Abstract
Purpose
To evaluate the effectiveness of acellular bovine pericardium grafts (Normal GEN®) used as scaffolds for conjunctival reconstruction.
Method
The acellular bovine pericardium graft and the amnion graft were implanted into the bulbar conjunctival defects of adult rabbits. Conjunctival samples of implanted materials and blank defect controls were observed at day 3, 7, 14, 21, 28, and 56 postoperatively. Histological examination was observed at day 14, 28, and 56 of surgery, including hematoxylin–eosin staining, periodic acid-Schiff staining, and Masson’s trichrome staining, while immunofluorescent microscopy was observed at 14 days and 28 days after surgery. Results were compared among the Normal GEN®, amnion, and blank defect controls.
Results
All three groups showed complete conjunctival reconstruction. Wounds that were not grafted closed by formation of conjunctival scar characterized by a linear array of densely packed collagen fibers in Tenon’s capsule. Subepithelial tissue in the grafted groups comprised a loosely organized network of randomly oriented collagen that resembled that of the normal bulbar conjunctiva. However, there was a dense layer of aligned collagen between the conjunctival Tenon’s capsule and the sclera in the NormalGEN® group, about 250 μm in thickness.
Conclusions
Implantation of the NormalGEN® graft promoted the formation of conjunctiva as a kind of scaffold both in structure and in function. It had more advantageous mechanical properties than the amnion, strong and elastic, during the period of conjunctival reconstruction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Conjunctival reconstruction is an essential part of ocular surface regeneration, because the conjunctiva is a natural barrier against pathogens and maintains the equilibrium of the tear film through the secretion of the mucins [1, 2]. As reported, the conjunctiva has the capacity of spontaneously healing upon injury. However, this is always accompanied with scarring and wound contraction, especially in extensive disorders, such as surgical trauma after lid surgeries, chemical/thermal burns, and ocular cicatricial pemphigoid, which could result in trichiasis, entropion, lagophthalmos, fornix shortening, and symblepharon and disturb the integrity of an adequate and stable tear film by obliterating the tear meniscus [1–4]. Ultimately, this can lead to severe ocular discomfort and even blindness from corneal opacity [1, 2]. So, an appropriate tissue substitute is needed as a scaffold for conjunctival reconstruction in these cases.
In recent years, several techniques have been developed to restore the conjunctival surface. Although the use of autologous tissue substitutes has made progress in clinical studies, and while synthetic matrices based on vitrified collagen, fibrin, poly(lactide-co-gycolide) (PLGA) have also been tested in animal models, they are limited for numerous reasons [2, 5–7] .For example, available donor grafts are limited in patients suffering from systemic autoimmune diseases. Donor site morbidity could be a concern if large grafts are required. Furthermore, the availability, cost, and standardization in the preparation of synthetic materials still remained issues. Otherwise, as the elasticity and foldability of the vitrified collagen were unknown, it was also unknown whether the vitrified collagen would separate from the sclera in bulbar conjunctival reconstruction. As for PLGA, it was not determined how much time was needed for it to degrade. Also, PLGA was non-elastic and non-transparent, and it was not suitable for carrying an epithelial layer [1–7].
Since 1995, the amniotic membrane (AM) has been used as the primary substitute for conjunctiva in human eyes. AM’s function is to protect the embryo and to surround and contain the amniotic fluid, and it has various kinds of promising biological effects, such as anti-inflammatory, anti-fibroblast, anti-microbial, and anti-angiogenic properties. Moreover, it promotes epithelialization by facilitating the migration of epithelial cells, reinforcing adhesion of basal epithelial cells, promoting epithelial differentiation, and producing growth factors that promote epithelial cell growth [8].However, AM has its limitations. First, amnion has poor biomechanical strength for sustaining the shape of the fornix. Second, it suffers from poor standardization (differences in protein expression dependent on donor age and length of pregnancy influence clinical results after transplantation) and donor-associated risks of infection transmission. Briefly, the availability, cost, and standardization in the preparation of AM still remain issues. This has prompted research for the development of new substitutes [2]. Until now, only a few studies have evaluated alternative substrates to AM transplantation. Our study aims to describe a new substitute for conjunctival reconstruction.
