Abstract
Background
To assess the effectiveness of OloGen (also named iGen), a porous, bioengineered, biodegradable, collagen-glycoaminoglycan matrix implant, in preventing poor bleb formation and early failure after trabeculectomy in eyes with a surgical wound defect.
Methods
The right eyes of 30 female New Zealand albino rabbits underwent trabeculectomy with OloGen implanted subconjunctivally on top of the scleral flap, while six right eyes received trabeculectomy without the implant to serve as a control group. A 1–2 mm diameter circular conjunctival defect was created in all eyes. Six rabbits in the group receiving the implant were sacrificed on days 3, 5, 7, 21, and 28. Rabbits in the control group were sacrificed on day 28. Perkins applanation tonometry, Seidel test and measurement of both the extent of the conjunctival defect and the anterior chamber depth were performed. Enucleated eyes were fixed in 4% formaldehyde and stained with hematoxylin and eosin (H&E) for general histological observation, and with Sirius and Fast-green stains to assess collagen deposition and cell migration.
Results
Seidel tests were negative for all operated and control eyes. No flat anterior chamber occurred in either group. With the exception of days 5 and 7, post-operative mean IOP difference is significant in both groups, (P > 0.05 for day 5, 7 and P < 0.05 for day 3, 14, 21 and 28). In the implant group, the mean IOP was reduced by between 42% and 35% at days 14, 21, and 28, whereas the mean IOP in the control group was reduced by between only 12% and 2%. In the implant group, histology showed randomized collagen deposition and microcyst formation in the bleb after the matrix had degraded completely at day 28. In the control group, histology showed dense collagen deposition subconjunctivally at day 28.
Conclusions
OloGen successfully serves as a 3-dimensional scaffold for cell migration and proliferation, and can prevent failure by maintaining the size of the bleb in the presence of a large wound defect. It might also be successful at repairing postoperative bleb leaks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tissue engineering involves the combination of a polymer scaffold with a population of stem, progenitor or precursor cells. Tissue growth is modeled to favor the development of a particular structure and, if the polymer scaffold used is biodegradable, can result in the formation of structures which are remarkably similar to normal tissue [1].
Long-term intraocular pressure (IOP) control following trabeculectomy may be limited by filtration failure, due to scarring at the level of the conjunctiva-Tenon’s-episcleral interface, the scleral flap or its overlying episclera, or the internal ostium [2, 3]. Adjunctive antifibrosis therapy with 5-fluorouracil (5-FU) or mitomycin-C (MMC) during or after filtering surgery has increased surgical success rates. Other agents, such as corticosteroids [4, 5], growth factor inhibition [6], and amniotic membrane [7] have been studied to improve surgical outcomes. Although antifibrotic agents have significant benefits in improving success rates of filtration surgery, late complications associated with these drugs, such as hypotony, bleb leaks and bleb-related infections, are common [8–10]. Accordingly, development of methods to overcome these complications has become an important challenge.
Tissue bioengineering using a three-dimensional, porous, biodegradable collagen-glycosaminoglycan matrix is a potential alternative method for controlling the wound healing process following filtration surgery and for avoiding complications with the administration of antifibrotic agents [11]. This technique directs wound healing toward tissue regeneration and away from scar formation by guiding the patterns of fibroblast migration, by normalizing secreted extracelluar matrix deposition, and by maintaining the size of the bleb, even in the face of a bleb leak by virtue of the 3-D structure of the implant, which functions prior to biodegradation as a reservoir. The physical properties of the collagen matrix may also have the ability to modify postoperative wound healing in patients with deficient conjunctiva due to previous repeated surgical intervention [12, 13], or an inadvertent conjunctival button-hole during surgery. These conditions risk postoperative bleb leaks and flat anterior chambers with poor bleb formation. The clinical applications of OloGen in trabeculectomy have been presented at conferences (European Congress of Ophthalmology, Vienna, Jun 9–12, 2007; World Glaucoma Congress, Singapore, July 18–21, 2007). In this study, we focus on create a three-dimensional wound healing process by implanting OloGen to get a better leaked bleb healing after trabeculectomy.
