La Roche Ltd, Basel, Switzerland) at least 2 hours after any food intake. Ten healthy controls and 36 diabetic patients consented to plasma glucose measurements. The duration of DM was based on the patient’s own report, and was represented by an ordinal parameter (DM diagnosed for less than 5 years, DM diagnosed for 5 to 10 years, DM diagnosed for more than 10 years).
All procedures of the study followed the tenets of the Declaration of Hesinki. This study was approved by the Ethics Committee of The Hong Kong Polytechnic University. Informed consent was obtained from each subject following full explanation of the experimental procedures.
Stimulus conditions
The VERIS Science 5.1 system (Electro-Diagnostic-Imaging, San Mateo, CA, USA) was used for mfERG measurement. The stimulus was shown on a high-luminance CRT monitor (FIMI Medical Electrical Equipment, Saronno, Italy). The stimulus pattern contained 103 scaled hexagons with an angular subtense of 44° vertically and 47° horizontally. The sequence of hexagonal pattern stimulation followed a pseudo-random binary m-sequence (213-1) with a video frame rate of 75 Hz. In each MOFO stimulation, there were four video frames: a pseudo-random m-sequence focal flash, followed by a full-screen dark frame, a full-screen global flash, and another full-screen dark frame. The duration of one MOFO stimulation sequence was 53.3 ms. The background luminance of the mfERG display was 100 cd/m2. At the high contrast level (98 %), both the luminance of the bright phase of the multifocal stimulus and the global flashes were set at 200 cd/m2 as suggested by our previous study, in order to obtain optimal DC and IC responses [23]. The dark phase was set at a luminance of 2 cd/m2. At the low contrast level (46 %), the bright phase of the multifocal stimulus was set at 166 cd/m2, while the dark phase was set at 61 cd/m2. A central cross on the stimulus pattern was used as a fixation target. One eye was randomly selected for mfERG measurement. The recording was carried out with room illuminance of about 100lux.
Recording conditions
Detailed eye examination (including subjective refraction, biomicroscopy and indirect ophthalmoscopy) with fundus photodocumentation was carried out for each subject. The Stratus optical coherence tomography (Carl Zeiss Meditec, Inc., Dublin, CA, USA) was used to measure the macular thickness in a fast scanning mode in order to rule out any patients with macular oedema. In the MOFO mfERG measurement, the pupil of the tested eye was dilated with 1 % tropicamide (Alcon, Fort Worth, TX, USA) to at least 7 mm diameter. The untested eye was occluded. A Dawson–Trick–Litzkow (DTL) electrode was placed on the lower bulbar conjunctiva to be the active electrode. Gold-cup electrodes were used as reference and ground electrodes, on the temporal side of the tested eye and forehead respectively. The ERG signal was amplified (×100,000) (Grass Instrument Co., Quincy, MA, USA) and band-pass filtered (3–300 Hz). The MOFO mfERG protocol was carried out at high (98 %) and low (46 %) contrast levels. The mfERG recording time for each contrast level was about 8 minutes, and was divided into 32 segments. Each segment lasted approximately 14 seconds, and a short break was provided between segments. The refractive error of the tested eye was corrected for the viewing distance of 33 cm. The recording quality was monitored using the real-time display of the VERIS program. Segments contaminated by poor fixation, eye movement, or blinks were rejected and re-recorded immediately.
Analysis
-
1)
Analysis of the MOFO mfERG signals
The 103 MOFO mfERG trace arrays were grouped into 35 regions as suggested by Bearse and colleagues. This grouping combines similar waveforms while maintaining their nasal, temporal, and eccentricity locations (Fig. 2) [17, 24]. For each region, signal amplitudes of the DC and IC were measured. The DC implicit time was measured from the onset of the multifocal stimulus to the peak of DC; the IC implicit time was measured from the onset of the global flash to the peak of IC (Fig. 1). Left-eye MOFO mfERG signals were transposed so that all eyes were apparently right eyes for the purposes of data analysis.
