Abstract
Background
This report describes the use of combined laser photocoagulation and intravitreal bevacizumab administration for aggressive zone I retinopathy of prematurity (ROP).
Methods
A male patient, born at 25 weeks gestation with a birth weight of 884 g, received indirect laser photocoagulation and a 0.75 mg intravitreal bevacizumab injection to each eye for aggressive stage 3 zone I ROP. Structural outcomes were evaluated 3 months after treatment.
Results
At 3-month follow-up, treatment had resulted in ROP regression, prompt resolution of plus signs and neovascular proliferation in both eyes, and no signs of systemic or ocular adverse events.
Conclusions
The combination of indirect laser photocoagulation and intravitreal bevacizumab injection was well tolerated and induced prompt regression of aggressive zone I ROP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Retinopathy of prematurity (ROP) is a leading cause of childhood blindness [1]. Zone I disease is known to have a worse prognosis even after cryotherapy or laser photocoagulation compared to zone II or III disease [2–7].
Vascular endothelial growth factor (VEGF) has been known as one of the major factors in ROP development and mediates the formation of new blood vessels in various retinal neovascular diseases [8–11]. Serum VEGF levels are reported to be higher in infants with stage 3 and threshold ROP than in those with less severe disease [12].
Bevacizumab is an anti-VEGF monoclonal antibody. Intravitreal bevacizumab injections have recently gained popularity as a potential treatment for several intraocular neovascular diseases without known serious ocular systemic adverse events [13, 14]. However, most of the reports on use and safety of intravitreal bevacizumab are data from treatment of adult patients, not children and especially not premature newborns with their specific situation, risks and needs.
The present report describes the use of intravitreal bevacizumab combined with indirect laser photocoagulation to treat aggressive zone I ROP in a premature (25-weeks gestation) male newborn.
Materials and methods
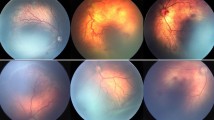
The male patient was born at 25 weeks gestation with a birth weight of 884 g. His first screening examination at 8 weeks of age showed bilateral stage 3 zone I ROP with severe plus disease and extensive epiretinal vascular proliferation (Fig. 1). The patient was, hence, scheduled for a combined treatment of intravitreal bevacizumab and indirect laser photocoagulation. The off-label use of bevacizumab and the potential risks were discussed with the parents of the patient. We explained potential drug-related adverse events such as systemic thromboembolic events, blood pressure increase, intraocular inflammation and a possible negative effect on normal retinal vasculogenesis and neurogenesis, as well as potential injection-related adverse events including lens injury, endophthalmitis, retinal detachment and vitreous hemorrhage. We also explained that although preliminary short-term data suggested intravitreal drug use was safe, there were no published data regarding safety in premature newborns. After a thorough discussion regarding the possible consequences of treatment, the parents agreed to proceed with the combination therapy.
Under general anesthesia, both eyes received a near confluent pattern of indirect diode laser photocoagulation to the avascular retina, immediately anterior to the border of the vascular zone extending to the ora serrata for 360°. After photocoagulation, 0.75 mg (0.03 cc) bevacizumab was injected intravitreally using a 30-gauge needle placed 1 mm behind the limbus in each eye. The perfusion of optic nerve heads was then confirmed using indirect ophthalmoscope.
The systemic condition of the patient was closely monitored during the perioperative and postoperative periods in the neonatal intensive care unit, including continuous monitoring of blood pressure, oxygen saturation and heart rate.
Results
The surgery was uneventful, and the general status of the patient during the operation and perioperative period was stable without any serious ocular or systemic events associated with drug or surgical procedures. A substantial decrease in vascular engorgement and tortuosity was noticeable from postoperative day 1, and neovascular proliferation regression was obvious in both eyes by postoperative 1 week. At 3 months follow-up, a fundus examination revealed clear media with well-regressed ROP in both eyes (Fig. 2).