Materials and methods
Implantation of biodegradable matrix
All investigations conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. A drug-free, biodegradable, porous collagen matrix of 1% collagen/C-6-S copolymer (OloGen) with a 7-mm diameter and a 4-mm thickness was used. The pore size of the collagen matrix ranged from 20 μm to 200 μm. Thirty-six female New Zealand albino rabbits weighing 2.5–3.5 kg were anesthetized by an intramuscular injection of ketamine (35 mg/kg) and xylazine (5 mg/kg). Thirty eyes underwent trabeculectomy with matrices implanted subconjunctivally, and six eyes underwent trabeculectomy alone to serve as a control group.
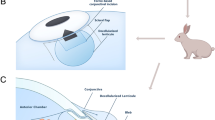
Trabeculectomy was performed using Watson’s modification of Cairns’ technique described in detail elsewhere [14]. A limbus-based conjunctival flap was fashioned, and a rectangular 3 × 4 mm scleral flap was prepared. A 1 × 2 mm sclerostomy was followed by a peripheral iridectomy, The scleral flap was closed with one relatively loose 10-0 nylon suture. To control the course of bleb leakage, re-epithelialization was controlled to heal at the same time by creating a different size of defect initially in this study. A 1–2 mm diameter circular conjunctival defect, 2 mm from limbus, was created by incompletely suturing the conjunctival incision. The OloGen was placed on top of the scleral flap, under the conjunctival defect in the implant group (Fig. 1). In the control group, a 1–2 mm circular conjunctival defect was created without implantation of the matrix. Tenonectomy was not performed, and antifibrotic agents were not used. On postoperative days 3, 5, 7, 14, 21, and 28, groups of six rabbits each in the implant group were evaluated. Groups of six rabbits were also sacrificed on the postoperative days 3, 5, 7, 21, and 28 for histologic examination. The six rabbits in the control group were evaluated clinically on postoperative days 3, 5, 7, 14, 21, and 28 and were sacrificed on postoperative day 28 for histologic examination.
Clinical evaluation
Rabbits were anesthetized with half dosage of intramuscular ketamine (35 mg/kg) and Xylazine (5 mg/kg) on the above post-operative days. A Seidel test was performed. The extent of the conjunctival defect was measured with a computer-drafted 0.2 mm grid ruler printed on a transparent slide. IOP was measured prior to surgery and on the above days using Perkins applanation tonometry, after the rabbits had been anesthetized with a half dose of intramuscular ketamine (35 mg/kg) and xylazine (5 mg/kg). The percent postoperative IOP reduction was calculated as follows:
Two-tailed Student’s t-test (P < 0.05) was used to compare IOP between the control and collagen implant eyes within each group over time.
Histological evaluation
Rabbits were sacrificed by excess CO2 after general anesthesia. The globe was enucleated and fixed overnight in 4% formaldehyde. The eyes were longitudinally bisected at the midline, and the anterior chamber depth measured using a ruler under the microscope. The tissue was processed for paraffin fixation. Microtome sections were cut at 7 μm, and stained with hematoxylin and eosin (H&E) for general histological observation, and with Sirius red and fast green stain to assess collagen deposition. Additional tissue sections were used for alpha-smooth muscle actin (SMA) immunocytochemistry to identify the distribution of myofibroblasts and the maturity of the wound.
Biomaterial characteristics-the relationship between strain and pressure
The change in height of a PBS-soaked OloGen matrix was measured following gradual application of an external force. The definition of strain is:
Results
Clinical evaluation and histology
Preoperative IOP (mean±SD) was 14.3 ± 2.4 mmHg in the implant group and 12 ± 1.9 mmHg in the control group. The mean size of the conjunctival defect was 3.8 ± 1.4 mm2 in the implant group and 6.91 ± 0.32 mm2 in the control group (P > 0.05). The postoperative clinical measurements are presented in Fig. 6 and Table 1. The conjunctival defect healed more than 90% at an average rate of 0.53 ± 0.03 mm2/day on day 7 in the implant group. In the control group, the conjunctiva also healed more than 90% on day 7; an average rate of 0.97 ± 0.03 mm2/day (Fig. 7). The difference in rate of conjunctival healing in the two groups was not significant by the two-tailed t-test (P > 0.05) The IOP of the implant group was significantly reduced throughout the 28 days of observation. However, the IOP of the control group was significantly reduced only when a conjunctival defect was present, and returned to the preoperative level around day 14. The difference in percent reduction of IOP in the two groups was significant at days 3, 14, 21 and 28 (Fig. 7). Seidel tests were negative throughout the 28 days of observation in both groups. No flat anterior chambers were found in either group throughout the 28 days of observation.