The multifocal stimuli pattern was mapped with the automated mosaic fundus photo (each circle indicates the fundus photo taken at a particular gaze. There are in total nine gazes, one central gaze and eight peripheral gazes, to form a mosaic fundus photo). Both were divided into 35 regions as in previous studies (about two to three hexagons were grouped as one region in the mfERG topography as indicated by the dark polygons). This figure illustrates the regional mfERG waveform of a diabetic patient with DR lesions at different locations. Those regional mfERG samples with DR (in red lines) are compared with the averaged regional samples from the control group (in blue lines)
The MOFO mfERG responses from the 35 regions were grouped according to the fundus photographs grading (see below) for further analysis. To account for the topographic asymmetry of the mfERG and provide the same baseline for comparison, a z-score scale was established for the MOFO mfERG responses [25, 26]. The MOFO mfERG responses in the control group were used to calculate the means and standard deviations for each specific location across the 35-division of the mfERG topography. The means and standard deviations obtained above were then used to calculate the z-score of the MOFO mfERG responses for each subject at that specific region (by subtracting the mean from the individual mfERG response and then dividing it by the standard deviation obtained from the control group, [i.e., (Individual mfERG response−mfERG mean response of the control group)/standard deviation].
-
2)
Relating the plasma glucose level, DM duration, and averaged mfERG responses in the control and diabetic subjects
The plasma glucose level was compared between the control and diabetic subjects by independent t-test. The MOFO mfERG responses from the 35-division array were averaged so that each subject gave a mean z-score of the mfERG responses. The correlation between the individual plasma glucose level and DM duration with the averaged mfERG responses of the diabetic subjects was then obtained.
-
3)
Grading of the fundus photographs
A Topcon IMAGEnet fundus camera was used to take colour fundus photographs with one central 45° field and eight peripheral surrounding fields. The fundus photographs from various fields were grouped into a single photograph in mosaic format. The 103-hexagon pattern of the mfERG topography was aligned with the mosaic of fundus photos for each subject. The blind spot depression and the central peak were aligned with the optic disc and fovea respectively. The 103 hexagons were then grouped into the 35-division pattern as shown in Fig. 2. (Calculations including the range of corrections used in these experiments and the range of axial lengths expected suggest that the variation in magnification of the retinal image of the stimulus pattern would be small, in the range of 3 %).
The regional retinal defects were then graded by a masked retinal specialist according to the following scales based on the severity of retinopathy:
-
Group 0: regional samples from control subjects
-
Group 1: regional samples from DM patients without retinopathy (“No NPDR” group) (equivalent up to the ETDRS level 10) [27, 28]
-
Group 2: regional samples containing hard exudates (“HE” group) (equivalent up to the ETDRS level 14) [27, 28].
For the regional samples with definite haemorrhage (equivalent up to the ETDRS level 35 and 43) [27, 28], they were further divided into two types (outer and inner retinal haemorrhage) based on the retinal depth of the haemorrhage
-
Group 3: regional samples containing outer retinal haemorrhage — Dot/ Blot haemorrhage, together with or without hard exudates (“Outer retinal haemorrhage ± HE” group)
-
Group 4: regional samples containing inner retinal defect — Flame haemorrhage, together with or without cotton-wool-spots (“Inner ± outer retinal haemorrhage ± CWS ± HE” group)
(Note that retinal regions with small drusen and the retinal regions lacking retinopathy signs from the NPDR groups were excluded).
-
4)
Mapping between fundus photographs and MOFO mfERG topography
Based on the above grading in the 35 retinal regions, the corresponding regional MOFO mfERG responses were then associated with these different retinal defect grades for multiple comparisons. It was assumed that the mfERG 35 divisions were independent of each other [16]. The statistical analysis was done using SPSS 16.0 (SPSS, Chicago, IL, USA). Repeated measures analysis of variance with Bonferroni's adjustment was applied to study the group difference (groups 0 to 4). The Bonferroni’s adjustment was based on the contrast levels (a within-subject factor with two levels) and retinal defect groups (a between-subject factor with five levels). In the case of the existence of interaction between factors, simple effect of the group factor was then reported.
-
5)
Evaluation of the diagnostic values of the MOFO mfERG parameters
For each MOFO mfERG parameter (amplitude and implicit time of the DC and IC) at each contrast level, a receiver-operating-characteristic curve was constructed and the area-under-the-curve was calculated (GraphPad Prism 5; GraphPad, San Diego, CA, USA) to estimate the predictive ability of each parameter in DR.
