Abstract
Background
Anterior segment cytomegalovirus (CMV) infection, which can be presented as anterior uveitis and corneal endotheliitis, has recently been reported in immunocompetent patients. We would like to access the validity of two presumed characteristic clinical profiles: profile 1, non-herpes simplex virus (HSV)/varicella zoster virus (VZV) corticosteroid-recalcitrant inflammatory ocular hypertensive syndrome (IOHS), and profile 2, corneal endotheliitis with specific coin-shaped keratic precipitates (KPs), that could be helpful in identifying CMV anterior segment intraocular infection.
Methods
Patients with either profile 1 or profile 2 or both were enrolled consecutively from the uveitis service in Chang Gung Memorial Hospital, Taoyuan, between January 1, 2006 and May 31, 2010. Diagnostic anterior chamber tapping was performed and followed by real-time quantitative polymerase chain reaction (PCR) to detect herpesviridae DNA including HSV I and II, VZV, CMV, and Epstein–Barr virus.
Results
Thirty-one eyes of 30 patients (21 males and nine females) were enrolled in this study. CMV DNA PCR was positive in 29 eyes of 28 patients (20 males and eight females). Nineteen of 20 eyes (19 patients) in profile 1 had positive CMV PCR. Ten of 11 eyes (11 patients) in profile 2 had positive CMV PCR. The positive predictive value of profile 1 and profile 2 was 94.7% and 90.9%, respectively. The positive predictive value of combining the two profiles was 93.3%.
Conclusions
Non-HSV/ZVZ corticosteroid-recalcitrant IOHS and corneal endotheliitis with specific coin-shaped KPs could be used as the screening tool for CMV anterior segment intraocular infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anterior segment cytomegalovirus (CMV) infections, which can be presented as anterior uveitis and corneal endotheliitis, have been reported recently in immunocompetent patients [1–12]. It has been identified as one of the disease entities recently in what was previously classified as idiopathic anterior uveitis and idiopathic corneal endotheliitis [11, 13]. CMV anterior uveitis has a wide spectrum of clinical manifestations, but all the previously reported patients presented as hypertensive anterior uveitis [1–3, 8, 9]. CMV corneal endotheliitis is characterized by mild iritis and corneal edema associated with coin-shaped and/or linear keratic precipitates (KPs) [10, 11]; Koisumi et al. thought coin-shaped KPs were a characteristic sign of CMV endotheliitis [5, 10]. To make a definite diagnosis of CMV anterior segment infection, aqueous tapping for polymerase chain reaction (PCR) analysis or intraocular antibody assay is necessary. However, the patients are reluctant to have aqueous tapping and these tests are expensive in our country. Besides, aqueous tapping on every patient with anterior uveitis, the major part in the uveitis service, might not be an acceptable ethical principle. A simple and useful screening tool for CMV anterior segment infection is mandatory.
In this study, we would like to evaluate the validity of the two presumed characteristic clinical profiles of CMV anterior segment infection: (1) non-herpes simplex virus (HSV)/varicella zoster virus (VZV) corticosteroid-recalcitrant inflammatory ocular hypertensive syndrome (IOHS), and (2) corneal endotheliitis with specific coin-shaped keratic precipitates (KPs), to see if they help the clinicians to identify such an infection, then make an accurate diagnosis and initiate a prompt treatment to prevent subsequent morbidities.
Patients and methods
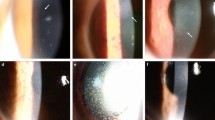
This study was conducted as a single-center retrospective review of patients with anterior segment intraocular inflammation between January 1, 2006 and May 31, 2010. Laboratory data collected for each enrolled patient included complete blood count with white blood cells differential count, erythrocyte sedimentation rate, chest radiography, tuberculin test, syphilis study, human leukocyte antigen B27 study, urine routine study, human immunocompromised virus serology and CD4/CD8 T cell count study. In addition, they were referred to an internal medicine physician for further evaluation if necessary. Inclusion criteria for aqueous tapping were: profile (1) Non- HSV/ VZV IOHS refractory to topical prednisolone acetate (1%) hourly for at least 1 week; profile (2) corneal endotheliitis with specific coin-shaped KPs, irrespective of the magnitude of the IOP. The corneal endotheliitis with coin-shaped KPs was defined as medium-sized KPs arranged in a circumferential pattern with associated localized corneal edema (Fig. 1) [10]. Exclusion criteria for this study were: (1) presence of posterior or intermediate uveitis; (2) history or typical clinical presentations of HSV or VZV ocular infections; (3) previous cataract surgery with residual lens material; and (4) a known or suspicious infectious uveitis etiology such as syphilis, human immunodeficiency virus, and tuberculosis. After obtaining informed consent from the patients, diagnostic anterior chamber tapping was performed followed by polymerase chain reaction (PCR) to detect herpesviridae DNA including HSV I and II, VZV, CMV, and Epstein–Barr virus (EBV). This clinical study followed the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of Chang Gung Memorial Hospital.