Day 3
In the implant group, epithelial cells combined with inflammatory cells, but no foreign body reaction grew from the edge of the conjunctival defect into the OloGen implant, forming a continuous structure (Fig. 2b,c). A shallow subconjunctival space was present (Fig. 2a). The OloGen implant under the conjunctival defect was stained with fluorescein, but no active leakage was noted. In the implant group, the reduction of IOP was 31 ± 28% and the average size of the conjunctival defect was 2.5 ± 0.77 mm2. In the control group, the reduction of IOP was 59 ± 8% and the average size of the conjunctival defect was 3.93 ± 1.0 mm2 (Table 1). The difference between the reduction of IOP of the two groups was significant (P < 0.05) (Fig. 7).
Day 3. a Large conjunctival defect (double arrow) over the area above the OloGen with a prominent subconjunctival space (white arrow). b Conjunctival epithelium (black arrows) grows from the edge of the defect into and on top of the OloGen (white arrow) (hematoxylin-eosin, 400×). c The Sirius red stain-positive area represents where the OloGen (white arrow) is filled with cells (black arrows) (Sirius red and fast green stain, 400×)
Day 5
In the implant group, the conjunctival defect was smaller and cells were growing over most of the matrix without foreign body reaction (Fig. 3b,c). While bleb formation was not complete, the size of the bleb was maintained by the OloGen (Fig. 3a). The Seidel tests were negative in both groups. In the implant group, the IOP reduction was 59 ± 4.8% and the average size of the conjunctival defect was 0.96 ± 0.93 mm2. In the control group, the IOP reduction was 43 ± 25% and the average size of the conjunctival defect was 1.5 ± 0.6 mm2 (Table 1). The difference of reduction of IOP between the two groups was significant (P < 0.05) (Fig. 7).
Day 7
In the implant group, the bleb was formed with complete coverage of the defect with less density of collagen stain (Fig. 4b) and SMA positive stained cells inside the bleb (Fig. 4c). The OloGen was embedded under the healed conjunctiva with less collagen density inside the area of degraded OloGen and scleral tunnel (Fig. 4a). Seidel testing was negative in both groups. In the implant group, the reduction of IOP was 42 ± 12.5%, and the average size of the conjunctival defect was 0.20 ± 0.50 mm2. In the control group, the reduction of IOP was 36 ± 21%, and the average size of the conjunctival defect was 0.40 ± 0.60 mm2 (Table 1). The difference of IOP reduction between the two groups was not significant (P > 0.05) (Fig. 7).
Day 14
The conjunctival defect was healed in both groups. The IOP reduction in the implant group was significantly greater (42 ± 13.1%) than in the control group (2.0 ± 10%) (P < 0.05%) (Table 1).
Day 21
The reduction of IOP in the implant group was 37 ± 13.2% and 3 ± 21% in the control group (Table 1). The difference of reduction of IOP between the two groups was significant (P < 0.05) (Fig. 7).
Day 28
In the implant group, the bleb was filled with dispersed, randomized, loose collagen with an unhealed wound inside the scleral tunnel as shown by sirius stain (Fig. 5a). Randomized collagen deposition was present subconjunctivally (Fig. 5b) and the reduction of IOP was 35 ± 24.2%. Smooth muscle actin staining showed absence of myofibroblasts inside the bleb. A localized but prominent bleb was seen clinically (Fig. 6). In the control group, the subconjunctival space was limited by dense collagen deposition (Fig. 5c) and the reduction of IOP was 12 ± 5%. The difference of reduction of IOP between the two groups was significant (P < 0.05) (Fig. 7). The anterior chamber depth was significantly less reduced in the implant group than in the control one (P < 0.05) (Table 1).