The NormalGEN® graft originates from the decellularized bovine pericardium, a biodegrading collagenous patch. This material has been used in the human urinary bladder regeneration, in the manufacture of valve bioprostheses, and in the breast reconstruction after skin-sparing mastectomy. All of these applications have proven the safety and availability of the material for use in clinical patients [9–11]. However, there is a lack of research about its use in conjunctival reconstruction. Considering its composition, collagen, which is proven to have the ability to improve epithelial cell growth, we speculated that a NormalGEN® graft could be used as the scaffold for conjunctival reconstruction [1, 2].
In summary, the goal of this study was to prove the possibility of using a NormalGEN® graft as a scaffold for conjunctival reconstruction. Meanwhile, it also compared the effect of NormalGEN® grafts with that of AM and un-grafted wounds in conjunctival reconstruction in rabbit models. The outcomes were evaluated both anatomically and physiologically.
Methods
Animals
All investigations conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and were approved by the Institutional Animal Care and Use Committee (IACUC) of the Zhongshan Ophthalmic Center, Sun-Yat University (2011-007). A total of 30 New Zealand albino rabbits weighing between 2.5 and 3.5 kg were used as animal transplant recipients. The rabbits were randomized into three groups (containing ten rabbits each): a test group with NormalGEN® scaffold implantation, a control group with AM implantation, and a blank group without implantation.
Preparation of conjunctival substitute
NormalGEN®, (supplied by Grandhope Biotech Co., Ltd. Guangdong, China), is derived from bovine pericardium, fixed with an epoxy cross-linking technique, decellularized, and modified with a series of biochemical techniques. The product is preserved by a defined process consisting of meticulous cleaning of the tissue and sterilization by gamma irradiation. The finished product is stored in saline solution in a package of double transparent plastic bags at a temperature range of 4–37 °C.
After the serial treatments, the material retains its basic structure, and acellular collagen is the main component. The product is a uniform flat sheet with a smoother surface on one side, varying from 0.05 mm to 0.5 mm in thickness.
Humane amnion (AM) was received from pregnant donors in accordance with the Declaration of Helsinki. Fetal membrane obtained on Caesarian section was washed in sterile saline solution, and the amnion was separated from chorion by blunt dissection. The membrane was rinsed 3 times in 0.01 M phosphate buffered saline (PBS) containing 0.1 mg/ml streptomycin (Sigma-Aldrich, St. Louis, MO, USA) and 0.25 μg/ml amphotericin B (Sigma–Aldrich), and then cut in pieces, measuring 5 × 5 cm. Then it was mounted on nitrocellulose paper with the epithelial side up and stored frozen at −80 °C. Before use, it was thawed, and washed three times with balanced salt solution (BSS). The AM was trimmed to fit the conjunctival defect.
Surgical operation
The conjunctival defect model was designed the same as that used in previous studies [3, 7]. Thirty adult New Zealand albino rabbits were anesthetized by intramuscular injection of ketamine (30 mg/kg) and chlorpromazine (15 mg/kg). After opening the eye by spectrum, a 7.0-mm diameter vacuum trephine was used to perform a circular bulbar conjunctival defect on the right or left eye of the animals, located at the superior lateral position at a distance of 2 mm away from the corneal-scleral limbus.
Thus, the conjunctival epithelium, substantia propria, and Tenon’s capsule were completely removed in the lesion to expose the bare sclera. Slight retraction of the wound edges resulted in a final wound diameter of approximately 8 mm (Fig. 1). All procedures were performed under a standard ophthalmic operating microscope (OPMI VISC 200 Plus, Carl Zeiss, Jena, Germany). Then, the animals were randomized into three groups (containing ten rabbits each): a NormalGEN® group, an amnion group, and an un-grafted group. In the NormalGEN® and amnion groups, the wounds were either grafted with an 8.0-mm disc of NormalGEN® with the coarse side up, or with AM with the epithelial side up, using eight interrupted 8-0 Vicryl violet monofilament sutures. In the un-grafted group, the wounds remained ungrafted. Postoperatively, topical tobramycin and dexamethasone (Tobradex®; Alcon Laboratories, Fort Worth, TX, USA) eye drops were administered 4 times daily for 2 weeks, tapered to twice daily for another 2 weeks, and then discontinued. Topical tobramycin and dexamethasone (Tobradex®; Alcon Laboratories) eye ointment was applied once per night for 1 month.