Results
Correlation of the plasma glucose level and DM duration with the averaged MOFO mfERG parameters
The plasma glucose levels of the diabetic subjects were significantly higher than those of the control subjects (independent t-test, p < 0.0001). Among the 38 diabetic subjects, no significant correlation was found between the averaged MOFO mfERG responses and the DM duration (Spearman’s r ranged from −0.09 to 0.1, p ranged from 0.57 to 0.94). For the plasma glucose level measured from the 36 diabetic subjects, significant correlation was only found with the mean z-score of IC implicit time at low contrast level. The higher the plasma glucose level was, the greater was the delay of the mean IC implicit time at low contrast level (Pearson’s r = 0.412; p = 0.012) (Table 1).
Local MOFO mfERG responses in different types of retinopathy defects
A total of 1019 MOFO regional samples was collected. The numbers of regional samples from each group were: group 0 — 486 regional samples (47.7 %), group 1 — 302 regional samples (29.6 %), group 2 — 28 regional samples (2.8 %), group 3 — 168 regional samples (16.5 %), group 4 — 35 regional samples (3.4 %).
DC and IC amplitude measures, and DC and IC implicit time measures, showed statistically significant effects of contrast levels (repeated measures ANOVA, p < 0.001), groups (p < 0.001) and their interaction (p < 0.001). The differences between the subgroups of subjects were further studied by applying one-way ANOVA and Bonferroni’s post-hoc test.
DC amplitude z-score (DCA_z)
There were significantly smaller DC amplitudes at high and low contrast levels for all diabetic groups than for the control subjects (p < 0.02) (Fig. 3). At the low contrast level, the DC amplitude showed a greater decrease in the presence of retinopathy signs. The DCA_z from the regions in group 4 deteriorated even more compared to the regional samples from group 1 (p = 0.011) (Fig. 3). It should be noted that the diabetic subjects showed considerable variation in response.
Comparison of the DC amplitude z-scores (DCA_z) for groups 0 to 4 at high (98 %) and low (46 %) contrast levels. * : p < 0.05 when compared with group 0; † : p < 0.05 when compared with group 1. Box plot: centre line — the mean; the edges of the box — ±1 standard deviation; the edges of the vertical bars — range
IC amplitude z-score (ICA_z)
At the high contrast level (Fig. 4), all the diabetic groups (groups 1–4) showed a reduction in ICA_z compared to group 0 (p < 0.001). With the presence of visible retinopathy, there was a further reduction in IC amplitude. Among the diabetic groups, the ICA_z of group 2 was significantly reduced compared to group 1 (p = 0.0034) and group 3 (p = 0.018).
Comparison of IC amplitude z-scores (ICA_z) for groups 0 to 4 at high (98 %) and low (46 %) contrast levels.* : p < 0.05 when compared with group 0; † : p < 0.05 when compared with group 1. Box plot: centre line — the mean; the edges of the box — ±1 standard deviation; the edges of the vertical bars — range
At the low contrast level (Fig. 4), the trend of the ICA_z was similar to that at the high contrast level. Again, with the existence of visible retinopathy, there was a further reduction in IC amplitude. However, statistical significance was only seen between these two pairs of comparison: group 0 and group 2, group 0 and group 3 (p < 0.02). The ICA_z of group 3 was also significantly smaller than group 1 (p < 0.00023). The lack of statistically significant findings between the “No NPDR” and control groups here may be attributed to the considerable variation in response.
DC implicit time z-score (DCIT_z)
For high contrast level stimuli (Fig. 5), all the locations with retinopathy (groups 2–4) showed a significant delay in response compared to group 0 (p < 0.002) and group 1 (p < 0.01). The existence of visible retinopathy led to a greater delay in the DC implicit time than the regions without retinopathy. However, no statistically significant difference was seen for the regional samples at low contrast level. This was largely due to the increased variability of the responses in the DR groups, with a few patients showing very much faster responses, especially to the low contrast stimuli (Fig. 5).
