Abstract
Background
Clinical trials have demonstrated that acute intensive insulin therapy may cause transient worsening of retinopathy in type 1 and type 2 diabetes patients. However, the related mechanism still remains controversial. The purpose of the present study was to investigate the effect of insulin on the mitochondrial membrane potential (△Ψm), reactive oxygen species (ROS) production, UCP-2 and VEGF expression in bovine retinal microvascular endothelial cells (BRECs) in the presence of normal or high glucose and the related mechanisms.
Methods
BRECs were isolated as primary cultures and identified by immunostaining. Passage BRECs were initially exposed to normal (5 mM) or high glucose (30 mM) for 3 days, with equimolar L-glucose supplemented for osmotic equation. Then the cells were treated with 1 nM, 10 nM, or 100 nM insulin for 24 h: △Ψm and ROS production were determined by JC-1 and CM-H2DCFDA, respectively. Expression of UCP-2 and VEGF mRNA was determined by real-time RT-PCR; expression UCP-2 and VEGF protein was determined by Western-blotting analysis. A general ROS scavenger N-acetylcysteine (NAC, 10 mM) and an NADPH oxidase inhibitor apocynin (1 mmol/l) were added 1 h before treatment with 100 nM insulin.
Results
Insulin increased △Ψm, ROS production, and expression of UCP-2 and VEGF in BRECs at normal glucose (5 mM) in a dose-dependent manner. Low-dose insulin (1 nM) decreased △Ψm, ROS production, and UCP-2, VEGF expression in BRECs at high glucose (30 mM); and high-dose insulin (10 nM, 100nM) recovered △Ψm, ROS production, and UCP-2, VEGF expression. Pretreatment of cells with NADPH oxidase inhibitor apocynin significantly suppressed 100 nM insulin-induced ROS production (p < 0.01, one-way ANOVA). Pretreatment of cells with ROS scavenger N-acetylcysteine completely blocked insulin-induced UCP-2 expression (p < 0.01, one-way ANOVA) and significantly suppressed VEGF expression (p < 0.01, one-way ANOVA).
Conclusions
High-dose insulin-induced ROS production and VEGF expression in BRECs in the presence of high glucose might be one of the reasons for the transient worsening of diabetic retinopathy during intensive insulin treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clinical trials have demonstrated that acute intensive insulin therapy may cause transient worsening of retinopathy in type 1 and type 2 diabetes patients [1–4]. The worsening result is in part due to hard exudates and macular edema pathologies of blood–retinal barrier breakdown. Several possible mechanisms have been reported for the phenomenon: first, diabetes was severe at the early stage; however, randomized clinical trails have ruled out this possibility [4]; second, insulin reduced retinal blood flow and subsequently led to retinal hypoxia, increased vascular permeability, and edema; third, insulin could cause arthrosclerosis and promote mitosis, thrombosis formation; finally, vascular endothelial growth factor was also thought to be involved in insulin-induced deterioration of retinopathy. Recently, insulin has been reported to increase intracellular reactive oxygen species (ROS) production in HepG2 cells [5], 3T3L1 adipocytes [6], thyroid cells [7], and human fibroblasts [8, 9].
ROS have been well documented as a signaling molecule stimulating cytokine secretion and cell proliferation, but at higher concentrations, ROS can induce cell injury/death by oxidant modification of proteins and carbohydrates, lipid peroxidation, and DNA strand nicks. Furthermore, ROS, like other edemagenic mediators (e.g., thrombin, histamine, and TNF-α), can cause intercellular gap formation, cell-shape change, and actin filament reorganization [10]; these morphological features implicate impaired cell–cell adhesion, which eventually results in impaired endothelial barrier function and increased leukocyte adhesion and extravasation [11]. Acute and chronic oxidant stress is a major cause of vascular endothelial dysfunction and plays an important role in the pathophysiology of several vascular diseases, including diabetes [11]. There is substantial evidence that oxidant stress increases vascular endothelial permeability [12–16] and leukocyte extravasation [17–19]; besides, oxidative stress has been shown to play a key regulatory role in the development of diabetic complications [20–23]. Recently, a unifying hypothesis has been proposed that uncoupling protein (UCP-2)-mediated mitochondrial ROS production in a chronic hyperglycemia setting may be a key initiator in increased polyol pathway flux, increased production of advanced glycation end-products, activation of protein kinase C, and increased hexosamine pathway flux [24, 25]. To the best of our knowledge, there have been no previous reports on the role of insulin in ROS production, UCP-2, and VEGF expression in endothelial cells at normal and high glucose. Moreover, hyperglycemia-induced ROS appears to be produced mainly from mitochondrial sources and through the enzyme NADPH oxidase [26]. However, the mechanisms by which insulin stimulates ROS production and their pathophysiological implications remain to be elucidated.
