Abstract
Purpose
To compare Icare ONE rebound self-tonometer (ICRBT) measurements with Goldman applanation tonometry (GAT).
Methods
A trained examiner instructed each of 60 normal subjects on use of the ICRBT. Each subject then took two measurements of his/her own pressure using the ICRBT. Finally, a different examiner, who was masked to the earlier readings, measured IOP by GAT. Bland–Altman limits of agreement (LOA), intraclass correlation coefficients (ICCs), Kappa values, and paired t-test were used to assess the agreement between the two methods. Pearson’s correlation coefficient was used for correlation analysis.
Results
All of the subjects were able to obtain correct measurements with ICRBT after three attempts. The mean intraocular pressure with ICRBT and GAT measurements were 16.0 ± 3.3 mmHg and 13.7 ± 2.5 mmHg respectively. The mean difference between patient’s ICRBT and technician’s GAT measurements was 2.3 mmHg (p < 0.001). In 63 % (38/60) of the cases the IOP difference (ICRBT − GAT) was within ± 3 mmHg. The weighted Kappa for the IOP measurements of the two methods was 0.49 (95% CI: 0.30–0.68, p < 0.001), indicating acceptable agreement. A significantly positive correlation was found between ICRBT IOP measurements and central corneal thickness (CCT) (r = 0.48, p < 0.001). In addition, the difference in IOP measurements (ICRBT − GAT) between the two methods was positively correlated with CCT (r = 0.31, p = 0.015), indicating that greater thickness is associated with greater differences between the two methods.
Conclusion
The ICRBT was reliable in the hands of normal subjects, and may be used for self-monitoring of IOP. ICRBT measurements generally overestimated GAT measurements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although Goldmann applanation tonometry (GAT) is considered the gold-standard method for determining intraocular pressure (IOP), several inherent systemic errors and limitations of this method have been described [1, 2]. Recently, several new instruments and methods of tonometry were introduced into clinical practice, aiming to obtain a more accurate estimation of true IOP [3]. The Icare rebound tonometer (TA01, Tiolat Oy, Helsinki, Finland) is a portable handheld tonometer, which does not require any topical anesthetic. The principles of rebound tonometry have been described in detail elsewhere [4–6]. Briefly, the Icare tonometer records IOP by detecting the deceleration of a rod probe as it is bounced off the cornea. As the IOP increases, the rod probe bounces off the cornea faster. This movement is detected by a solenoid inside the instrument. It uses a disposable rod probe which has a plastic tip to minimize corneal injury. Several studies have shown that the Icare tonometer provided good results in terms of ease of use, precision, and reproducibility of its measurements. It has been also shown to correlate well with GAT, even in the hands of inexperienced tonometrists [7–20]. Recently a version of the Icare tonometer (Icare ONE) for home measurement of IOP by patients became commercially available [21]. The purpose of the present study was to compare Icare ONE measurements with GAT.
Methods
Sixty normal subjects were recruited to participate in the study. Subjects had no significant ocular history, were free of ocular symptoms and were not taking any medication. Subjects with physical or mental disability that prevented them from using the instrument were excluded from the study. The study used an Athens Eye Hospital Institutional Review Board-approved protocol. Before the measurements, all subjects received an anterior segment examination to ensure that no ocular disease existed and the cornea was clear. Central corneal thickness (CCT) was assessed using a non-contact optical low coherence reflectometry (OLCR) pachymeter (Haag–Streit, Switzerland).
ICRBT’s indicator displays 11 different pressure zones between 5 and 50 mmHg. The pressure zones are: 5–7, 7–10, 10–14, 14–18, 18–21, 21–24, 24–27, 27–30, 30–35, 35–40 and 40–50 mmHg. A series of six measurements are obtained for an indication (specific pressure zone) to be displayed, and an indication sounds at the last proper measurement. Only correct values are taken into consideration, since warning indications, for probe errors or wrong position of tonometer, are displayed and sounded during measurement. A built-in inclination sensor corrects the result automatically, eliminating the tonometer’s position sensitivity.