Comparison of DC implicit time z-scores (DCIT_z) for groups 0 to 4 at high (98%) and low (46%) contrast levels. * : p < 0.05 when compared with group 0; † : p < 0.05 when compared with group 1. Box plot: centre line — the mean; the edges of the box — ±1 standard deviation; the edges of the vertical bars — range
IC implicit time z-score (ICIT_z)
At the high contrast level (Fig. 6), the mfERG responses from the diabetic groups were, on average, slower than those from group 0. With visible retinopathy (group 2–4), the delay was larger than those without DR (group 1). Group 2 and group 3 showed a significant delay compared to group 0 in the IC implicit time (p < 0.02). The IC implicit time of group 3 also had a significant delay in response compared to group 1 (p = 0.0022).
Comparison of IC implicit time z-scores (ICIT_z) for groups 0 to 4 at high (98%) and low (46%) contrast levels. * : p < 0.05 when compared with group 0; † : p < 0.05 when compared with group 1. Box plot: centre line — the mean; the edges of the box — ±1 standard deviation; the edges of the vertical bars — range
At the low contrast level (Fig. 6), only Group 1 showed a significant delay in IC implicit time compared to Group 0 (p = 0.0013).
Diagnostic value of the MOFO mfERG parameters
In order to determine the diagnostic value of the MOFO mfERG parameters, receiver operating characteristics (ROC) curves were plotted and the area-under-the-curve (AUC) was calculated; these values are summarized in Table 2. When differentiating the retinopathy groups (groups 2–4) from group 0, DCIT_z at 98 % contrast level resulted in the highest AUC (76.6 %). However, the AUC value of DCIT_z declined to 62.6 % if it was used to differentiate the diabetic groups (groups 1–4) from group 0 (Table 2).
DCA_z at low and high contrast levels and the ICA_z at high contrast level showed the highest potential for screening out functional defects due to diabetes (including those without visible vascular defects) having AUC values ranging from 69.1 to 70.9 % (Table 2).
Discussion
This study illustrated the variations of MOFO mfERG responses under high (98 %) and low (46 %) contrast levels in the diabetic retina. By inserting a periodic global flash between two successive multifocal stimuli, adaptation activity in the retina should be enhanced [21]. In MOFO mfERG assessment, there are two main components: the direct component (DC) and the induced component (IC). The DC response is the average response to the focal stimulation, while the IC response shows the effect of the preceding focal stimulation on the response to the global flash [15].
In this study, using the high-contrast MOFO paradigm, the delay and reduction of the mfERG responses suggests that both middle and inner retinal layers (i.e., DC and IC responses respectively) were impaired even in diabetic patients without signs of retinopathy. Greater delay of response (on average) and reduction of response amplitude in the mfERG were seen when retinopathy signs were present. This implies that certain local functional deterioration started before the visible signs of vascular retinopathy could be detected in the clinical screening assessment.
Previous studies have reported reduced responses in DR in the pattern ERG [29], the second order kernel responses of mfERG [14], the oscillatory potentials (OPs) and photopic negative responses (PhNR) of the standard full-field ERG [10, 30–33] and mfERG [17, 34–36]. All these previous studies proposed that the inner retinal functions were affected in diabetic retina. In the animal study, by pharmacological dissection, the IC was found to be contributed from the third-order neurons and ganglion cells [22]. The reduction of IC response here further supported the functional changes in the inner retina of the diabetic patients in the localized aspect. However, such situation was not observed in the study by Shimada et al. [15]. It might be due to the difference in the subject inclusion. In their study, both type I and II diabetic patients were put together for analysis, while only type II diabetic patients were recruited in our current study.
The DC in MOFO mfERG was found to be predominantly from the bipolar cells with partial contribution from the third-order neurons [22]. Its amplitude reduction among diabetic patients before observable vascular lesions in this study suggests that the middle retinal layers may deteriorate early in DR. Shimada et al. [15] reported similar findings for the high contrast mfERG. Considering that the minimum oxygen supply appears at the area near the inner nuclear layer (INL) [37], our results agree with the hypothesis that the middle retina is at risk of hypoxic damage in diabetic patients.