The real-time polymerase chain reaction protocol was based on the method by Stocher et al. [14]. The CMV PCR primers were designed as GenBank accession numbers NC001347 (CMV-L-for 5’-GACACAACACCGTAAAGC-3’, CMV-L-rev 5’-CAGCGTTCGTGTTTCC-3’) suggested by Gault et al. [15]. The PCR primers for HSV were designed as GenBank accession numbers M16721 (HSV-for 5’-GCTCGAGTGCGAAAAAACGTTC-3’, HSV-rev 5’-CGGGGCGCTCGGCTAAC-3’) suggested by Espy et al. [16]. The PCR primers for VZV were designed as VZV -for 5’-TGTCCTAGAGGAGGTTTTATCTG-3’, VZV-ref 5’-CATCGTCTGTAAGACTTAACCAG-3’) suggested by Stocher et al. [14]. The PCR primers for EBV were designed as EBV -for 5’-GGTAGGTCCCCTGGACCTGCCGCT-3’, EBV-rev 5’-CCCACCCACAGATCCAACGA-3’ as suggested by Chang et al. [17]. The detection limit was reported at 250 virus-derived DNA copies per millilter with high sensitivity and specificity [14–17]. The detection limit was reconfirmed by the standard curve of real-time PCR of our laboratory.
Patients were examined at 1 week, 1 month, and then monthly thereafter. Additional visits were arranged depending upon the patient’s condition. The treatment trials included intravitreal ganciclovir injection only, oral valganciclovir (Valcyte™, F. Hoffmann-La Roche Ltd, Basel, Switzerland) only or intravitreal ganciclovir injection followed by oral valganciclovir. The CMV infection was considered controlled if the aqueous inflammation was less than 1+ for more than 1 month after the cessation of treatment. We graded the aqueous inflammation on the basis of the grading scheme developed by the SUN working group [18]. The age, gender, follow-up length, best-corrected visual acuity (BCVA), intraocular pressure, intraocular inflammation activity, viral DNA PCR results and treatment strategy were reviewed and recorded at the pre-tapping and final post-tapping visits.
Descriptive statistics were reported as mean ± SD or median ± SD for continuous variables. Differences between two normally distributed groups were evaluated by Student’s t test. Paired Wilcoxon signed-ranks test was used to compare two values which were paired, but far from normal distribution. All statistical assessments were two-sided and evaluated at the 0.05 level of significance. A p value adjusted by the multiple comparison tests was calculated in each experiment. SPSS software version 12.0.1 for Windows (SPSS, Inc., Chicago, IL, USA) was used for calculation.
Results
Thirty-one eyes of 30 patients conformed to profile 1 or 2. All of the enrolled patients had neither systemic immunodeficiency nor leukocyte antigen B27 allele. The enrolled patients included 21 males and nine females. The median age of the enrolled patients was 54.5 ± 16.0 years (range 25–85 years). The average follow-up period after aqueous tapping was 8.0 months (range 1–36 months). One patient had bilateral eyes involvement. Demographics and summary of the patients are listed in Table 1.
Twenty eyes of 19 patients (patients 1–19) fit profile 1, i.e., non-HSV/ZVZ corticosteroid-recalcitrant IOHS. Nineteen eyes of 18 patients (patients 1–18) had positive CMV DNA PCR result. The average genomic copy number of CMV in the eyes with profile 1 was 3.8 × 105 ± 1.0 × 106 /ml. One patient (patient 4) had positive PCR result for both EBV and CMV. The positive predictive value of profile 1 for CMV iritis was 94.7%.