A trained technician instructed the participant how to use ICRBT. As the indicator led light displays a leeway per each measurement zone, the mean value was recorded as the respective IOP (e.g., an indication of IOP between 18–21 mmHg was recorded as 19.5 mmHg). Two measurements of the IOP taken from the participants, with a 10-minute difference, were evaluated. Similarly, two IOP measurements using the GAT with a 10-minute time interval between them were performed by an experienced technician who was unaware of the previous ICRBT measurements. All measurements (GAT& ICRBT) were conducted within 1 hour.
Only one eye’s IOP values for each participant, randomly chosen, were used for the statistical analysis. The average IOP values of each device are used to illustrate the comparison of the two devices. The difference in IOP measurements was calculated by subtraction: ICRBT − GAT.
Statistical analysis
Quantitative variables are expressed as mean values ± SD. Study variables were normally distributed as verified by the Kolmogorov–Smirnov test. Intraclass correlation coefficients (ICCs) for random effects models were used to assess interuser and intermethod agreement [22]. For intermethod agreement, the mean values of the first and second IOP measurements of the ICRBT and GAT methods were used. ICCs equal or lower to 0.40 indicate poor to fair agreement, 0.41–0.60 moderate agreement, 0.61–0.80 good agreement and over 0.80 excellent agreement [23]. Interuser and intermethod agreement were further assessed by Bland–Altman 95% confidence intervals (CI) for limits of agreement (LOA) [24]. The 95% CI for LOA indicates that 95% of the differences fall between these two limits [24]. Additionally, the repeatability coefficient was calculated as twice the standard deviation of the differences. The repeatability coefficient specifies the range within which the 95% of the differences between two IOP measurements of the same user are likely to fall.
Paired t-tests were also used to investigate differences between the measurements obtained by the two methods.
Kappa values were calculated to assess the agreement between the two methods across the 11 specific pressure zones of ICRBT. GAT measurements were classified accordingly for the analysis in the aforementioned pressure zones. Kappa is a measure of the level of agreement obtained in excess of that which would be observed by chance. Given the ordered nature of the data, weighted Kappa statistics were calculated. This allows more weight to be placed on the two measurements that are assigned to adjacent categories than on measurements assigned to non-adjacent categories. Large weighted Kappa values indicate small disagreement, in comparison with chance levels. The maximum is value 1, corresponding to perfect agreement; values greater or equal to 0.75 are considered as excellent agreement, and values greater than 0.4 indicate acceptable reliability [23].
Pearson correlation coefficients were used in order to explore the association of IOP measurements with CCT. P values reported are two-tailed. Statistical significance level was set at .05, and analysis was conducted using STATA 8.0 Statistical Software.
Results
The mean age of the participants was 55.5 ± 17.5 years, ranging from 17 to 82 years. The mean corneal thickness was 548.3 ± 36.9 μm. All of the subjects were able to obtain correct measurements with ICRBT after three attempts. The mean intraocular pressure with ICRBT and GAT measurements were 16.0 ± 3.3 mmHg and 13.7 ± 2.5 mmHg respectively. Table 1 presents the mean IOP measurements, the ICCs, and LOAs from ICRBT and GAT methods.
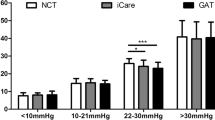
A significantly good agreement was found between the two methods (Fig. 1). Furthermore, an overestimation was found in ICRBT measurements compared to GAT ones as defined from paired comparisons (p < 0.001). Linear regression analysis indicated a significant slope (β = 0.57, p < 0.001) for the association of IOP ICRBT with GAT. Sixty-three percent (38/60) of the paired differences between ICRBT and GAT measurements were within the range of 3 mmHg. The agreement of the two measurements of the same methods is shown in Table 2. ICCs and LOAs indicated excellent agreement for both ICRBT and GAT methods. The weighted Kappa for the IOP measurements of the two methods was 0.49 (95% CI: 0.30–0.68, p < 0.001), indicating acceptable agreement. The weighted Kappa for the first and second IOP measurements of ICRBT method was 0.60 (95% CI: 0.43–0.77, p < 0.001), while the weighted Kappa for the first and second IOP measurements of GAT method was 0.54 (95% CI: 0.37–0.72, p < 0.001), indicating acceptable agreement.