In the present study, we aim to verify whether insulin can increase the ROS production and UCP-2 expression in BRECs at normal or high glucose and to discuss the related mechanisms, so as to pave the way for investigating the damages caused by acute intensive insulin therapy. We examined the mitochondrial membrane potential (△Ψm) and ROS production in BRECs exposed to 5 or 30 mmol/l glucose after they were pretreated with different concentrations of insulin; intracellular ROS was examined by fluorescent inverted microscope and flow cytometry. Flow cytometry was also used to measure △Ψm in BRECs. Real-time PCR was used to examine mRNA expression of UCP-2 and VEGF, and Western blotting was used to examine the UCP-2 protein expression.
Materials and methods
Cell culture
BRECs were isolated by homogenization and by a series of filtration steps as described previously [27], and were subsequently cultured in Dulbecco’s modified Eagle’s medium (DMEM, GIBCO) supplemented with 10% FBS (GIBCO), 100 mg/l heparin (Sigma), 10 mmol/l HEPES (GIBCO), and 15 mg/LECGS (Sigma). The culture plate was coated with Gelatin (Sigma) beforehand. Cells were cultured in 5% CO2 at 37°C and media were changed every 3 days. Thereafter, the cells were characterized for their homogeneity using an immunoreactivity test with anti-factor VIII antibody for BRECs. Only cells of passages 2–5 were used for the following experiments. BRECs were cultured for 3 days in DMEM containing either 5 or 30 mmol/l glucose. L-glucose (25 mmol/l) was supplemented in the 5 mmol/l group as osmotic agent. Cells were serum-deprived overnight in Dulbecco’s modified Eagle’s medium containing 0.2% w/v bovine serum albumin and then incubated in the absence or presence of different concentrations of insulin for 24 h. A general ROS scavenger N-acetylcysteine (NAC, 10 mM) or an NADPH oxidase inhibitor apocynin were added 1 h before treatment with 100nM insulin.
Measurement of mitochondrial membrane potential and ROS production
Mitochondrial membrane potential (△Ψm) in BRECs was measured by flow cytometry (Coulter Epics XL; Beckman-Coulter, Fullerton, CA) as described in a previous study [28]. ROS production in the cells was determined using fluorescent probe 5-(and-6)-chloromethyl-2′, 7′-dihydrodichlorofluorescein diacetate acetyl ester (CMH2DCFDA; Molecular Probes, Eugene, OR). After being incubated with 2 μmol/l CMH2DCFDA at 37°C for 30 min, the cells were washed twice with fresh pre-warmed medium and imaged using a Zeiss inverted fluorescence microscope. Cells were collected from growth media via centrifugation or pipetting for flow cytometry (Coulter Epics XL; Beckman-Coulter, Fullerton, CA). Cells were resuspended in pre-warmed PBS containing the probe to provide a final working concentration of 2 μM dye. After incubation in 5% CO2 at 37°C for 30 min, the loading buffer was removed and the cells were given prewarmed growth medium for incubation at 37°C for 10 min. Cells in 5 mM glucose group were used for determination of the baseline fluorescence intensity. After flow cytometry, the cells were counted and fluorescence was expressed as per 105 cells. All the results were tested in triplicate.