Day 28. a Patent scleral tunnel (black arrow) (Sirius red stain). b Random collagen deposition (arrows) in the bleb after the matrix has degraded completely (in the implant group, day 28) (Sirius red and fast green stain, 200×). c Less subconjunctival space with dense collagen deposition in the control group (black arrows) above sclera (double arrow) (Sirius red and fast green stains, 200×)
Conjunctival wounds heal more than 90% at day 7 in both groups (P > 0.05, two-tailed t-test). In the implant group, IOP was maintained at approximately a 40% reduction throughout the 28-day observation period. In the control group, IOP goes back to pre-operative level at day 14 (P > 0.05 for day 5, 7 and P < 0.05 for day 3, 14, 21and 28 (two-tailed t-test))
Biomaterial characteristics of soaked OloGen
When OloGen is compressed by external pressure, its height reduces gradually. In a greater percentage of height change, more pressure is needed to deform it. During the application of increased external force on top of the matrix, the change of strain increased gradually without obvious PBS leakage from matrix. The only exception is when the PBS is suddenly released at 3.2 Kpa with the simultaneous drop of external force (Fig. 8).
Discussion
The success of trabeculectomy depends on inhibiting the healing process. Inadequate closure of the conjunctival incision because of limited available tissue or early wound dehiscence may cause failure by collapse of the bleb, adhesions within the scleral tunnel, or collapse of the anterior chamber. Prevention of early flat anterior chamber and limited bleb formation can be achieved by controlling the speed and amount of the aqueous humor leak. A number of methods have been attempted to repair bleb leaks, including amniotic membrane grafting [15], conjunctival grafting [16–18], conjunctival advancement [19], fibrinogen glue, and application of a bandage contact lens [20] or a collagen shield [21]. Nevertheless, complete coverage of the bleb leak is difficult, risking a flat anterior chamber and early scleral tunnel closure during the healing period.
In the current study, a bioengineered, biodegradable soaked implant was placed below a surgically induced conjunctival defect, forming a temporary dome shape and producing hydraulic pressure on the scleral flap in order to avoid sudden onset of leakage of aqueous humor and hypotony in the early postoperative stage. The incisions in the both groups were closed with scleral sutures of identical tension and IOP checked before implantation in the OloGen group (Fig. 7). We chose to control the parameter of wound healing based on the study design. If the conjunctival defect could not heal simultaneously, it would be difficult to assess the outcome, especially the effect of prolonging a shallow anterior chamber. We can control the speed of conjunctival growth over the implant and make both groups heal at around the same time by creating different sized conjunctival defects in the two groups. The 2–3 mmHg difference in IOP on day 3 between the implant and control groups originated from the extra hydraulic pressure provided by the soaked OloGen, offering a protective barrier from a sudden change in IOP under conditions of a surgically defective bleb. In general, IOP was reduced about 40% in the implant group throughout the 28 days of observation; however, low IOP in the control group lasted only 7–14 days. Histologically, a shallow but formed bleb was created by the OloGen matrix (Figs. 2a, 3a and 4a).
Healing of a surgically induced wound with the OloGen differs from the healing without the matrix in an eye with a spontaneous bleb leak or surgical wound dehiscence. In the latter situation, the extracellular matrix involved in wound healing is directly over the sclera, similar to a two-dimensional environment, and induces a more anterior scar line. In a large leaking wound with a flat bleb, the ECM necessary to promote cell migration is absent in the bleb space, and healing occurs more like a pure conjunctival defect on the basis of the location of the ECM on top of the scleral bed. Similarly, decreased aqueous within the scleral tunnel increases the chance of early wound healing and a flat bleb. Therefore, it is important to reepithelialize the conjunctival defect and to maintain a transient bleb before the scleral tunnel heals (Figs. 4b and 5a). The rate of conjunctival healing with the application of OloGen was 0.5 mm2/day, which is slower than the speed of a surgically induced conjunctival wound but was not significant (Fig. 7).
