Abstract
Purpose To test how the PhNR of the flash ERG is affected in human nonproliferative diabetic retinopathy (NPDR). Methods The PhNR was elicited with red stimuli (5 cd s/m2 with 4-min duration) and blue background (10 cd/m2). Standard Ganzfeld flash ERGs were recorded according to the ISCEV standard for the clinical electroretinogram (2004). A total of 81 diabetic patients with different severity levels of NPDR were examined. Forty-three age-matched normal controls were also studied. Results The amplitude of PhNR decreased significantly as DR progressed, while the implicit time was prolonged. Amplitudes of the PhNR in the control group, no DR, mild NPDR, moderate NPDR, and severe NPDR were 78.1 ± 15.1 μV, 69.0 ± 17.8 μV, 64.5 ± 13.2 μV, 45.9 ± 9.0 μV, and 33.7 ± 10.8 μV respectively, and the implicit times of PhNR were 71.5 ± 5.0, 72.0 ± 6.2, 73.6 ± 5.0, 75.7 ± 6.1, and 82.9 ± 7.8 min respectively. Compared to the control group, the reduction of PhNR amplitude in all diabetic groups was statistically significant. However, except for the OPs, the percent reduction of the amplitude of standard ERG waves was far less than that of the PhNR. The percent decrease in amplitude of the PhNR and ∑OPs was not significantly different in any diabetic group. However, the reduction of the amplitude of ∑OPs (and other standard ERG waves) was statistically significant only in moderate and severe NPDR groups. Conclusions PhNR is a sensitive indicator of the function of inner retina in diabetic patients. There is a potential role for the PhNR in assessing inner retinal damage and evaluating the effect of treatment in NPDR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic retinopathy (DR) is a leading cause of adult blindness and is the most common complication of diabetes. It affects more than 90% of people with diabetes, ultimately leading to retinal edema, neovascularization, and vision loss in some patients. DR is defined clinically by microvascular changes, and the primary pathogenesis is generally assumed to involve the retinal vessels. However, it has become increasingly clear that diabetic retinopathy affects not only retinal vasculature, but also the neural elements of the retina [1–5]. Retinal ganglion cells (RGC) are particularly susceptible to glutamate excitotoxicity, which plays an important role in ischemic diseases such as vessel occlusion and diabetic retinopathy [6].
Electroretinography provides an objective measure of the loss of retinal function in affected eyes. Studies in monkeys and cats [7, 8] have shown that the photopic negative response (PhNR) of flash full-field ERG, which follows the b-wave, represents the activity of RGCs and their axons. In the past several years, PhNR has been used in studies of glaucoma [8–11], retinal vascular disease [12–14], and optic neuropathy [15–17]. All of these experimental and clinical results indicate that PhNR is a very sensitive index in evaluating the function of inner retina. In the study by Kizawa et al. [14], using a white background and white stimuli, they found that amplitudes of the oscillatory potentials (OPs) and PhNR progressively decreased with the progression of DR. They also found amplitudes and implicit times of OPs are better indicators than those of the PhNR in detecting functional decreases in patients with early DR. In the present study, we recorded PhNR using blue background and red stimuli in patients with nonproliferative diabetic retinopathy (NPDR).
Material and methods
Subjects
A total of 81 diabetic patients with nonproliferative diabetic retinopathy (NPDR) at different severity levels and 43 age-matched normal controls were studied in the present report.
Patients were grouped according to the stage of retinopathy which was determined by the funduscopic findings and was based on the latest international clinical diabetic retinopathy disease severity scale [18]. According to this classification system, the first level is no apparent retinopathy, and the second is mild NPDR, in which only microaneurysms are seen. The third level is moderate NPDR and includes eyes with more than just microaneurysms but fewer features than severe NPDR. The fourth level is severe NPDR and includes any of the following ocular findings but no sign of proliferative diabetic retinopathy (PDR): >20 intraretinal hemorrhages in each of the four quadrants; definitive venous beading in two quadrants; or prominent intraretinal microvascular abnormalities in one quadrant. The fifth level is PDR and includes all eyes with distinct neovascularization.