Clinical examination and postoperative assessment
General observation and photography of the three groups were performed at day 3, 7, 14, 21, 28, and 56 postoperatively, using a slit lamp (SL-D7; Topcon Co., Japan), including observations of conjunctival congestion, the time required for complete graft epithelialization, clinical signs of fibrosis and scarring, the presence of complications including infections, and dissolution of the grafts, to evaluate and record the conjunctival wounds and examine the anterior segment. Meanwhile, conjunctival congestion was scored in masked fashion by the same examiner according to a modification of the MacDonald–Shadduck scoring system: grade 0, normal; grade 1, mild, a flushed reddish color; grade 2, moderate, a bright red color; and grade 3, severe, a dark, beefy red color [12].
Histology and immunohistochemistry
Groups of rabbits were killed at 14 days (n = 2), 28 days (c 2), and 56 days (n = 2) postoperatively via the intramuscular injection of an overdose of ketamine and chlorpromazine (1:1) for histologic examination. Intact eyes, including eyelids, were removed en bloc by orbital exenteration and fixed by immersion in 4 % formaldehyde overnight. The portion of each eye that included the wound site, along with the underlying scleral bed, was dissected and embedded in paraffin. The samples were sectioned at 5 μm and stained with hematoxylin & eosin (H&E) for general cell morphology, periodic acid-Schiff’s reaction (PAS) to identify goblet cells, and Masson’s trichrome (MT) to assess collagen deposition and remodeling. The identification of specific cell types was based on the cell morphology observed in sections stained with H&E. Polymorphonuclear neutrophils (PMNs) were identified by their characteristic multi-lobed nuclei and fine cytoplasmic granules. Eosinophils were characterized by bi-lobed or multi-lobed nuclei and larger eosinophilic granules. Mononuclear cells possessed a large, dark-staining nucleus or a kidney-shaped or notched nucleus with sky-grey cytoplasm. Fibroblasts were characterized by elongated morphology and oval nuclei. The infiltrated cells were counted in five randomly selected fields (×400) of the H&E-stained slides.
Tissue sections from the groups of rabbits, which were euthanized on day 14 (n = 2) and day 28 (n = 2), were stained with an antibody for immunohistochemical staining, including the grafted or ungrafted wound sites and the underlying scleral beds. The sections were collected and frozen in an optimal cutting temperature (OCT) compound (Whiga Technology Co., Guangdong, China) and stored at −80 °C. Frozen specimens were sectioned at 7 μm with a cryostat and air-dried. Sections were fixed in dry acetone for 15 minutes, evaporated, and rehydrated in PBS for 15 minutes. To block nonspecific binding, the tissues were incubated with 10 % normal goat serum for 30 minutes. Subsequently, the sections were incubated at 4 °C overnight with either a 1:300 dilution of mouse αCK 4 (GTX11215, GeneTex, USA) or a 1:30 dilution of mouse αMucin-5 AC (BM5547, Acris Antibodies, Inc., San Diego, CA, USA) in blocking buffer. The sections were washed three times in PBS and then stained for 1 hour with a 1:150 dilution of fluorescein (FITC)-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology, Inc., USA). After several washings with PBS, the sections were counterstained (DAPI, Roche, Germany), mounted, and examined by confocal microscopy (Zeiss LSM 510, ZWSIS, Göttingen, Germany). Control slides were prepared as described and were stained with primary or secondary antibodies only.
Statistical analysis
Statistical analyses were performed with SPSS 13.0. Wilcoxon-rank test was used to analysis the amount of mononuclear cells, eosinophils, and fibroblastic-like cells in different groups for the pair-wise comparisons.