A significantly positive correlation was found between ICRBT IOP measurements and CCT (r = 0.48, p < 0.001) and between GAT IOP measurements and CCT (r = 0.38, p = 0.003). Also the difference in IOP (ICRBT − GAT) measurements between the two methods was positively correlated with CCT (r = 0.31, p = 0.015), indicating that greater thickness is associated with greater differences between the two methods. The graphical presentation of correlation between ICRBT − GAT vs CCT is shown in Fig. 2.
Discussion
Management of glaucoma is currently based on the determination of IOP. The current approach is to measure IOP at a routine office visit. We know however that IOP varies over a course of a day and from day to day. Thus the sporadic determination of IOP in the office might be of similar value for the management of glaucoma as is the random sugar measurement for the management of diabetes [25]. The impact of 24-hour IOP fluctuations on glaucoma progression is a controversial issue, partly because there is not yet a reliable measurement technology for the determination of the 24-hour IOP. There is compelling evidence, however, that peak IOP may occur outside office hours [26].
Thus a self-tonometer that would give us access to IOP measurements during after- office hours, especially during the regular activities of the patient, outside an artificial clinical setting, would be clinically useful.
Self-tonometry may be used to uncover IOP fluctuations, monitor the effect of medications and reinforce patient compliance [27]. Hughes et al. in 2003 [28] reported that 24-hour IOP monitoring changed the clinical management of 79.3% of the patients in their study.
The present study indicated that the Icare-ONE tonometer may be used as a self-tonometer from well-motivated and adequately trained subjects. One of its advantages is that it does not require topical anesthesia, and it may be used by patients who have difficulties in instilling drops. All of the participants were able to obtain technically correct ICRBT measurements under the guidance of the technician. This is in agreement with the results of previous reports [21, 29].
The LOA between repeated ICRBT readings were ± 5.5 mmHg. This compares very well with the repeatability of rebound tonometer measurements obtained by trained technicians described in previous studies. Davies et al. [8] indicated that the 95% LOA between repeated ICRBT readings were ± 5.11 mmHg. This also agrees with the repeatability coefficient of measurements obtained by patients which was reported by Asrani et al. [21] to be between 5.98 and 7.58 mmHg.
The present study found a reasonable overall correlation between ICRBT and GAT: the weighted Kappa for the IOP measurements of the two methods was 0.49. Nevertheless, ICRBT overestimated IOP by 2.3 mmHg. This was similar to most of the previous studies, which reported overestimations from 0.2 to 3.4 mmHg [7–20]. Recently, Vasanthan Mutuvelou et al. [30] showed that central ICare measurements overestimated IOP by 2 mmHg and that peripheral measurements underestimated IOP by approximately 1.4–2 mmHg. Underestimation of IOP increased with angled measurements. According to their hypothesis, lesser degrees of overestimation reported in previous studies might be attributed to angled or peripheral measurements.
The study by Vasanthan Mutuvelou et al. [30] indicates that errors of self-tonometry with ICRBT may result from the inaccurate centration of the instrument. Self-ICRBT measurements may be obtained from the peripheral cornea rather than from the center. However, the impact of the correct centration on the accuracy of ICRBT measurements is not clear. Chui et al. [15] showed that although the peripheral cornea was significantly thicker than the central cornea, IOPs obtained by the ICare at the two regions were similar, and on average 1.94 mmHg higher than GAT measurements.