In the present study, there were 19 patients (12 females and 7 males, aged 45–70, mean 60.5 ± 7.0-year old) with no apparent retinopathy, 19 patients (10 females and 9 males, aged 41–67, mean 54.6 ± 8.3-year old) with mild NPDR, 21 patients (11 females and 10 males, aged 42–77, mean 60.1 ± 10.9-year old) with moderate NPDR, and 22 patients (12 females and 10 males, aged 41–77, mean 61.3 ± 10.0-year old) with severe NPDR. The age-matched control group consisted of 43 normal controls (14 females and 29 males, aged 40–74, mean 57.0 ± 9.6-year old).
Each subject received comprehensive ophthalmological examination, including visual acuity, slit-lamp biomicroscopy, and indirect ophthalmoscopy. In addition, fundus fluorescein angiography (FFA) was performed in both eyes 1–7 days before the ERG was examined in all patients. None of the patients had laser photocoagulation or surgery history.
Only the data from the right eye were used for the analyses.
Informed consent was obtained from every subject or patient after the procedures used in the study were fully explained.
Stimulus
The PhNR and standard Ganzfeld ERG were recorded using the Espion visual testing system (Diagnosis). The PhNR was recorded with blue background (10 cd/m2, peak wavelength 465 nm) and red flashes (5 cd s/m2 with 4-min duration, peak wavelength 635 nm). Flashes were produced by light-emitting diodes. Standard Ganzfeld ERG was produced according to the ISCEV standard for the clinical electroretinogram (2004).
Recording
After the pupils were maximally dilated with topical 0.5% tropicamide and 0.5% phenylephrine hydrochloride, topical corneal anesthesia was induced with 1% dicaine hydrochloride. ERGs were recorded binocularly with contact-lens electrodes referenced to both ear lobes, and the forehead was grounded. Scotopic ERGs were first recorded after 20-min dark adaptation, and then the patient was light adapted under the background light of 30 cd/m2 for 10 min before recording photopic ERGs. After another 1-min blue background light adaptation, PhNRs were recorded with red flashes.
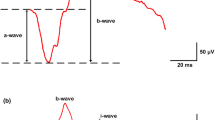
The amplitude of PhNR was measured from baseline to the trough immediately after the b-wave according to the study of Viswanathan et al. [7, 8, 11]. The sum amplitude of OP1, OP2, OP3, and OP4 was calculated. Implicit time of OP2, which is the most stable wavelet among the OPs, was measured.
Statistical evaluation
The data for each ERG response were analyzed with SPSS 11.0. The statistical significance of the data was calculated using the independent-samples t test. Receiver operating characteristic (ROC) analysis was performed by computer on the amplitude of PhNR and ∑OPs to determine the area under the ROC curve.
Results
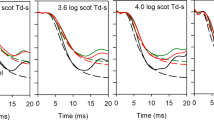
Representative PhNR and standard ERG responses recorded from one control and diabetic patients with different stages of DR are shown in Fig. 1. The amplitude of PhNR decreased obviously with the progression of DR, while the implicit time was prolonged. Among the standard ERG responses, OPs also were observed to decline, while other responses of the standard ERG did not change as much as PhNR and OPs.
Table 1 shows the ERG amplitudes (mean ± SD) of normal controls and diabetic patients with different stages of DR. Compared to the control group, the amplitude of PhNR in no DR, mild NPDR, moderate NPDR, and severe NPDR decreased by 11.8, 17.5, 41.3, and 56.9% respectively (see Fig. 2). While the amplitude of ∑OPs decreased by 12.2, 11.9, 37.1, and 59.5% respectively. The reduction of PhNR amplitude was statistically significant in all diabetic groups. However, despite the large mean change in amplitude, the drop of ∑OPs was statistically significant only in moderate and severe NPDR groups. However, the percent reduction for PhNR and ∑OPs was not significantly different for any diabetic groups. Other waves of the ERG were affected in moderate and severe NPDR. Figure 2 and Table 1 shows that in the moderate NPDR group, except for the combined (rod–cone)-b wave and rod-b wave, the amplitude reduction of all other measures was statistically significant. In the severe NPDR group, except for the combined-b wave, the amplitude reduction of all other measures was statistically significant.