Results
Characterization of experimental materials
The NormalGEN® graft, a kind of opaque white tissue, consists of dense collagen fibers in various directions without cellular structure, varying from 400 μm to 500 μm in thickness, which could imply that it has obvious toughness. The transparent amnion grafts comprises three layers: the epithelium, basement membrane, and stroma, and are easily destroyed during the surgical procedure, with a thickness of 300 μm to 350 μm. The epithelial cells are polygonal in shape and closely adherent to the basement membrane. To emphasize, the collagen structure of the AM stroma was loosely arranged, which complied with its fragile mechanical characteristic. As the positive control, normal transparent bulbar conjunctiva consists of a columnar epithelial layer 2 to 3 cells thick. The stroma, with a thickness of about 400 μm, is composed of randomly oriented collagen with sparse fibroblastic-like cells and capillary vessels (Fig. 2).
Re-epithelial membrane
Through gross-eye evaluation, it was observed that the wounds were completely covered by epithelium, with a thin smooth layer of epithelium at 14 days in the NormalGEN® group. Meanwhile, the conjunctiva-like tissue showed at 28 days after surgery in the NormalGEN® group and at 14 days in both the amnion group and the un-grafted group, with scarring (Fig. 3). The reconstructed tissue consisted of a thin layer of epithelium cells in the NormalGEN® group, of four to five layers of epithelium cells in the AM group, and of a thin layer of epithelium cells in the un-grafted group (Fig. 4).
Changes in mean conjunctival congestion score. a Comparison among the non-dissolved NormalGEN®, AM, and un-grafted groups. b Comparison between non-dissolved and dissolved states in NormalGEN® groups. Scores in non-dissolved state gradually decreased. The dissolved state showed an abrupt increase in score at 2 weeks after the surgery, and score gradually increased as time progressed
Meanwhile, PAS was used to evaluate the scattering of goblet cells in the re-epithelial membrane. Strong PAS-positive staining, representing conjunctival goblet cells, was found in these stratified epithelial layers, and the amount of stained mucin in the three groups was also comparable to the positive control. At 28 days postoperatively, there were a few goblet cells, with smaller nuclei than normal, scattered on the reconstructed conjunctiva with NormalGEN® graft implantation. Meanwhile, goblet cells were identified at 14 days postoperatively in both the amnion group and the blank control group. In comparison, the amount of goblet cells in the reconstructed epithelium was smaller than in normal conjunctival epithelium (Fig. 5).
Otherwise, the presence of goblet cells and the phenotypes of reconstructed epithelium was further proven by immunohistochemistry. The staining of CK4, a conjunctival-specific cytokeratin, suggested a continuous epithelium in all groups, and MUC5AC staining recognized the specific secretory conjunctival mucin (5 AC subtype) and selectively stained goblet cells [2]. Through the use of immunofluorescence to detect CK4, it was revealed that the defect was totally re-epithelialized in the NormalGEN® group at 28 days post-operation, and in the amnion group and un-grafted group it was re-epithelialized at 14 days post-operation. Examination of MUC5AC staining in the defect site after transplantation revealed a significant difference in goblet cell percentages among the groups. The defect site grafted with the NormalGEN® graft was repopulated by MUC5AC-positive goblet cells to the same degree as that of the native conjunctiva and the un-grafted group. However, the MUC-5 AC of the epithelium layer stained more positively in the amnion group than in the normal group (Fig. 6).
The reconstructed fibrous connective tissue
Apart from the re-epithelium, the reconstructed tissue also included substantia propria, Tenon’s capsule, and a collagen layer (Fig. 4). In the NormalGEN® group, it was observed that there was a thin conjunctival substantia propria similar to that of normal conjunctiva, a dense Tenon’s capsule of about 200 μm (containing newly born capillary vessels), and a collagen layer of about 400 μm. In the AM group, there was a conjunctival matrix about two times thicker than that of normal conjunctiva, a Tenon’s capsule of about 200 μm (containing less newly born capillary vessels), and a collagen layer of about 150 μm. In the un-grafted group, a conjunctival matrix similar to that of normal conjunctiva, a thick and dense Tenon’s capsule of about 300 μm (containing the least amount of newly born capillary vessels), and a collagen layer of about 150 μm.