In the presence of leakage, bleb healing based on the scleral bed as the level of ECM has difficulty in forming a prominent bleb. We used a three-dimensional aqeous humor-soaked artificial scaffold to supply the intercellular skeleton for cells to migrate, proliferate and differentiate, resulting in formation of a temporary bleb (Figs. 2a, 3a and 4a). The hydraulic pressure of the aqeous humor-soaked matrix also functions to supply resistance at the outlet of the scleral tunnel and to maintain the anterior chamber. This also was proven by the significant difference of depth of the anterior chamber in both groups at day 28 (Table 1) and the higher post-operative IOP in the implant group with the same tension of scleral sutures on day 3 (Fig. 7). The data from the biomaterial characteristics of this study shows the stiffness of OloGen (initial thickness is 4 mm) can maintain its 50% height and keep PBS inside it with a pressure less than 7–8 mmHg pressure (Fig. 8). By this observation, we can predict that the wound healing will proceed at a higher level—and under a semi-permeable condition above the scleral flap—than in an eye without the matrix. In addition, the three-dimensional aspect of the artificial scaffold permitted the conjunctiva with a large defect to be elevated from the scleral bed, retaining a prominent space underneath it (Figs. 2a and 3a).
Both physical and physiological factors influence the wound healing process. Due to the relationship between myofibroblasts and inflammation, prominent fibrosis can be expected in the bleb with a conjunctival defect [22–24]. Myofibroblasts are induced in the acute inflammatory stage, with the potential to close the wound by contraction and secrete collagen before they undergo apoptosis after the wound becomes mature, leaving the secreted collagen inside the bleb. The larger the wound, the more myofibroblasts are needed to repair it. In filtering surgery, this phenomenon increases the chance of bleb failure by contracting the depth and size of the bleb [25]. This is one of the reasons why trabeculectomy fails in some refractory glaucoma cases [26]. With certain diseases, a persistent foreign body reaction induces a prolonged inflammation, postponing the maturity of the wound and increasing the incidence of fibrosis. A biodegradable material can counteract the contractile effect of myofibroblasts until the period of inflammation passes. In this study, the cells migrated into both the surface and the inner structure of the scaffold until the defect of the conjunctiva healed with a bleb formed by the volume of the degraded scaffold. After complete degradation of OloGen, a randomized collagen deposition (Fig. 5b) and no myofibroblasts were found inside the functional and mature bleb (Table 1). Even though we did not directly observe the wound healing process in the scleral tunnel, keeping the bleb by providing a temporary dome shape at the early stage or by normalizing the bleb structure throughout the 28-day observation can possibly maintain the dehesive condition of the sceral tunnel with humors aqueous inside, and help prevent scar formation (Fig. 5a).
In conclusion, successful filtering surgery should have the following characteristics: (1) the conjunctiva should function with a normal structure, (2) the bleb should be maintained in a physiologically stable condition, (3) the scleral tunnel should remain patent after the wound becomes mature, and (4) a stable anterior chamber depth should be maintained. In bleb leakage, all four criteria stated above are difficult to satisfy. Tissue engineering without antifibrotic medication can meet all four requirements in a surgically defective bleb, resulting in a successful trabeculectomy in both physical and physiologic aspects.