Analysis of UCP-2 and VEGF mRNA expression by real-time RT-PCR
Total RNA was extracted from BRECs with reagent (Trizol; Invitrogen Life Technologies, Gaithersburg, MD) and was stored at −80°C until use. A quantitative polymerase chain reaction (qPCR) kit (DyNAmo Flash SYBR Green; Finnzymes Oy, Espoo, Finland) was used according to the manufacturer’s instructions. The primer sequences (sense/antisense) were used as follows: UCP-2, 5′- ACGGGACACCTTTAGAGAAGCTT-3′/5′- ACTTTCTCCTTGGATCTGTAACCG-3′; VEGF, 5′- GCAGAATCATCACGAAGTGG-3′/5′- GCATGGTGATGTTGGACTCC-3′; β-actin, 5′- AAAGACCTGTACGCCAACAC-3 ′/5 ′-GTCATACTCCTGCTTGCTGAT-3 ′. The specificity of the amplification product was determined by a melting curve analysis. Standard curves were generated for each gene by preparing serial dilutions of the respective cDNA gene template of known quantities. Relative quantities of each gene were obtained by normalizing their signals to that of β-actin.
Analysis of UCP-2 and VEGF protein expression by Western-blotting assay
BRECs (4 × 106 ) were collected and lysed in lysis buffer (NP40 1%, Tris 10 mM, NaCl 200 mM, EDTA 5 mM, glycerol 10%, and protease inhibitors; pH 7). Cell samples were centrifuged at 12,000 rpm for 20 min at 4°C, and clear supernatants were collected. Fifty micrograms of protein from each sample was subjected to SDS-PAGE using a Bio-Rad miniature slab gel apparatus and was electrophoretically transferred onto a nitrocellulose sheet. The sheet was blocked with 5% nonfat dried milk solution and incubated overnight with partially purified mouse anti-UCP-2 polyclonal antibody (Sigma Chemical Co; 1:500), mouse monoclonal anti-vascular endothelial growth factor antibody (Sigma V4758; 1:200), β-Actin (monoclonal anti-β-actin; Sigma; 1:1000) expression was used as an internal control to confirm equivalent total protein loading. After blotting for UCP-2, the membrane was stripped and was blotted for VEGF.
Statistical analysis
The results were expressed as mean ± SD from three separate experiments and the data were subjected to one-way ANOVA analysis. A p value less than 0.05 was considered statistically significant. All analyses were done with a software system (Prism 4.0; GraphPad, San Diego, CA) and a statistical software program (SPSS13.0 for Windows; SPSS, Chicago, IL).
Results
Insulin increased △Ψm and ROS in BRECs at normal glucose in a dose-dependent manner
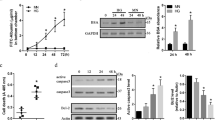
Flow cytometry showed that insulin increased △Ψm in BRECs at normal glucose in a dose-dependent manner (Fig. 1a). Compared to the NG group (11.17 ± 1.72), insulin at 1 nM, 10 nM, and 100 nM induced a 1.18-fold (13.23 ± 0.91), 1.33-fold (14.90 ± 0.92), and 1.49-fold (17.40 ± 1.31) △Ψm in BRECs at normal glucose, respectively, with △Ψm in group NG significantly lower than those in groups NG + 10nM insulin (p < 0.05, one-way ANOVA) and NG + 100nM insulin (p < 0.01, one-way ANOVA). Consistent with the increase of △Ψm, insulin also dose-dependently increased ROS production in BRECS (Fig. 1-b); insulin at 1 nM, 10 nM, and 100 nM induced a 1.51-fold (126.2 ± 14.4), 1.98-fold (165.6 ± 9.1), and 2.31-fold (193.2 ± 21.1) ROS production in BRECS at normal glucose, respectively, with the ROS abundance in group NG (83.6 ± 12.8) significantly lower than those in groups NG + 1 nM insulin (p < 0.05, one-way ANOVA), NG + 10nM insulin (p < 0.01, one-way ANOVA), NG + 10 0nM insulin (p < 0.01, one-way ANOVA). Moreover, Pearson correlation analysis indicated that ROS production was positively correlated with the △Ψm (r = 0.97, p = 0.0136) (Fig. 1c).