The present study indicated a positive correlation between ICRBT and CCT as well as between the difference in IOP measurements (ICRBT − GAT) and CCT (r = 0.31, p = 0.015), pointing out that greater thickness is associated with greater differences between the two methods. Several previous studies [10, 11, 18] reported that rebound tonometry measurements were influenced by CCT. In concordance with the present study, Brusini et al. [10] found that the deviation of ICare measurements from the CCT corrected GAT measurements correlated with CCT (r = 0.63, p < 0.01). Thus we must keep in mind that ICRBT measurements may be affected by the biomechanical properties of the cornea to a greater degree than GAT measurements.
There are several potential limitations in the present study that prevent us from drawing conclusions about how our results may apply to glaucoma patients. The participants were normal subjects with normal IOPs. Additionally, they were volunteers, highly motivated without any disability. In contrast, glaucoma patients tend to be older with significant comorbidity, which might affect their ability for self-tonometry [31]. Furthermore, although the present study indicated good agreement between ICRBT and GAT in a moderate IOP range, this may not be the case in high IOP values. A previous study indicated that in high IOP values, measurements with the ICare tonometer do not correlate well with GAT [14]. Finally, we must take into account that this study was based on mean IOP measurements, and that huge leeway is allowed by the examined version of the instrument, which might be too high for patients in need for a very tight IOP control.
In conclusion, the present study indicated that the Icare ONE self-tonometer may provide proper measurements in the hands of well-trained highly motivated normal subjects. Its measurements generally correlate well with GAT, overestimating IOP by approximately 2.3 mmHg. However, the difference between ICRBT and GAT should be measured for each individual patient, as it is affected by CCT.
References
Whitacre MM, Stein R (1993) Sources of error with use of Goldmann type tonometers. Surv Ophthalmol 38:1–30
Whitacre MM, Stein RA, Hassanein K (1993) The effect of corneal thickness on applanation tonometry. Am J Ophthalmol 115:592–596
ElMallah MK, Asrani SG (2008) New ways to measure intraocular pressure. Curr Opin Ophthalmol 19:122–126
Kontiola AI (2000) A new induction-based impact method for measuring intraocular pressure. Acta Ophthalmol Scand 78:142–145
Danias J, Kontiola AI, Filippopoulos T, Mittag T (2003) Method for the noninvasive measurement of intraocular pressure in mice. Invest Ophthalmol Vis Sci 44:1138–1141
Cervino A (2006) Rebound tonometry: new opportunities and limitations of non-invasive determination of intraocular pressure. Br J Ophthalmol 90:1444–1446
Martinez-de-la-Casa JM, Garcia-Feijoo J, Castillo A, Garcia Sanchez J (2005) Reproducibility and clinical evaluation of rebound tonometry. Invest Ophthalmol Vis Sci 46:4578–4580
Davies LN, Bartlett H, Mallen EA, Wolffsohn JS (2006) Clinical evaluation of rebound tonometer. Acta Ophthalmol Scand 84:206–209
Nakamura M, Darhad U, Tatsumi Y, Fujioka M, Kusuhara A, Maeda H, Negi A (2006) Agreement of rebound tonometer in measuring intraocular pressure with three types of applanation tonometers. Am J Ophthalmol 142:332–334
Brusini P, Salvetat ML, Zeppieri M, Tosoni C, Parisi L (2006) Comparison of ICare tonometer with Goldmann applanation tonometer in glaucoma patients. J Glaucoma 15:213–217
Martinez-de-la-Casa JM, Garcia-Feijoo J, Vico E, Fernandez-Vidal A, Benitez del Castillo JM, Wasfi M, Garcia-Sanchez J (2006) Effect of corneal thickness on dynamic contour, rebound, and Goldmann tonometry. Ophthalmology 113:2156–2162