Figure 3 illustrate the ROC curves of PhNR and OPs in patients with different stages of DR. The area under the ROC curves of PhNR in no DR, mild NPDR, moderate NPDR, and severe NPDR was 0.671, 0.765, 0.972, and 0.996 respectively. The area under the ROC curves of OPs in no DR, mild NPDR, moderate NPDR, and severe NPDR was 0.636, 0.608, 0.875, and 0.985 respectively.
Receiver operating characteristic curves of photopic negative response and oscillary potentials in patients with different stages of diabetic retinopathy. The area under the Receiver operating characteristic curves of photopic negative response is larger than that of oscillary potentials in any patient group with different stages of diabetic retinopathy
Table 2 shows the ERG implicit time (mean ± SD) in normal controls and diabetic patients with different stages of DR. The implicit time of PhNR increased with the progression of DR. Compared to control group, the increase in implicit time of PhNR was statistical significant only in moderate and severe NPDR groups. Similar results could be found in other ERG responses, including OP2.
Discussion
Atrophy of ganglion cells and degeneration of the INL in the retinas of humans with diabetes was described by Wolter [19] over 40 years ago and at the same time Bloodworth [20] found degeneration of the inner plexiform layer (IPL) and ganglion cell layer histologically. Neuroretinal cell biology has demonstrated abnormalities early after the onset of diabetes, before development of ophthalmoscopically defined retinopathy, that involves ganglion cells and cells of the inner nuclear and inner plexiform layers, where apoptosis has been demonstrated in rats and humans [5]. In the present study, the PhNR progressively decreased with the progression of DR, which is consistent with the hypothesis that retinal neuropathy may be an early and important component of the pathogenesis of diabetic retinopathy.
To record the PhNR, we used red flashes on blue background according to the method of Viswanathan et al. [7, 8, 11], who noticed the PhNR by accident with such recording conditions in a macaque study [7]. However, this does not necessarily mean that stimuli must be red flashes on a blue background in order to produce the PhNR. Monochromatic full-field test stimuli may produce more obvious PhNR than broadband stimuli because they provide less opportunity for inhibitory center-surround interactions in the responses of spectrally opponent retinal ganglion cells. This could enhance ganglion cell responses and increase the PhNR. Furthermore, when both background and flash are both spectrally broadband; more opportunity exists for light adaptation of the cone pathways that produce responses to the test flashes [21]. If inner retinal signals were adapted by backgrounds weaker than those affecting outer retinal signals, then signals originating from hyperpolarizing bipolar cells, photoreceptors, and perhaps horizontal cells, rather than from ganglion cells would provide the dominant negative potentials in the ERG.
In the present study, it was found that OPs and PhNR were affected more obviously than other waves. Compared to normal controls, the percent reduction in mean amplitude in different DR stages was greater and more similar for ∑OPs and PhNR than for the other waves. However, the amplitude decrease of ∑OPs was statistically significant only in moderate and severe NPDR, whereas it was statistically significant in all NPDR groups for PhNR. The present results are different from those of Kizawa et al. [15] who found that the amplitude of OPs was a better indicator than amplitude of the PhNR in detecting functional decreases in patients with early diabetic retinopathy. Part of the reason may be the different stimuli used to record PhNR. They use white flashes and white background which may be better stimuli for OPs while we use red flashes and blue background, which, depending on stimulus strength, can provide larger PhNRs.
Although there is consensus that OPs originate from the inner retina, the mechanisms by which they are generated are unresolved, with evidence demonstrated for both involvement of feedback circuitry and for intrinsic membrane mechanisms of amacrine cells [22, 23]. However, more and more evidence shows that the PhNR originates from the RGCs and their axons. So PhNR may be more sensitive than other ERG parameters in evaluating and detecting early functional changes to ganglion cells in DR.