The reductions in the DC and IC responses provide crucial evidence that the middle and inner retina are actually impaired at an early stage in diabetic patients. Recent studies have reported that hypoxia can affect the photoreceptors and the INL [37–41], while excito-toxicity also plays a role in affecting the neurotransmission among amacrine and glial cells in the diabetic retina [42–44]. It seems that multiple retinal layers are affected in diabetes at the early stage [45]. Here in this study, low-contrast MOFO mfERG stimulation was applied to avoid saturation of the non-linear retinal response. Hood and co-workers [20] reported that mfERG stimulation at a contrast level of 50 % evokes a waveform with more involvement of the inner human retina [21, 46]. The low-contrast mfERG stimulus has been used to investigate inner retinal activity in glaucoma [47, 48]. Our diabetic patients demonstrated reduced DC and IC amplitudes in both high- and low-contrast conditions. However, reducing contrast of the stimulus does not appear to improve discrimination between the control and diabetic patients, and additional delay of implicit time for low-contrast stimuli was not obvious; this might be due to a large inter-subject variability or, more likely, the problems in the inner retina induced by DR are different from those induced by glaucoma. Since amplitude and implicit time responses under different contrast levels appear to be different in diabetic and glaucoma conditions [47, 48], the basis of these two diseases is believed to be different (e.g., cell loss or cellular dysfunction). It further supports the hypothesis by Greenstein and co-workers [45] that the mechanism of the retinal dysfunction at the early stage of DM is unlike that of glaucoma.
Previous mfERG studies reported that the implicit time was a more “sensitive” parameter in detecting functional anomalies than response amplitude in DM patients [16, 34, 49–51]. In our study, the implicit time of DC was only maximally sensitive in screening retinal locations with retinopathy signs with delayed response in average. However, some very fast responses were obtained from the diabetic patients, especially at the low-contrast condition. This could not be fully explained by the range of plasma glucose levels among subjects as there was no significant correlation with the DCIT. Moreover, Klemp and co-workers [52] found that the short-term hyperglycemia leads to a shorter implicit time in the first- and second-order mfERG responses, presumably because of increased retinal metabolism. This seems to contradict to the positive correlation here between the plasma glucose level and the ICIT_z at low-contrast level. The discrepancy may be due to the effect of chronic instead of short-term hyperglycemia in our diabetic subjects. Study on the effect of the stability of the plasma glucose level (e.g., glycated hemogloblin) in type II diabetic patients should also be considered.
Based on our current findings, to screen out functional abnormalities at a very early stage in the diabetic patients without retinopathy, the amplitudes of IC from high contrast stimuli, and DC at both high- and low-contrast levels were preferred. Compared with the longitudinal study by Harrison and co-authors [51] which showed the implicit time as a more sensitive parameter than amplitude, different possibilities may account for our difference from the previous studies:
-
1) The MOFO paradigm dissociates the original mfERG retinal responses into two different components. The MOFO mfERG with the insertion of a global flash is used to enhance the retinal adaptation mechanism. By splitting the retinal components, subtle changes in the waveform amplitude may thus become more obvious.
-
2) Modifying the electrophysiological protocols may favor the activities of different types of retinal cells [23, 43, 53]. The mfERG protocol used in the study by Harrison et al. [51] was the standard mfERG. Without the dissociation of the inner retinal responses by the global flash, the standard mfERG responses would mix the middle and inner retinal responses together; some subtle changes on the resultant waveform might thus be masked. And the bandpass filter applied in the study by Harrison et al. was 10–100 Hz, which screened out some high-frequency oscillatory potentials contributed from the retinal ganglion cells and third-order neurons [54]; while in our study, a bandpass filter of 3–300 Hz was applied to cover the range of both high- and low-frequency retinal responses, to study the middle and inner retinal layer performance. The involvement of the high-frequency component would thus lead to the difference.
-
3) In our study, only type II diabetic patients were recruited, while both type I and II diabetic patients were recruited in the study by Harrison et al. The different subject pool may cause this discrepancy. It is because the underlying mechanism, medical treatment ,and prevalence of DR progression of type I and type II DM showed different patterns [1, 4, 42].
The MOFO mfERG paradigm provides more detailed information in terms of the retinal adaptive changes or the retinal recovery rate than the conventional paradigm. However, as this study was limited by its cross-sectional nature, a longitudinal follow-up study should be carried out in order to find out the prediction ability of the MOFO mfERG for the DR onset. It is surprising that the duration of DM does not correlate with the individual mean MOFO responses, but this may be due to the variability of the latent period before DM was diagnosed for our patients.