Sahin A, Niyaz L, Yildirim N (2007) Comparison of the rebound tonometer with the Goldmann applanation tonometer in glaucoma patients. Clin Experiment Ophthalmol 35:335–339
Ruokonen PC, Schwenteck T, Draeger J (2007) Evaluation of the impedance tonometer TGDc-01 and iCare according to the international ocular tonometer standards ISO 8612. Graefes Arch Clin Exp Ophthalmol 245:1259–1265
Munkwitz S, Elkarmouty A, Hoffmann EM, Pfeiffer N, Thieme H (2008) Comparison of the iCare rebound tonometer and the Goldmann applanation tonometer over a wide IOP range. Graefes Arch Clin Exp Ophthalmol 246:875–879
Chui WS, Lam A, Chen D, Chiu R (2008) The influence of corneal properties on rebound tonometry. Ophthalmology 115:80–84
Jóhannesson G, Hallberg P, Eklund A, Lindén C (2008) Pascal, ICare and Goldmann applanation tonometry–a comparative study. Acta Ophthalmol 86:614–621
Abraham LM, Epasinghe NC, Selva D, Casson R (2008) Comparison of the ICare reboundtonometer with the Goldmann applanation tonometer by experienced and inexperienced tonometrists. Eye 22:503–506
Poostchi A, Mitchell R, Nicholas S, Purdie G, Wells A (2009) The iCare rebound tonometer: comparisons with Goldmann tonometry, and influence of central corneal thickness. Clin Experiment Ophthalmol 37:687–691
Vandewalle E, Vandenbroeck S, Stalmans I, Zeyen T (2009) Comparison of ICare, dynamic contour tonometer, and ocular response analyzer with Goldmann applanation tonometer in patients with glaucoma. Eur J Ophthalmol 19:783–789
Scuderi GL, Cascone NC, Regine F, Perdicchi A, Cerulli A, Recupero SM (2010) Validity and limits of the rebound tonometer (ICare®): clinical study. Eur J Ophthalmol 21:251–257
Asrani S, Chatterjee A, Wallace DK, Santiago-Turla C, Stinnett S (2011) Evaluation of the ICare rebound tonometer as a home intraocular pressure monitoring device. J Glaucoma 20:74–79
McGraw K, Wong S (1996) Forming Inferences about some intraclass correlation coefficients. Psychological Methods 1:30–46
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Altman DG, Bland JM (1983) Measurement in medicine: the analysis of method comparison studies. Statistician 32:307–317
Bagga H, Liu JH, Weinreb RN (2009) Intraocular pressure measurements throughout the 24 h. Curr Opin Ophthalmol 20:79–83
Konstas AG, Topouzis F, Leliopoulou O, Pappas T, Georgiadis N, Jenkins JN, Stewart WC (2006) 24-hour intraocular pressure control with maximum medical therapy compared with surgery in patients with advanced open-angle glaucoma. Ophthalmology 113:761–765
Liang SY, Lee GA, Shields D (2009) Self-tonometry in glaucoma management—past, present and future. Surv Ophthalmol 54:450–462
Hughes E, Spry P, Diamond J (2003) 24-hour monitoring of intraocular pressure in glaucoma management: a retrospective review. J Glaucoma 12:232–236
Tarkkanen A, Ulfves K, Ulfves T (2010) Self-tonometry in glaucoma. Graefes Arch Clin Exp Ophthalmol 248:1679–1681
Vasanthan Muttuvelu VD, Baggesen K, Ehlers N (2010) Precision and accuracy of the ICare tonometer - Peripheral and central IOP measurements by rebound tonometry. Acta Ophthalmol Sep 15 [Epub ahead of print]
Balkrishnan R, Bond JB, Byerly WG, Camacho FT, Anderson RT (2003) Medication-related predictors of health-related quality of life in glaucoma patients enrolled in amedicare health maintenance organization. Am J Geriatr Pharmacother 1:75–81
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have no proprietary interest in any aspect of this study.
Rights and permissions
About this article
Cite this article
Halkiadakis, I., Stratos, A., Stergiopoulos, G. et al. Evaluation of the Icare-ONE rebound tonometer as a self-measuring intraocular pressure device in normal subjects. Graefes Arch Clin Exp Ophthalmol 250, 1207–1211 (2012). https://doi.org/10.1007/s00417-011-1875-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-011-1875-6