Mitochondrial membrane potential (△Ψm) and ROS production in BRECs treated with insulin in presence of normal glucose (NG). a △Ψm in BRECs cultured in NG (5 mM glucose) treated with 0, 1, 10, or 100 nm insulin. b ROS production in BRECs treated with 0, 1, 10, or 100 nm insulin in presence of normal glucose. c Correlation analysis between △Ψm level and intracellular ROS generation in BRECs. Mean ± SD for three separate experiments (*p < 0.05 vs. NG; **p < 0.01 vs. NG)
Low-dose insulin decreased and high-dose insulin recovered △Ψm and ROS production in BRECs
As shown in Figs. 2a and b, compared with the HG group (16.63 ± 0.80), 1 nM, 10 nM, and 100 nM insulin induced a 0.74-fold (12.33 ± 1.33), 1-fold (16.57 ± 1.33), 1.33-fold (22.10 ± 2.36) of △Ψm at high glucose (30 mM), respectively. In parallel with increased △Ψm, 1nM, 10nM, and 100nM insulin also induced a 0.79-fold (135.2 ± 6.8), 0.96-fold (165.4 ± 12.1), and 1.12-fold (191.5 ± 9.9) ROS production, respectively. Compared with the control group (171.7 ± 12.2), 1nM insulin significantly decreased △Ψm (p < 0.05, one-way ANOVA) and ROS (p < 0.05, one-way ANOVA) production. However, when the cells were treated with 10 nM and 100 nM insulin, there was a gradual recovery of △Ψm and ROS production. Pearson correlation analysis also indicated that ROS production was positively correlated with the △Ψm (r = 0.94, p = 0.029) (Fig. 2c).
Mitochondrial membrane potential (△Ψm) and ROS production in BRECs treated with insulin in presence of high glucose (HG). a △Ψm in BRECs treated with 0, 1, 10, or 100 nm insulin cultured in the presence of high glucose (30 mM glucose). b ROS production in BRECs treated with 0, 1, 10, or 100 nm insulin in the presence of high glucose (30 mM glucose). c Correlation analysis between △Ψm level and intracellular ROS generation in BRECs. Mean ± SD for three separate experiments (# p < 0.05 vs. HG)
Insulin upregulated UCP-2, VEGF mRNA, and protein expression in BRECs at normal glucose
To determine the effects of insulin on UCP-2, VEGF expression at normal glucose, serum-starved endothelial cells were treated with 0, 1 nM, 10 nM, or 100 nM insulin for 24 h, and UCP-2, VEGF mRNA, and protein expression were examined by real-time PCR and Western-blotting analysis. Compared to the NG group, 1 nM, 10 nM, and 100 nM insulin increased UCP-2, VEGF mRNA, and protein expression in a dose-dependent manner (Fig. 3). NAC at 10 mM completely blocked 100 nM insulin-induced UCP-2 mRNA (0.97 ± 0.12 vs. 2.80 ± 0.36; p < 0.01, one-way ANOVA) and protein (0.630 ± 0.060 vs. 1.043 ± 0.091; p < 0.01, one-way ANOVA) expression, and partially blocked 100 nM insulin-induced VEGF mRNA (2.06 ± 0.11 vs. 4.03 ± 0.36; p < 0.01, one-way ANOVA) and protein (1.397 ± 0.060 vs. 1.780 ± 0.095; p < 0.01, one-way ANOVA) expression.
UCP-2 and VEGF expression in BRECs treated with insulin in the presence of normal glucose (5 mM glucose). a UCP-2 mRNA expression in BRECs treated with 0, 1, 10, or 100 nM insulin, 10 mM NAC + 100 nM insulin. b VEGF mRNA expression in BRECs treated with 0, 1, 10, or 100 nM insulin, 10 mM NAC + 100 nM insulin. c UCP-2 protein expression in BRECs treated with 0, 1, 10, or 100 nM insulin, 10 mM NAC + 100 nM insulin. d VEGF protein expression in BRECs treated with 0, 1, 10, or 100 nM insulin, 10 mM NAC + 100 nM insulin. Mean ± SD for three separate experiments (*p < 0.05 vs. NG; **p < 0.01 vs. NG)
Low-dose insulin decreased and high-dose insulin recovered UCP-2 and VEGF expression in BRECs at high glucose
To further determine the effects of insulin on UCP-2 and VEGF at high glucose, serum-starved endothelial cells at high glucose were treated with 0, 1 nM, 10 nM, and 100 nM insulin for 24 h, and UCP-2 mRNA and protein expression was quantified by real-time PCR and Western blotting. Compared to the HG group, insulin at 1 nM and 10 nM significantly decreased mRNA and protein expression of UCP-2 and VEGF (p < 0.01, one-way ANOVA) (Fig. 4). However, 100 nM insulin completely or partially recovered UCP-2 and VEGF expression (p > 0.05, one-way ANOVA).