Meanwhile, MT staining was performed to further visualize the conjunctival stroma fibrillar organization. The collagen of the NormalGEN® graft softened, and was in a hypocellular condition from day 14 to day 28, which resulted in highly aligned collagen fibers without inflammatory action at day 56. Constant deposition of new collagen was found at day 14 and day 28 in the un-grafted group, while the elastic fiber was constantly formed. In both treated groups, randomly aligned fibrils and loosely packed fibroblasts were found in the reconstructed substantia propria, resembling the uninjured normal tissue. However, it was interesting to find in the NormalGEN® group that there was a dense layer of aligned collagen between the conjunctival stroma and the sclera, about 250 μm in thickness. As a result, it was shown that the density of the reconstructed fiber tissue decreased in the following order: the NormalGEN® group > the ungrafted group > the amnion group > the normal conjunctiva (Fig. 5).
The dissolving of grafts in the NormalGEN® group and the amnion group
In the NormalGEN® group, it was observed by gross examination that five implanted grafts (50 % of the group) began to dissolve into yellow-white and soft material at 14 days, while the dissolving phenomenon was not observed in the other 50 % during the entire reconstructed period. The rabbits with dissolved NormalGEN® graft began to die from 28 days after surgery. The amnion began to dissolve at 7 days and disappeared at 28 days, and it remained transparent during the entire period (Fig. 3).
Conjunctival scarring
The conjunctival scar showed at 28 days in the NormalGEN® group, at 14 days in the amnion group, and at 7 days in the un-grafted group after surgery. However, it became stable at 56 days in all three groups. Finally, the negative control group showed much more significant conjunctival contractions and scarring than the NormalGEN® group, while the amnion group restored its nearly normal bulbar conjunctival appearance under naked-eye observation (Fig. 3).
Inflammatory reaction of the operated areas
In each group, a decreasing inflammatory action was observed over time. Moreover, the inflammatory action after surgery could be represented by the mean conjunctival congestion score, as shown in Fig. 7. This indicates that the degree of inflammatory congestion decreased in the following order: the amnion group < the un-grafted group < the NormalGEN® group. Moreover, when the biological dural graft dissolved, the inflammatory reaction lessened.
Periodic Acid Schiff’s reaction (PAS) to the number of goblet cells and Masson’s trichrome (M-T) to the alignment of collagen of grafted and un-grafted wounds in representative cases and of native conjunctiva. a–b at day 28 in the NormalGEN® group; c–d at day 14 in the Amnion group; e–f at day 14 in the un-grafted group; g–h Normal conjunctiva (200×)
Via histological staining, a hypercellular condition was noted in both grafted wounds and un-grafted wounds at day 14 and day 28 (Tables 1, 2 and 3). The inflammatory reaction on day 28 was more severe than on day 14, and it disappeared at day 56 in both the NormalGEN® group and the amnion group. Meanwhile, the un-grafted group showed a regularly decreasing trend in inflammatory reaction. In the NormalGEN® group, the amount of mononuclear cells increased from 0.1 % to 11.9 %, while that of fibroblastic-like cells decreased from 99.9 % to 88.1 % from day 14 to day 56, and the amount of eosinophile cells stayed at 0 %. In the amnion group, it was interesting to find that the eosinophile cells reached 21.2 % at day 14 and then decreased in a sharp trend, the amount of mononuclear cells reached a peak of 50.1 % at day 28, and the amount of fibroblastic-like cells was 69.6 % at day 14, 44.5 % at day 28, and 93 % at day 56. In the un-grafted group, the amount of mononuclear cells was 1.4 % at day 14 and deceased to 1.4 % at day 28; meanwhile, the amount of eosinophile cells stayed at 0 %, and the amount of fibroblastic-like cells stayed at above 95 % (Fig. 8).