References
Lieth E, Gardner TW, Barber AJ et al (2000) Retinal neurodegeneration: early pathology in diabetes. Clin Exp Ophthalmol 28:3–8
Barber AJ (2003) A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry 27:283–290
Martin PM, Roon P, VanElls TK et al (2004) Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci 45:3330–3336
Lopes de Faria JM, Russ H, Costa VP (2002) Retinal fibre layer loss in patients with type 1 diabetes mellitus without retinopathy. Br J Ophthalmol 86(7):725–728
Barber AJ, Lieth E, Khin SA et al (1998) Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest 102:783–791
Nakazawa T, Takahashi H, Nishijima K (2007) Pitavastatin prevents NMDA-induced retinal ganglion cell death by suppressing leukocyte recruitment. J Neurochemistry 100:1018–1031
Viswanathan S, Frishman LJ (1997) Evidence that negative potentials in the photopic electroretinograms of cats and primates depend upon spiking activity of retinal ganglion cell axons. Soc Neurosci Abstr 23:1024
Viswanathan S, Frishman LJ, Robson JG et al (1999) The photopic negative response of the macaque electroretinogram is reduced by experimental glaucoma. Invest Ophthalmol Vis Sci 40:1124–1136
Drasdo N, Aldebasi YH, Chiti Z et al (2001) The S-cone PhNR and pattern ERG in primary open angle Glaucoma. Invest Ophthalmol Vis Sci 42:1266–1272
Colotto A, Falsini B, Salgarello T et al (2000) Photopic negative response of the human ERG: losses associated with glaucomatous damage. Invest Ophthalmol Vis Sci 41:2205–2211
Viswanathan S, Frishman LJ, Robson JG et al (2001) The photopic negative response of the flash electroretinogram in primary open angle glaucoma. Invest Ophthalmol Vis Sci 42:514–522
Machida S, Gotoh Y, Tanaka M et al (2004) Predominant loss of the photopic negative response in central retinal artery occlusion. Am J Ophthalmol 137:938–940
Chen H, Wu D, Huang S et al (2006) The photopic negative response of the flash electroretinogram in retinal vein occlusion. Doc Ophthalmol 113:53–59
Kizawa J, Machida S, Kobayashi T et al (2006) Changes of oscillatory potentials and photopic negative response in patients with early diabetic retinopathy. Jpn J Ophthalmol 50:367–373
Li B, Barnes GE, Holt WF (2005) The decline of the photopic negative response (PhNR) in the rat after optic nerve transection. Doc Ophthalmol 111:23–31
Gotoh Y, Machida S, Tazawa Y (2004) Selective loss of the photopic negative response in patients with optic nerve atrophy. Arch Ophthalmol 122:341–346
Miyata K, Nakamura M, Kondo M et al (2007) Reduction of oscillatory potentials and photopic negative response in patients with autosomal dominant optic atrophy with OPA1 mutations. Invest Ophthalmol Vis Sci 48:820–824
Wilkinson CP, Ferris FL, Klein RE et al (2003) Proposed international diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 101:1677–1682
Wolter J (1961) Diabetic retinopathy. Am J Ophthalmol 51:1123–1141
Bloodworth JMB Jr. (1962) Diabetic retinopathy. Diabetes 11:1–22
Rangaswamy NV, Shirato S, Kaneko M et al (2007) Effects of spectral characteristics of Ganzfeld stimuli on the photopic negative response (PhNR) of the ERG. Invest Ophthalmol Vis Sci 48:4818–4828
Kenyon GT, Moore B, Jeffs J et al (2003) A model of high-frequency oscillatory potentials in retinal ganglion cells. Vis Neurosci 20:465–480
Vigh J, Solessio E, Morgans CW et al (2003) Ionic mechanisms mediating oscillatory membrane potentials in wide field retinal amacrine cells. J Neurophysiol 90:431–443
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, H., Zhang, M., Huang, S. et al. The photopic negative response of flash ERG in nonproliferative diabetic retinopathy. Doc Ophthalmol 117, 129–135 (2008). https://doi.org/10.1007/s10633-008-9114-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-008-9114-0