UCP-2, VEGF expression in BRECs treated with insulin in presence of high glucose (30 mM glucose). a UCP-2 mRNA expression in BRECs treated with 0, 1, 10, or 100 nM insulin. b VEGF mRNA expression in BRECs treated with 0, 1, 10, or 100 nM insulin. c UCP-2 protein expression in BRECs treated with 0, 1, 10, or 100 nM insulin. d VEGF protein expression in BRECs treated with 0, 1, 10, or 100 nM insulin. Mean ± SD for three separate experiments (*p < 0.05 vs. HG; **p < 0.01 vs. HG)
Apocynin inhibited insulin-induced ROS production
To explore the mechanism by which insulin induces ROS upregulation, we measured the ROS production in BRECs pretreated with apocynin, an NADPH oxidase inhibitor. As illustrated in Fig. 5a and b, insulin-induced ROS appears to be produced mainly from mitochondrial sources and through the enzyme NADPH oxidase. Since apocynin significantly decreased 100 nM insulin-induced ROS production (77.70 ± 14.87 vs. 199.40 ± 33.80; p < 0.01, one-way ANOVA).
NADPH oxidase inhibitor apocynin inhibited insulin-induced ROS production in presence of normal glucose (5 mM glucose). a Intracellular ROS generation in BRECs treated with 0, 100 nm insulin, or 1 mmol/l apocynin +100 nM insulin with flow cytometry. Mean ± SD for three separate experiments (**p < 0.01 vs. NG +100nM insulin). b Intracellular ROS generation in BRECs treated with 0 (a), 100 nm insulin (b), or 1 mmol/l apocynin + 100 nM insulin (c) under a Zeiss inverted fluorescence microscope. a Weak fluorescence for ROS. b Increased fluorescence for ROS. c Apocynin greatly reduced fluorescence for ROS
Discussion
We found in the present study that insulin increased △Ψm, ROS production, and UCP-2 expression in BRECs in a dose-dependent manner in the presence of normal glucose. Low-dose insulin (1 nM) decreased △Ψm, ROS production, and UCP-2, VEGF expression in BRECs in presence of high glucose, and high-dose insulin (10 nM, 100 nM) recovered △Ψm, ROS production, and UCP-2, VEGF expression. Pretreatment of cells with apocynin, an NADPH oxidase inhibitor, significantly suppressed the insulin-induced ROS production (p < 0.01, one-way ANOVA). Pretreatment of cells with ROS scavenger N-acetylcysteine significantly suppressed the insulin-induced UCP-2 and VEGF expression (p < 0.01, one-way ANOVA).
In diabetes, the main sources of ROS generation in the vasculature include glucose auto-oxidation, the polyol pathway, AGE, mitochondrial electron transport chain (ETC), uncoupled eNOS, and NAd (P)H oxidases, with the latter enzymes arguably a major source of ROS generation in hypertension, atherosclerosis, and diabetes [26, 29]. Recently, insulin has been reported to increase intracellular ROS production, possibly through phosphatidylinositol 3´- kinase-dependent mechanism and protein kinase C-dependent mechanism [5–9]. Our study demonstrated that insulin could increase intracellular ROS production in BRECs also through mitochondria and NADPH oxidase, as administration of NAd (P)H inhibitor apocynin can counteract insulin-induced overproduction of ROS. Consequently, insulin also upregulates UCP-2 expression, a sensor and a negative regulator of ROS production, in BRECs. UCP-2 is a moiety known to compensatively dissipate △Ψm and to uncouple oxidative respiration. We found that insulin upregulated UCP-2 expression through modulating ROS production, since ROS scavenger NAC could completely blocked the insulin-induced UCP-2 expression. Moreover, insulin also could also upregulate the expression of VEGF in BRECs, which was the possible cause responsible for the transient worsening of retinopathy in diabetes patients. Since the ROS scavenger NAC could partially suppress the insulin-induced VEGF expression, we believe insulin also can upregulate VEGF expression through ROS production.