Immunofluorescence to detect CK4 and MUC-5AC to represent the structure in the conjunctiva of grafted and ungrafted wounds in representative cases and in native conjunctiva. a–b NormalGEN® group (28 days post-operation): N: NormalGEN® graft; S: sclera; c–d Amnion group (14 days post-operation); e–f Un-grafted group (14 days post-operation); g–h Normal conjunctiva (400×)
Discussion
Conjunctival reconstruction is necessary for severe disorders of the ocular surface that induce conjunctival fornix shortening, conjunctival defect, and orbital implant exposure, because of its importance in maintaining the normal function of the tear film and cornea and maintaining good appearance, as well as preventing infection of the eyes or the orbit [1–5, 13, 14]. A scaffold for conjunctival reconstruction has been urgently needed, as the self-cure of conjunctival defects has unsatisfactory results because of the formation of conjunctival scars [2–7] .An ideal conjunctival equivalent must meet several criteria. It should be sufficiently elastic and well-tolerated, without causing inflammation or stimulating rejection. Further, it should carry an epithelium with the distinctive goblet cell phenotype for better mucin production and secretion, as many ocular surface disorders are associated with mucin-deficient dry-eye diseases [2].
Here, we have reported the development of the NormalGEN® graft for the treatment of ocular surface disorders involving conjunctival defects, and indicated the defect of AM as a substitute in some cases of conjunctival reconstruction.
Our study indicates that the conjunctival epithelium was able to grow over the NormalGEN® graft, which served as a collagen scaffold, as proved by HE staining and the expression of maker CK4. At 14 days after surgery, the re-epithelialization of NormalGEN® grafted wounds was nearly complete, with a thinner layer, whereas there were several layers of epithelium forming in the amnion group and the un-grafted group. It is possible that the dense collagen array slowed down the speed of the spreading of the nutrients needed for re-epithelialization [3, 15]. In addition, it was difficult to judge whether the epithelium originated from the conjunctival progenitor or was owned by the graft itself in the amnion group [15]. Finally, the severe inflammatory reaction killed the fragile epithelium cells [15]. In the gross examination, all the wounds were fully repaired with the formation of conjunctival-like tissue by 28 days after surgery, but a complete stable epithelial layer that contained goblet-like cells covered both un-grafted and grafted wounds at 56 days, which can be attributed to the inflammation. Our findings concerning the time scale of re-epithelialization of un-grafted control wounds and the amnion grafted wounds are consistent with other studies [3, 15]. One of the most important functions of the conjunctival epithelium is mucin production and secretion, which is mainly fulfilled by goblet cells. Therefore, the number and secretory level of goblet cells are crucial indications of the epithelium’s functionality [1, 2, 16]. Our study proved that the NormalGEN® graft successfully supported the growth of goblet cells, which were scattered in the epithelium the same as in the normal conjunctiva.
Cells that are characteristic of an acute inflammatory response were present in both un-grafted and grafted wounds during the period of the conjunctival reconstruction. A higher degree of inflammatory reaction was observed in the conjunctiva reconstructed with a NormalGEN® graft, which may be attributable to the fact that both collagen degradation and reorganization could induce inflammatory reaction. The histological results showed that the dissolving of the graft induced the migration and proliferation of mononuclear cells, which also promoted the absorption of these substances. In general, there was only normal inflammation from the reaction to healing in the NormalGEN® group [3, 17]. However, in the amnion group, eosinophile cells took a great amount at the early stage after surgery, which may indicate that the amnion graft was more inclined to induce a slight level of allergic reaction. The amount of mononuclear cells peaked at about 50 %, with the lowest percentage of fibroblastic cells at day 28, which may indicate that the amnion graft played an important role in anti-inflammatory action prior to day 28, and then the amnion dissolved when the normal inflammation from the curing reaction appeared [15, 18]. In all three groups, the inflammatory reaction did not became stable at day 28, even in the amnion group and in the un-grafted group, which already had the obvious formation of re-epithelium at that time. The authors thought that the early time before day 28 was mainly for the formation of epithelium, and the period between day 28 and day 56 was mainly for collagen organization in the conjunctival reconstruction. Moreover, though it could be indicated that the normal inflammatory reaction was not beneficial to re-epithelialization, the slight allergic reaction was beneficial, possibly because the mononuclear cells consumed some of the new growing epithelial cells.