Eleven eyes of 11 patients (patients 20–30) were presented as profile 2, i.e., corneal endotheliitis with specific coin-shaped KPs. Ten patients had positive CMV DNA PCR result. The average genomic copy number of CMV in the eyes with profile 2 was 9.4 × 105 ± 2.2 × 106 /ml. The positive predictive value of profile 2 for CMV corneal endotheliitis was 90.9%. All the patients with profile 2 had associated mild aqueous reaction and all except five patients (patients 18, 26, 27, 28, and 29) had elevated IOP.
When we combined the two profiles together, the positive predicative value was 93.3%.
All the 28 patients with positive CMV PCR results showed poor corticosteroid response for iridocyclitis and endotheliitis. All patients except one (patient 26 in the first trimester of pregnancy) received anti-CMV treatments. Four eyes of three patients (patients 1, 6, and 22) received oral valganciclovir treatment only (900 mg twice a day; mean duration: 9.3 months; range 3–13 months); 11 eyes of 11 patients (patients 2–4, 7, 10, 11, 16–18, 23, 24) had the intravitreal ganciclovir injection once without the following oral valganciclovir; 12 eyes of 12 patients (patients 5, 8, 9, 12–15, 20, 21, 25, 27–29) received intravitreal ganciclovir followed by oral valganciclovir (mean duration: 2.5 months, range 0.5–6 months). All treated patients had good inflammation control after anti-CMV treatments, but one patient (patient 1) who was treated with oral valganciclovir solely, had recurrent inflammation 1 month after cessation of the medication. Four patients (patients 1, 14, 22, 27) had persistent diffuse bullous corneal edema after treatments and two patients (patients 22 and 27) received penetrating keratoplasty for persistent diffuse corneal edema after anti-CMV treatment and patient 22 had cataract extraction and intraocular lens implantation simultaneously. Patient 28 had two previous grafts that failed; CMV corneal endotheliitis was diagnosed on the third graft. The IOP of the patients was well controlled after anti-CMV treatments, and no patients needed further filtering surgery.
Case reports
Patient 5
A 63-year-old male had hypertensive anterior uveitis in the left eye since 1999, and had been treated with topical corticosteroids and IOP-lowering agents. There was nothing contributory in the right eye. The aqueous inflammation responded poorly to the medication. He received trabeculectomy and cataract extraction with intraocular lens insertion surgeries in the left eye in April 2006 at another hospital. The anterior uveitis persisted after the operation. He was referred to us in November 2007. The BCVA was 6/12 in the left eye. The conjunctival bleb was high and avascular in the nasal upper quadrant. The IOP was 33.6 mmHg in the left eye. The aqueous inflammation was 1+ without flare. No iris atrophy or posterior synechiae was detected. The fundus was normal but glaucomatous cupping was observed.
The infection survey was negative for HIV, tuberculosis, and syphilis and the HLA-B27 allele was negative. The aqueous viral DNA PCR was positive for CMV. We did the intravitreal injection of ganciclovir (2 mg/0.05 ml) in the right eye followed by 4 weeks of oral valganciclovir (900 mg twice a day). The iritis subsided and did not recur. The BCVA was 6/12 and the last IOP was 20 mmHg at the 5-month follow-up.
Patient 20
A 32-year-old male was diagnosed with glaucomatocyclitis crisis in the right eye in October 2004. The left eye was normal and not contributory. The uveitis had been poorly controlled for the following 2 years. The intraocular pressure had been fluctuating between 25 and 40 mmHg. He came to our service on January 2007. The ocular examination showed BCVA was 6/30 and intraocular pressure was 38.7 mmHg. Corneal endotheliitis with specific coin-shaped keratic precipitates was noted in the right eye (Fig. 2a). The vitreous was clear. The fundus was normal except for the glaucomatous optic cupping change. The infection survey was negative for HIV, tuberculosis, and syphilis. HLA-B27 was negative. We performed aqueous tapping and found that the viral DNA PCR was positive for CMV but negative for HSV and EBV.
He received intravitreal injection of ganciclovir (2 mg/0.05 ml) 2 weeks later after diagnosis, followed by oral maintenance valganciclovir (900 mg twice daily) for 3 months. The keratic precipitates and corneal edema subsided with the treatment regimens (Fig. 2b). The visual acuity was 6/15 after 5 months of treatment. The IOP decreased to 7.4 mmHg, but the BCVA also decreased to 6/20 due to hypotensive choroidopathy at 17-month follow-up.