The multifocal electroretinogram, together with the MOFO paradigm, provides a means of detecting early functional anomalies in the diabetic retina before visible vascular defects appear. Comparing with the other standard electrophysiological assessments (full-field ERG, pattern ERG and VEP), the MOFO mfERG not only provides the retinal adaptation assessment but with topographic details. It aids in differentiating the early functional deterioration(s) at the middle and inner retina in diabetic retina. This result suggests potential retinal sites (middle and inner retina) for future pharmaceutical therapies. The MOFO mfERG is helpful in monitoring the disease progression before sight-threatening retinopathy supervenes. It may be useful in evaluating the effectiveness of the potential therapies [55].
Conclusion
DM can lead to early impaired adaptation in the retina before the presence of visible vascular lesions. With an increase in the severity of retinopathy, there is a more dramatic deterioration in the mfERG responses. Amplitudes of DC and IC components of the MOFO mfERG assessment appear to be better parameters than implicit time measures in screening for diabetes without retinopathy. As far as the implicit time in MOFO mfERG is concerned, it is a better parameter for screening the diabetic retina with vascular lesions. The MOFO technique in mfERG provides a means of early detection of retinal anomalies in DM, and its findings also provide information about the severity of anomalies in the diabetic retina.
References
LeRoith D, Taylor S, Olefsky J (2004) Diabetes mellitus - a fundamental and clinical text. 3rd edition. Lippincott Williams & Wilkins, Philadelphia, pp 458–459
Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27:1047–1053
Porta M, Bandello F (2002) Diabetic retinopathy - A clinical update. Diabetologia 45:1617–1634
Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL 3rd, Klein R (2003) Diabetic retinopathy. Diabetes Care 26:226–229
Carmichael TR, Carp GI, Welsh ND, Kalk WJ (2005) Effective and accurate screening for diabetic retinopathy using a 60 degree mydriatic fundus camera. S Afr Med J 95:57–61
Aldington SJ, Kohner EM, Meuer S, Klein R, Sjolie AK (1995) Methodology for retinal photography and assessment of diabetic-retinopathy - the Eurodiab IDDM Complications Study. Diabetol 38:437–444
Harding SP, Broadbent DM, Neoh C, White MC, Vora J (1995) Sensitivity and specificity of photography and direct ophthalmoscopy in screening for sight threatening eye disease: the Liverpool Diabetic Eye Study. BMJ 311:1131–1135
Yamamoto S, Kamiyama M, Nitta K, Yamada T, Hayasaka S (1996) Selective reduction of the S cone electroretinogram in diabetes. Br J Ophthalmol 80:973–975
Holopigian K, Greenstein VC, Seiple W, Hood DC, Carr RE (1997) Evidence for photoreceptor changes in patients with diabetic retinopathy. Invest Ophthalmol Vis Sci 38:2355–2365
Kizawa J, Machida S, Kobayashi T, Gotoh Y, Kurosaka D (2006) Changes of oscillatory potentials and photopic negative response in patients with early diabetic retinopathy. Jpn J Ophthalmol 50:367–373
Luu CD, Szental JA, Lee SY, Lavanya R, Wong TY (2010) Correlation between retinal oscillatory potentials and retinal vascular caliber in type 2 diabetes. Invest Ophthalmol Vis Sci 51:482–486
Kern TS, Engerman RL (1995) Vascular lesions in diabetes are distributed non-uniformly within the retina. Exp Eye Res 60:545–549
Sutter EE, Tran D (1992) The field topography of ERG components in man: I. The photopic luminance response. Vision Res 32:433–446
Palmowski AM, Sutter EE, Bearse MA Jr, Fung W (1997) Mapping of retinal function in diabetic retinopathy using the multifocal electroretinogram. Invest Ophthalmol Vis Sci 38:2586–2596
Shimada Y, Li Y, Bearse MA Jr, Sutter EE, Fung W (2001) Assessment of early retinal changes in diabetes using a new multifocal ERG protocol. Br J Ophthalmol 85:414–419