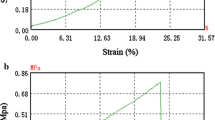
It was proven that there was not only re-epithelialization, but also collagen reconstruction in the conjunctival reconstruction, which might be controlled by myofibroblasts. The pattern of collagen deposition, as well as the subsequent cross-linking and remodeling of collagen, are factors that may determine the final collagen configuration and the functional characteristics of the repair or scar tissue [2–4, 19]. In our study, we found that there was a thick and dense layer of aligned collagen between the conjunctival stroma and the sclera, which indicated that the collagen reconstruction with NormalGEN® grafts involves two processes: the graft’s collagen dissolution and refactoring. We speculated that the use of a thinner NormalGEN® graft in conjunctival reconstruction would reduce the collagen deposition. In other words, the thinner NormalGen® grafts might have better results in conjunctival reconstruction, and the best thickness of the NormalGen® grafts would be the point of our further study.
In terms of mechanical characteristics, it was found that the NormalGEN® graft had a better supporting ability than the amnion graft, due to the fact that the NormalGEN® graft has a special period of collagen deposition in the conjunctival reconstruction. In fact, we had already used the NormalGEN® grafts in clinic for repairing the exposure of orbital impants, finding that all the artificial dural grafts observed were covered by new conjunctiva completely after surgery in 3 to 6 months, which meant the NormalGEN® grafts could be used in the anophthalmia conjunctival reconstruction [20]. And due to the fragile mechanical characteristics, the AM has never been used for repairing the exposure of orbital implants, although it was universally used in conjunctival reconstruction after pterygium sugery in clinic [13, 14]. Consequently, we predicted that the NormalGEN® graft might be more suitable for anophthalmologic shortening fornix reconstruction than AM.
The limitations of this study include the following. First, it was a preliminary study, and the conjunctival defect was small. It might be more efficient to display the mechanical advantages of the NormalGEN® graft in anophthalmia models. Second, the graft was too thick compared to the conjunctiva. A thinner layer of the NormalGEN® graft would better satisfy the ideal criteria for conjunctival reconstruction. Third, xenorejection depends on the two species involved in the experiment. Therefore, investigations using primates could better assess the immune reaction when the material is implanted in human beings.
Conclusion
Implantation of the NormalGEN® graft promoted the formation of nearly normal conjunctiva as a kind of scaffold both in structure and in function. However, there was a dense layer of aligned collagen between the conjunctival Tenon’s capsule and the sclera in the NormalGEN® group, about 250 μm in thickness. Wounds that were not grafted closed by formation of conjunctival scar, characterized by a linear array of densely packed collagen fibers in Tenon’s capsule. In the presence of NormalGEN® grafts and amnion grafts, the wound-healing response was modified toward regeneration of nearly physiological subepithelial stroma. Subepithelial tissue in the grafted group comprised a loosely organized network of randomly oriented collagen that resembled that of the normal bulbar conjunctiva.
References
Schrader S, Notara M, Beaconsfield M, Tuft SJ, Daniels JT, Geerling G (2009) Tissue engineering for conjunctival reconstruction: established methods and future outlooks. Curr Eye Res 34(11):913–924
Zhou H, Lu Q, Guo Q, Chae J, Fan X, Elisseeff JH, Grant MP (2014) Vitrified collagen-based conjunctival equivalent for ocular surface reconstruction. Biomaterials 35:7398–7406
Hsu WC, Spilker MH, Yannas IV, Rubin PA (2000) Inhibition of conjunctival scarring and contraction by a porous collagen–glycosaminoglycan implant. Invest Ophthalmol Vis Sci 41:2404–2411
Kumar A, McCormick A, Bhargava JS (2011) Temporary Gortex(polytetrafluoroethylene) spacer for the treatment of fornix shortening following severe alkali chemical injury. Orbit 30(5):252–254