Discussion
In this study, we assessed two clinical manifestations: non-HSV/VZV corticosteroid-recalcitrant IOHS (profile 1) and corneal endotheliitis with coin-shaped KPs (profile 2) that could be predictors of anterior segment CMV infection. We used real-time quantitative PCR detection of CMV DNA as the definitive diagnostic tool and the positive predictive value of profile 1 and profile 2 was 94.7 and 90.9%, respectively. The overall positive predictive value for the 2 profiles for CMV anterior segment infection was 93.3%. In addition, most of our CMV DNA-positive patients had responded to ganciclovir or valganciclovir treatment clinically, which lent further credence to CMV as the causative agent.
Two different presentations in CMV anterior segment infection have been reported in immunocompetent patients in the literature. One is corneal endotheliitis [5, 6, 10, 19–21] and the other is anterior uveitis [3, 7, 8, 22, 23]. In the reports of patients with CMV anterior uveitis, high IOP was one of the clinical manifestations [7, 8, 21]. In the patients with CMV endotheliitis, however, high IOP was not described in every case. In Koizumi's eight-case series of PCR-proven CMV endotheliitis, four had good IOP under medical control and two had normal IOP as in our five patients (patients 22, 26, 27, 28, and 29) [10]. Contrary, in Chee's study of ten cases of PCR-proven CMV endotheliitis, all of them presented with high IOP. The IOP was included in profile 1 because anterior uveitis is the most common type of uveitis and the high IOP could narrow down the screening range. Topical corticosteroid has been commonly used in anterior uveitis with good therapeutic achievements. It is also a good diagnostic tool for differentiating infectious from non-infectious etiology if the treatment response is poor [24]. We added it into this profile as a criteria to further narrow down the candidates for study inclusion. Nevertheless, high IOP was not included in the cases in profile 2 because the coin-shaped KP is unique. Koizumi et al. believed that the coin-shaped KPs were a characteristic sign of CMV corneal endotheliitis and may represent the viral plaques generally seen in virus titration procedures [10]. Shiraishi et al. inspected one of the coin-shaped lesions under a confocal microscope and identified a group of large endothelial cells with owl's morphology, which is a pathognomonic sign of CMV infections [19]. When we add elevated IOP to profile 2, the positive predicative value decreased to 54.5% in our study.
Chee et al. devised a similar screening profile for hypertensive anterior uveitis [8]. They enrolled 105 eyes with hypertensive anterior uveitis for the aqueous viral DNA PCR and 24 eyes (22.8%) tested positive for CMV. The different positive predictive value between their study and ours could be the different clinical profile criteria. Although the risk of aqueous tapping is not high, it is not allowed by our IRB committee to be routinely applied to every patient with anterior uveitis clinically, so we did not include all cases of all hypertensive anterior uveitis but narrowed down the inclusion population with the criterion of “refractory to topical corticosteroid treatment”. This is also the reason why we did not have the control group of uveitis or non-uveitis patients and no negative predictive value could be determined in this study.
The virus DNA titer on real-time PCR had a wide variation in our study. Because of the retrospective study, it was hard for us to know the real interval between the onset of symptoms and the aqueous tapping, which might be associated with viral DNA concentrations. However, most our patients responded well to anti-CMV treatment and repeat tap for the patients, who had persistent corneal edema after treatment, revealed no detectable CMV DNA, so this indicates that CMV might be the etiologic, not bystander virus. We did not do intraocular CMV antibody assay because of the limited volume of the specimens. In addition, intraocular antibody assay has not been found to be a more sensitive test for CMV anterior segment infection [3, 7].