Fortune B, Schneck ME, Adams AJ (1999) Multifocal electroretinogram delays reveal local retinal dysfunction in early diabetic retinopathy. Invest Ophthalmol Vis Sci 40:2638–2651
Bearse MA Jr, Han Y, Schneck ME, Barez S, Jacobsen C, Adams AJ (2004) Local multifocal oscillatory potential abnormalities in diabetes and early diabetic retinopathy. Invest Ophthalmol Vis Sci 45:3259–3265
Bronson-Castain KW, Bearse MA Jr, Neuville J, Jonasdottir S, King-Hooper B, Barez S, Schneck ME, Adams AJ (2009) Adolescents with type 2 diabetes early indications of focal retinal neuropathy, retinal thinning, and venular dilation. Retina 29:618–626
Sutter EE, Bearse MA Jr (1998) The retinal topography of local and lateral gain control mechanisms. Vision Science and its Applications, OSA Technical Digest Series 1:20–23
Hood DC, Greenstein V, Frishman L, Holopigian K, Viswanathan S, Seiple W, Ahmed J, Robson JG (1999) Identifying inner retinal contributions to the human multifocal ERG. Vision Res 39:2285–2291
Sutter EE, Shimada Y, Li Y, Bearse MA Jr (1999) Mapping inner retinal function through enhancement of adaptation components in the M-ERG. Vision Science and Its Applications, OSA Technical Digest Series 1:52–55
Chu PH, Chan HH, Ng YF, Brown B, Siu AW, Beale BA, Gilger BC, Wong F (2008) Porcine global flash multifocal electroretinogram: possible mechanisms for the glaucomatous changes in contrast response function. Vision Res 48:1726–1734
Lung JC, Chan HH (2010) Effects of luminance combinations on the characteristics of the global flash multifocal electroretinogram (mfERG). Graefes Arch Clin Exp Ophthalmol 248:1117–1125
Bearse MA Jr, Han Y, Schneck M, Adams A (2003) Enhancement and mapping of inner retinal contributions to the human multifocal electroretinogram (mfERG). Invest Ophthalmol Vis Sci 44: ARVO E-abstract 2696
Wu S, Sutter EE (1995) A topographic study of oscillatory potentials in man. Vis Neurosci 12:1013–1025
Sutter EE, Bearse MA Jr (1999) The optic nerve head component of the human ERG. Vision Res 39:419–436
Early Treatment Diabetic Retinopathy Study Research Group (1991) Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology 98:786–806
Early Treatment Diabetic Retinopathy Study Research Group (1991) Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology 98:823–833
Parisi V, Uccioli L (2001) Visual electrophysiological responses in persons with type 1 diabetes. Diabetes Metab Res Rev 17:12–18
Chen H, Zhang M, Huang S, Wu D (2008) The photopic negative response of flash ERG in nonproliferative diabetic retinopathy. Doc Ophthalmol 117:129–135
Vadala M, Anastasi M, Lodato G, Cillino S (2002) Electroretinographic oscillatory potentials in insulin-dependent diabetes patients: a long-term follow-up. Acta Ophthalmol 80:305–309
Brinchmann-Hansen O, Dahl-Jorgensen K, Hanssen KF, Sandvik L (1992) Oscillatory potentials, retinopathy, and long-term glucose control in insulin-dependent diabetes. Acta Ophthalmol 70:705–712
Simonsen SE (1980) The value of the oscillatory potential in selecting juvenile diabetics at risk of developing proliferative retinopathy. Acta Ophthalmol 58:865–878
Kurtenbach A, Langrova H, Zrenner E (2000) Multifocal oscillatory potentials in type 1 diabetes without retinopathy. Invest Ophthalmol Vis Sci 41:3234–3241
Onozu H, Yamamoto S (2003) Oscillatory potentials of multifocal electroretinogram in diabetic retinopathy. Doc Ophthalmol 106:327–332
Shinoda K, Rejdak R, Schuettauf F, Blatsios G, Volker M, Tanimoto N, Olcay T, Gekeler F, Lehaci C, Naskar R, Zagorski Z, Zrenner E (2007) Early electroretinographic features of streptozotocin-induced diabetic retinopathy. Clin Experiment Ophthalmol 35:847–854
Alder VA, Cringle SJ, Constable IJ (1983) The retinal oxygen profile in cats. Invest Ophthalmol Vis Sci 24:30–36
Arden GB, Gunduz MK, Kurtenbach A, Volker M, Zrenner E, Gunduz SB, Kamis U, Ozturk BT, Okudan S (2010) A preliminary trial to determine whether prevention of dark adaptation affects the course of early diabetic retinopathy. Eye 24:1149–1155