References
Young MJ, Borras T, Walter M, Ritch R (2005) Potential applications of tissue engineering to glaucoma. Arch Ophthalmol 123:1725–1731
Azuara-Blanco A, Katz LJ (1998) Dysfunctional filtering blebs. Surv Ophthalmol 43:93–126
Liebmann JM, Ritch R (1988) 5-Fluorouracil in glaucoma filtration surgery. Ophthalmol Clin N Amer 1:125–131
Ball SF (1988) Corticosteroids, including subconjunctival triamcinolone, in glaucoma filtration surgery. Ophthalmol Clin N Amer 1:143–156
Sugar HS (1965) Clinical effect of corticosteroids on conjunctival filtering blebs. Am J Ophthalmol 59:854–860
Cordeiro MF, Gay JA, Khaw PT (1999) Human anti-transforming growth factor antibody: a new glaucoma anti-scarring agent. Invest Ophthalmol Vis Sci 40:2225–2234
Barton K, Budenz DL, Khaw PT, Tseng SCG (2001) Glaucoma filtration surgery using amniotic membrane transplantation. Invest Ophthalmol Vis Sci 42:1762–1768
DeBry PW, Perkins TW, Heatley G, Kaufman P, Brumback LC (2002) Incidence of late-onset bleb-related complications following trabeculectomy with mitomycin. Arch Ophthalmol 120:297–300
Greenfield DS, Liebmann JM, Jee J, Ritch R (1998) Late-onset bleb leaks after glaucoma filtering surgery. Arch Ophthalmol 116:443–447
Soltau JB, Rothman R, Budenz DL, Greenfield DS, Feuer W, Liebmann JM, Ritch R (2000) Risk factors for glaucoma filtering bleb infections. Arch Ophthalmol 118:338–343
Chen HSL, Ritch R, Krupin T, Hsu WC (2006) Control of filtering bleb structure through tissue bioengineering: An animal model. Invest Ophthalmol Vis Sci 47:5310–5314
Broadway DC, Chang LP (2001) Trabeculectomy, risk factors for failure and the preoperative state of the conjunctiva. J Glaucoma 10:237–249
Broadway DC, Grierson I, Sturmer J, Hitchings RA (1996) Reversal of topical antiglaucoma medication effects on the conjunctiva. Arch Ophthalmol 114:262–267
Watson PG, Barnett F (1975) Effectiveness of trabeculectomy in glaucoma. Am J Ophthalmol 79:831–845
Budenz DL, Barton K, Tseng SCG (2000) Amniotic membrane transplantation for repair of leaking glaucoma filtering blebs. Am J Ophthalmol 130:580–588
Buxton JN, Lavery KT, Liebmann JM, Buxton DF, Ritch R (1994) Reconstruction of filtering blebs with free conjunctival autografts. Ophthalmology 101:635–639
Schnyder CC, Shaarawy T, Ravinet E et al (2002) Free conjunctival autologous graft for bleb repair and bleb reduction after trabeculectomy and nonpenetrating filtering surgery. J Glaucoma 11:10–16
Wilson MR, Kotas-Neumann R (1994) Free conjunctival patch for repair of persistent late bleb leak. Am J Ophthalmol 117:569–574
Burnstein AL, WuDunn D, Knotts SL, Catoira Y, Cantor LB (2002) Conjunctival advancement versus nonincisional treatment for late-onset glaucoma filtering bleb leaks. Ophthalmology 109:71–75
Blok MD, Kok JH, van Mil C, Greve EL, Kijlstra A (1990) A use of the megasoft bandage lens for treatment of complications after trabeculectomy. Am J Ophthalmol 110:264–268, Sep
Fourman S, Wiley L (1989) Use of a collagen shield to treat a glaucoma filter bleb leak. Am J Ophthalmol 107:673–674
Hsu WC, Yannas MHS IV, Rubin PA (2000) Inhibition of conjunctival scarring and contraction by a porous collagen-glycosaminoglycan implant. Invest Ophthalmol Vis Sci 41:2404–2411
Chang L, Crowston JG, Cordeiro MF, Akbar AN, Khaw PT (2000) The role of the immune system in conjunctival wound healing after glaucoma surgery. Surv Ophthalmol 45:49–68
Grinnell F (1994) Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol 124:401–403
Kook MS, Lee DA (1995) Improving the success of glaucoma surgery by controlling wound healing. Ophthalmol Clin N Amer 8:393–400
Lieberman M, Ewing R (1990) Drainage implant surgery for refractory glaucoma. Int Ophthalmol Clin 19:802–810
Author information
Authors and Affiliations
Corresponding author
Additional information
Financial interest: Life Spring Biotech Co. Ltd has no financial relationship with any authors.
All authors have full control of all primary data, and agree to allow Graefe’s Archive for Clinical and Experimental Ophthalmology to review their data if requested.
Rights and permissions
About this article
Cite this article
Hsu, WC., Ritch, R., Krupin, T. et al. Tissue bioengineering for surgical bleb defects: an animal study. Graefes Arch Clin Exp Ophthalmol 246, 709–717 (2008). https://doi.org/10.1007/s00417-007-0744-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-007-0744-9