Chun YS, Park IK, Kim JC (2011) Technique for autologous nasal mucosa transplantation in severe ocular surface disease. Eur J Ophthalmol 21:545–551
Safinaz MK, Norzana AG, Hairul Nizam MH, Ropilah AR, Faridah HA, Chua KH, Ruszymah BH, Jemaima CH (2014) The use of autologous fibrin as a scaffold for cultivating autologous conjunctiva in the treatment of conjunctival defect. Cell Tissue Bank 15(4):619–626
Lee SY, Oh JH, Kim JC, Kim YH, Kim SH, Choi JW (2003) In vivo conjunctival reconstruction using modified PLGA grafts for decreased scar formation and contraction. Biomaterials 24:5049–5059
Cirman T, Beltram M, Schollmayer P, Rozman P, Kreft ME (2014) Amniotic membrane properties and current practice of amniotic membrane use in ophthalmology in Slovenia. Cell Tissue Bank 15:177–192
Pokrywczynska M, Adamowicz J, Sharma AK, Drewa T (2014) Humane urinary bladder regeneration through tissue engineering — an analysis of 131 clinical cases. Exp Biol Med (Maywood) 239(3):264–271
Goissis G, Giglioti Ade F, Braile DM (2011) Preparation and characterization of an acellular bovine pericardium intended for manufacture of valve bioprostheses. Artif Organs 35(5):484–489
Gubitosi A, Docimo G, Parmeggiani D, Pirozzi R, Vitiello C, Schettino P, Avellino M, Casalino G, Amato M, Ruggiero R, Docimo L (2014) Acellular bovine pericardium dermal matrix in immediate breast reconstruction after skin sparing mastectomy. Int J Surg 12(Suppl 1):S205–S208
Altmann S, Emanuel A, Toomey M, McIntyre K, Covert J, Dubielzig RR, Leatherberry G, Murphy CJ, Kodihalli S, Brandt CR (2010) A quantitative rabbit model of vaccinia keratitis. Invest Ophthalmol Vis Sci 51:4531–4540
Kamal S, Kumar S, Goel R, Bodh SA (2013) Serial sub-conjunctival 5-Fluorouracil for early recurrent anophthalmic contracted socket. Graefes Arch Clin Exp Ophthalmol 251:2797–2802
Jung S-K, Paik J-S, Sonn U-H, Yang S-W (2013) Surgical outcomes of acellular human dermal grafts for large conjunctiva defects in orbital implant insertion. Graefes Arch Clin Exp Ophthalmol 251:1849–1854
Meller D, Tseng SC (1999) Conjunctival epithelial cell differentiation on amniotic membrane. Invest Ophthalmol Vis Sci 40:878–886
Barabino S, Rolando M (2003) Amniotic membrane transplantation elicits goblet cell repopulation after conjunctival reconstruction in a case of severe ocular cicatricial pemphigoid. Acta Ophthalmol Scand 81:68–71
Pagoulatou E, Triantaphyllidou IE, Vynios DH, Papachristou DJ, Koletsis E, Deligianni D, Mavrilas D (2012) Biomechanical and structural changes following the decellularization of bovine pericardial tissues for use as a tissue engineering scaffold. J Mater Sci Mater Med 23:1387–1396
Koizumi NJ, Inatomi TJ, Sotozono CJ, Fullwood NJ, Quantock AJ, Kinoshita S (2000) Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res 20:173–177
Lai PH, Chang Y, Chen SC, Wang CC, Liang HC, Chang WC, Sung HW (2006) Acellular biological tissues containing inherent glycosaminoglycans for loading basic fibroblast growth factor promote angiogenesis and tissue regeneration. Tissue Eng 12(9):2499–2508
Danping H, Nuo X, Yidan H, Zhaoguang L (2009) The clinical application of repairing the exposure of orbital implants with the artificial biological dural graft. Ophthalmol CHN 18(6)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The National Natural Science Foundation of China (Grant No. 81371051) and Guangdong Technology Project Foundation of China (Grant No. 2010B031100012) provided financial support.
The sponsor had no role in the design or conduct of this research.
Conflict of Interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Animal Experiments
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Rights and permissions
About this article
Cite this article
Huang, D., Xu, B., Yang, X. et al. Conjunctival structural and functional reconstruction using acellular bovine pericardium graft (Normal GEN®) in rabbits. Graefes Arch Clin Exp Ophthalmol 254, 773–783 (2016). https://doi.org/10.1007/s00417-015-3201-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-015-3201-1