Linsenmeier RA (1986) Effects of light and darkness on oxygen distribution and consumption in the cat retina. J Gen Physiol 88:521–542
Linsenmeier RA, Braun RD, McRipley MA, Padnick LB, Ahmed J, Hatchell DL, McLeod DS, Lutty GA (1998) Retinal hypoxia in long-term diabetic cats. Invest Ophthalmol Vis Sci 39:1647–1657
Wangsa-Wirawan ND, Linsenmeier RA (2003) Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol 121:547–557
Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW (1998) Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest 102:783–791
Kaneko M, Sugawara T, Tazawa Y (2000) Electrical responses from the inner retina of rats with streptozotocin-induced early diabetes mellitus. J Jpn Ophthalmol Soc 101:775–778
Barber AJ (2003) A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry 27:283–290
Greenstein VC, Hood DC, Ritch R, Steinberger D, Carr RE (1989) S (blue) cone pathway vulnerability in retinitis pigmentosa, diabetes and glaucoma. Invest Ophthalmol Vis Sci 30:1732–1737
Bearse MA Jr, Sutter EE (1999) Contrast dependence of multifocal ERG components. Vision Science and its Applications, OSA Technical Digest Series 1:24–27
Chan HH, Ng YF, Chu PH (2011) Applications of the multifocal electroretinogram in the detection of glaucoma. Clin Exp Optom 94:247–258
Chu PH, Chan HH, Brown B (2006) Glaucoma detection is facilitated by luminance modulation of the global flash multifocal electroretinogram. Invest Ophthalmol Vis Sci 47:929–937
Schneck ME, Bearse MA Jr, Han Y, Barez S, Jacobsen C, Adams AJ (2004) Comparison of mfERG waveform components and implicit time measurement techniques for detecting functional change in early diabetic eye disease. Doc Ophthalmol 108:223–230
Bearse MA Jr, Han Y, Schneck ME, Adams AJ (2004) Retinal function in normal and diabetic eyes mapped with the slow flash multifocal electroretinogram. Invest Ophthalmol Vis Sci 45:296–304
Harrison WW, Bearse MA Jr, Ng JS, Jewell NP, Barez S, Burger D, Schneck ME, Adams AJ (2011) Multifocal electroretinograms predict onset of diabetic retinopathy in adult patients with diabetes. Invest Ophthalmol Vis Sci 52:772–777
Klemp K, Larsen M, Sander B, Vaag A, Brockhoff PB, Lund-Andersen H (2004) Effect of short-term hyperglycemia on multifocal electroretinogram in diabetic patients without retinopathy. Invest Ophthalmol Vis Sci 45:3812–3819
Masland RH (2001) Neuronal diversity in the retina. Curr Opin Neurobiol 11:431–436
Zhou W, Rangaswamy N, Ktonas P, Frishman LJ (2007) Oscillatory potentials of the slow-sequence multifocal ERG in primates extracted using the Matching Pursuit method. Vision Res 47:2021–2036
Simo R, Hernandez C (2009) Advances in the medical treatment of diabetic retinopathy. Diabetes Care 32:1556–1562
Acknowledgement
This study was supported by the Associated Fund (Research Postgraduate) from the Hong Kong Polytechnic University, Internal Research Grants (GU585, GU858) and the Niche Areas Myopia Research (J-BB7P) and Glaucoma Research (J-BB76) from the Hong Kong Polytechnic University. Special thanks to Prof. Brian Brown for his valued opinions.
Disclaimer
None of the authors have any proprietary interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Summary statement
In this study, a modified multifocal electroretinogram paradigm was applied to investigate the human diabetic retina. Early functional deterioration was detected before any clinically visible retinopathy.
Presented in part at the July 2009 ISCEV meeting, Abano Terme, Padova, Italy.
Rights and permissions
About this article
Cite this article
Lung, J.C.Y., Swann, P.G. & Chan, H.H.L. Early local functional changes in the human diabetic retina: a global flash multifocal electroretinogram study. Graefes Arch Clin Exp Ophthalmol 250, 1745–1754 (2012). https://doi.org/10.1007/s00417-012-2010-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-012-2010-z