We recommend that patients with CMV anterior segment infection should receive treatment when morbidity, such as corneal edema or glaucomatous change, develops. Koizumi et al. reported two patients with CMV endotheliitis experienced persistent bullous keratopathy due to no or delayed ganciclovir treatment and Kandori et al. reported three additional such cases, as in our four patients (patients 1, 14, 22, and 27) [10, 11, 25], because the clinical corneal remission after anti-CMV treatment may depend on the amount of normal endothelium at the start of treatment. Chee et al. treated the CMV DNA-positive patients with ganciclovir if they had progressive glaucoma optic disc damage or increasingly frequent attacks of hypertensive uveitis with the attendant risk of developing glaucoma [8]. This is also one of the reasons that we defined these two clinical profiles. However, the standard treatment has not yet been established since CMV anterior segment infection is an entity observed only fairly recently. Oral valganciclovir [7], topical ganciclovir [10], intravenous ganciclovir, and intravitreal ganciclovir injection [8] have been suggested as treatment options to suppress the intraocular CMV infection. We tried several treatment protocols on our patients and found intravitreal ganciclovir (2 mg/0.05 ml) as the loading dosage combined with oral valganciclovir, which was prescribed according to the severity of the post-injection inflammation index, may be a good treatment strategy for CMV anterior uveitis [26].
In conclusion, non-HSV/ZVZ corticosteroid-recalcitrant IOHS and corneal endotheliitis with specific coin-shaped KPs could be used as the screening profiles for the diagnosis of CMV infections. Patients with such ocular manifestations deserve a further laboratory study. Viral DNA PCR for aqueous humor could serve as an effective diagnostic tool for CMV infection. Treatment with antivirals may be warranted in patients when morbidity such as corneal edema or glaucomatous change is observed.
References
Mietz H, Aisenbrey S, Ulrich Bartz-Schmidt K, Bamborschke S, Krieglstein GK (2000) Ganciclovir for the treatment of anterior uveitis. Graefes Arch Clin Exp Ophthalmol 238:905–909
Markomichelakis NN, Canakis C, Zafirakis P, Marakis T, Mallias I, Theodossiadis G (2002) Cytomegalovirus as a cause of anterior uveitis with sectoral iris atrophy. Ophthalmology 109:879–882. doi:S0161-6420(02)00961-2
de Schryver I, Rozenberg F, Cassoux N, Michelson S, Kestelyn P, Lehoang P, Davis JL, Bodaghi B (2006) Diagnosis and treatment of cytomegalovirus iridocyclitis without retinal necrosis. Br J Ophthalmol 90:852–855. doi:10.1136/bjo.2005.086546
Kim EC, Margolis TP (2006) Hypertensive iridocyclitis. Br J Ophthalmol 90:812–813. doi:10.1136/bjo.2006.091876
Koizumi N, Yamasaki K, Kawasaki S, Sotozono C, Inatomi T, Mochida C, Kinoshita S (2006) Cytomegalovirus in aqueous humor from an eye with corneal endotheliitis. Am J Ophthalmol 141:564–565. doi:10.1016/j.ajo.2005.09.021
Chee SP, Bacsal K, Jap A, Se-Thoe SY, Cheng CL, Tan BH (2007) Corneal endotheliitis associated with evidence of cytomegalovirus infection. Ophthalmology 114:798–803. doi:10.1016/j.ophtha.2006.07.057
van Boxtel LA, van der Lelij A, van der Meer J, Los LI (2007) Cytomegalovirus as a cause of anterior uveitis in immunocompetent patients. Ophthalmology 114:1358–1362
Chee SP, Bacsal K, Jap A, Se-Thoe SY, Cheng CL, Tan BH (2008) Clinical features of cytomegalovirus anterior uveitis in immunocompetent patients. Am J Ophthalmol 145:834–840. doi:10.1016/j.ajo.2007.12.015
Chee SP, Jap A (2008) Presumed Fuchs heterochromic iridocyclitis and Posner-Schlossman syndrome: comparison of cytomegalovirus-positive and negative eyes. Am J Ophthalmol 146:883–889 e881. doi:10.1016/j.ajo.2008.09.001