It is interesting to notice that different effects of low-dose insulin and high-dose insulin on the △Ψm, ROS production, and UCP-2 expression in BRECs at high glucose (30 mM). On one hand, insulin can directly increase intracellular ROS production through mitochondria and NADPH oxidase; on the other hand, insulin-induced decrease of glucose concentration could indirectly down-regulate ROS production, and the final outcome is decided by which effect is stronger. When low-dose insulin (1 nM) was used, the upregulation ROS production is slight, and there is a decrease of ROS production and UCP-2 expression in BRECs. However, when high-dose (10 nM, 100 nM) insulin was used, the upregulation ROS production was strong, and there was a recovery of ROS production and UCP-2 expression.
The mechanism by which acute intensive insulin therapy cause a transient worsening of retinopathy in type 1 and type 2 diabetes patients still remains controversial. Lately, VEGF has been found to be involved in insulin-induced deterioration of retinopathy [30]. The present study found that when BRECs were treated with high-dose (100 nM) insulin at high glucose, the ROS production and VEGF expression were re-increased. Therefore, clinical intensive treatment with insulin may, while controlling the blood glucose, induce more ROS production and VEGF expression, leading to the transient worsening of retinopathy in diabetes patients.
In conclusion, insulin can increase △Ψm, ROS production, and expression of UCP 2 and VEGF expression in BRECs in presence of normal glucose in a dose-dependent manner ex vivo. High-dose insulin-induced ROS production and VEGF expression in BRECs in the presence of high glucose might be one of the reasons for the transient worsening of diabetic retinopathy during intensive insulin treatment.
References
The Diabetes Control and Complications Trial Research Group (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977–986
Roysarkar TK, Gupta A, Dash RJ, Dogra MR (1993) Effect of insulin therapy on progression of retinopathy in noninsulin-dependent diabetes mellitus. Am J Ophthalmol 115:569–574
Henricsson M, Janzon L, Groop L (1995) Progression of retinopathy after change of treatment from oral antihyperglycemic agents to insulin in patients with NIDDM. Diabetes Care 18:1571–1576
The Diabetes Control and Complications Trial (1995) The effect of intensive diabetes treatment on the progression of diabetic retinopathy in insulin-dependent diabetes mellitus. Arch Ophthalmol 113:36–51
Carnesecchi S, Carpentier JL, Foti M, Szanto I (2006) Insulin-induced vascular endothelial growth factor expression is mediated by the NADPH oxidase NOX3. Exp Cell Res 312:3413–3424
Mahadev K, Wu X, Zilbering A, Zhu L, Lawrence JT, Goldstein BJ (2001) Hydrogen peroxide generated during cellular insulin stimulation is integral to activation of the distal insulin signaling cascade in 3 T3-L1 adipocytes. J Biol Chem 276:48662–48669
Krawiec L, Pizarro RA, Aphalo P (2004) Role of peroxidase inhibition by insulin in the bovine thyroid cell proliferation mechanism. Eur J Biochem 271:2607–2614
Ceolotto G, Papparella I, Lenzini L (2006) Insulin generates free radicals in human fibroblasts ex vivo by a protein kinase C-dependent mechanism, which is inhibited by pravastatin. Free Radic Biol Med 41:473–483
Ceolotto G, Bevilacqua M, Papparella I (2004) Insulin generates free radicals by an NAd (P)H, phosphatidylinositol 3′-kinase-dependent mechanism in human skin fibroblasts ex vivo. Diabetes 53:1344–1351
Lum H, Malik AB (1994) Regulation of vascular endothelial barrier function. Am J Physiol 267:L223–L241
Lum H, Roebuck KA (2001) Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol 280:C719–C741
Johnson A, Phillips P, Hocking D, Tsan MF, Ferro T (1989) Protein kinase inhibitor prevents pulmonary edema in response to H2O2. Am J Physiol 256:H1012–H1022
Barnard ML, Matalon S (1992) Mechanisms of extracellular reactive oxygen species injury to the pulmonary microvasculature. J Appl Physiol 72:1724–1729
Shasby DM, Lind SE, Shasby SS, Goldsmith JC, Hunninghake GW (1985) Reversible oxidant-induced increases in albumin transfer across cultured endothelium: alterations in cell shape and calcium homeostasis. Blood 65:605–614
Ochoa L, Waypa G, Mahoney JR Jr, Rodriguez L, Minnear FL (1997) Contrasting effects of hypochlorous acid and hydrogen peroxide on endothelial permeability: prevention with cAMP drugs. Am J Respir Crit Care Med 156:1247–1255
Holman RG, Maier RV (1990) Oxidant-induced endothelial leak correlates with decreased cellular energy levels. Am Rev Respir Dis 141:134–140
Gaboury JP, Anderson DC, Kubes P (1994) Molecular mechanisms involved in superoxide-induced leukocyte-endothelial cell interactions in vivo. Am J Physiol 266:H637–H642
Hotter G, Closa D, Prats N, Pi F, Gelpi E, Rosello-Catafau J (1997) Free radical enhancement promotes leucocyte recruitment through a PAF and LTB4 dependent mechanism. Free Radic Biol Med 22:947–954
Scalia R, Lefer AM (1998) In vivo regulation of PECAM-1 activity during acute endothelial dysfunction in the rat mesenteric microvasculature. J Leukoc Biol 64:163–169
Baynes JW, Thorpe SR (1999) Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes 48:1–9
Haskins K, Bradley B, Powers K (2003) Oxidative stress in type 1 diabetes. Ann NY Acad Sci 1005:43–54
Watts GF, Playford DA, Croft KD, Ward NC, Mori TA, Burke V (2002) Coenzyme Q (10) improves endothelial dysfunction of the brachial artery in type II diabetes mellitus. Diabetologia 45:420–426
Kowluru RA, Tang J, Kern TS (2001) Abnormalities of retinal metabolism in diabetes and experimental galactosemia. VII. Effect of long-term administration of antioxidants on the development of retinopathy. Diabetes 50:1938–1942
Forbes JM, Coughlan MT, Cooper ME (2008) Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 57:1446–1454
Brownlee M (2005) The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54:1615–1625
Gao L, Mann GE (2009) Vascular NAd (P)H oxidase activation in diabetes: a double-edged sword in redox signalling. Cardiovasc Res 82:9–20
Wu H, Xia X, Jiang C, Wu J, Zhang S, Zheng Z, Liu W, Zhang Y, Ren H, Wei C, Xu X (2010) High glucose attenuates insulin-induced VEGF expression in bovine retinal microvascular endothelial cells. Eye 24:145–151
Cui Y, Xu X, Bi H, Zhu Q, Wu JF, Xia X, Ren Q, Patrick C (2006) Expression modification of uncoupling proteins and MnSOD in retinal endothelial cells and pericytes induced by high glucose: the role of reactive oxygen species in diabetic retinopathy. Exp Eye Res 83:807–816
Cave AC, Brewer AC, Narayanapanicker A (2006) NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal 8:691–728
Poulaki V, Qin W, Joussen AM, Hurlbut P, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP (2002) Acute intensive insulin therapy exacerbates diabetic blood-retinal barrier breakdown via hypoxia-inducible factor-1alpha and VEGF. J Clin Invest 109:805–815
Acknowledgements
This work was supported by grants from the National Basic Research Program of China (973 program), (2007 CB512205), National Basic Research grants of China (30872825, 2008), and the Plan of the Best Disciplines Leaders in Shanghai (09XD1400900).
Disclosure statement
None to declare.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, H., Jiang, C., Gan, D. et al. Different effects of low- and high-dose insulin on ROS production and VEGF expression in bovine retinal microvascular endothelial cells in the presence of high glucose. Graefes Arch Clin Exp Ophthalmol 249, 1303–1310 (2011). https://doi.org/10.1007/s00417-011-1677-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-011-1677-x