Koizumi N, Suzuki T, Uno T, Chihara H, Shiraishi A, Hara Y, Inatomi T, Sotozono C, Kawasaki S, Yamasaki K, Mochida C, Ohashi Y, Kinoshita S (2008) Cytomegalovirus as an etiologic factor in corneal endotheliitis. Ophthalmology 115:292–297 e293
Kandori M, Inoue T, Takamatsu F, Kojima Y, Hori Y, Maeda N, Tano Y (2010) Prevalence and features of keratitis with quantitative polymerase chain reaction positive for cytomegalovirus. Ophthalmology 117:216–222. doi:10.1016/j.ophtha.2009.06.059
Miyanaga M, Sugita S, Shimizu N, Morio T, Miyata K, Maruyama K, Kinoshita S, Mochizuki M (2010) A significant association of viral loads with corneal endothelial cell damage in cytomegalovirus anterior uveitis. Br J Ophthalmol 94:336–340. doi:10.1136/bjo.2008.156422
Van Gelder RN (2008) Idiopathic no more: clues to the pathogenesis of Fuchs heterochromic iridocyclitis and glaucomatocyclitic crisis. Am J Ophthalmol 145:769–771. doi:10.1016/j.ajo.2008.02.010
Stocher M, Leb V, Bozic M, Kessler HH, Halwachs-Baumann G, Landt O, Stekel H, Berg J (2003) Parallel detection of five human herpes virus DNAs by a set of real-time polymerase chain reactions in a single run. J Clin Virol 26:85–93
Gault E, Michel Y, Dehee A, Belabani C, Nicolas JC, Garbarg-Chenon A (2001) Quantification of human cytomegalovirus DNA by real-time PCR. J Clin Microbiol 39:772–775
Espy MJ, Uhl JR, Mitchell PS, Thorvilson JN, Svien KA, Wold AD, Smith TF (2000) Diagnosis of herpes simplex virus infections in the clinical laboratory by LightCycler PCR. J Clin Microbiol 38:795–799
Chang YS, Tyan YS, Liu ST, Tsai MS, Pao CC (1990) Detection of Epstein–Barr virus DNA sequences in nasopharyngeal carcinoma cells by enzymatic DNA amplification. J Clin Microbiol 28:2398–2402
Jabs DA, Nussenblatt RB, Rosenbaum JT (2005) Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol 140:509–516
Shiraishi A, Hara Y, Takahashi M, Oka N, Yamaguchi M, Suzuki T, Uno T, Ohashi Y (2007) Demonstration of “owl's eye” morphology by confocal microscopy in a patient with presumed cytomegalovirus corneal endotheliitis. Am J Ophthalmol 143:715–717. doi:10.1016/j.ajo.2006.11.026
Suzuki T, Hara Y, Uno T, Ohashi Y (2007) DNA of cytomegalovirus detected by PCR in aqueous of patient with corneal endotheliitis after penetrating keratoplasty. Cornea 26:370–372. doi:10.1097/ICO.0b013e31802d82fa00003226-200704000-00024
Yamauchi Y, Suzuki J, Sakai J, Sakamoto S, Iwasaki T, Usui M (2007) A case of hypertensive keratouveitis with endotheliitis associated with cytomegalovirus. Ocul Immunol Inflamm 15:399–401. doi:10.1080/09273940701486795
Hart WM Jr, Reed CA, Freedman HL, Burde RM (1978) Cytomegalovirus in juvenile iridocyclitis. Am J Ophthalmol 86:329–331
Teoh SB, Thean L, Koay E (2005) Cytomegalovirus in aetiology of Posner-Schlossman syndrome: evidence from quantitative polymerase chain reaction. Eye 19:1338–1340. doi:10.1038/sj.eye.6701757
Ohashi Y, Kinoshita S, Mano T, Kiritoshi A, Ohji M (1985) Idiopathic corneal endotheliopathy. A report of two cases. Arch Ophthalmol 103:1666–1668
Hwang YS, Hsiao CH, Tan HY, Chen KJ, Chen TL, Lai CC (2009) Corneal endotheliitis. Ophthalmology 116:164–164 e161. doi:10.1016/j.ophtha.2008.09.030
Hwang YS, Lin KK, Lee JS, Chang SH, Chen KJ, Lai CC, Huang JC, Kuo YH, Hsiao CH (2010) Intravitreal loading injection of ganciclovir with or without adjunctive oral valganciclovir for cytomegalovirus anterior uveitis. Graefes Arch Clin Exp Ophthalmol 248:263–269. doi:10.1007/s00417-009-1195-2
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hwang, YS., Shen, CR., Chang, S.H.L. et al. The validity of clinical feature profiles for cytomegaloviral anterior segment infection. Graefes Arch Clin Exp Ophthalmol 249, 103–110 (2011). https://doi.org/10.1007/s00417-010-1510-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-010-1510-y