Discussion
Zone I ROP is an uncommon disease, occurring in approximately 10% of all premature infants with retinopathy that requires treatment. Even after conventional treatment, patients with zone I disease often progress to unfavorable outcomes, such as a posterior fold, retinal detachment involving the posterior pole or a retrolental mass obstructing the view of the retina. The Cryotherapy for Retinopathy of Prematurity Cooperative Study reported a 77.8% unfavorable outcome rate, using cryotherapy, and the Early Treatment for Retinopathy of Prematurity Cooperative Group reported a 55.2% unfavorable outcome rate, using laser photocoagulation in zone I disease [2–4]. Other studies using diode laser photocoagulation for ROP showed unfavorable outcome rates, ranging from 40–77.8% [5–7]. One study showed a 100% unfavorable outcome rate following use of laser photocoagulation for posterior zone I ROP [7].
The frequent failure of conventional ablative therapy has been suggested to reflect the different mechanism underlying zone I ROP, involving aberrant vasculogenesis less dependent on VEGF165-mediated angiogenesis [4]. An alternative interpretation could be a second source of VEGF165, such as vitreal macrophages as reported by Naug et al. [15]. Thus, as suggested by other authors, we hypothesized that a combination therapy of conventional laser photocoagulation and intravitreal injection of monoclonal antibodies against VEGF, such as bevacizumab, might be more effective [4]. The drug may not only inhibit tyrosine kinase proteins and attack the process of abnormal vasculogenesis upstream from VEGF165, but may also block secondary sources of intraocular VEGF165 [4]. Bevacizumab was used in the present study as it was the only anti-VEGF drug available at the time at our institute. A more selective VEGF inhibitor, such as pegaptanib (Macugen®), might be a safer option for ROP treatment. Since VEGF is known to play a role in physiological retinal neurogenesis and vasculogenesis, blocking all VEGF isoforms may have negative effects on normal development in premature newborns [16, 17].
The present patient underwent both procedures on the same day as we believed it was safer to perform the injections under general anesthesia in sterile operating room conditions rather than under local anesthesia. Although the optimum intravitreal bevacizumab dose remains to be established as yet, previous reports describe using up to 2.5 mg without serious systemic or ocular adverse events [13, 14]. Animal studies have shown that typical doses of intravitreal bevacizumab did not affect visually evoked potentials and electroretinogram patterns in albino rabbits, nor were they associated with any histological retinal toxicity [18–20]. Considering the smaller vitreous volume in premature newborns, we chose to administer 0.75 mg bevacizumab rather than the usual adult intravitreal dose of 1.25 mg. Previous case reports also used 0.75 mg in premature newborns with aggressive posterior ROP without any serious complications [21, 22]. Since recent reports suggest that even smaller doses might be sufficient to inhibit intravitreal VEGF and neovascular proliferation, further studies are warranted to establish the optimal doses for premature newborns [23, 24].
In the current case, one combined treatment of drug injection and laser photocoagulation was sufficient to induce ROP regression. Unlike other neovascular diseases, such as choroidal neovascularization associated with age-related macular degeneration and proliferative diabetic retinopathy, ROP is less likely to necessitate repeated injections since the disease is known to undergo spontaneous involution in 90% of patients before 44 weeks of postmenstrual age, and the intravitreal therapeutic drug concentration is maintained for up to 4 weeks [24, 25]. These observations further support the use of intravitreal VEGF inhibitors for ROP since the newborn may not require repeated injections that create a cumulative risk of complications. However, further studies appear essential to determine the timing, interval and drug dose required to provide the optimal intravitreal concentration and to identify the most effective agent with the minimum toxicity.
The present study found that combined intravitreal bevacizumab and laser photocoagulation treatment promptly and strikingly halted the progression of vascular proliferation. Although new vessel regression and the disappearance of plus disease may be achieved by laser photocoagulation alone, the prompt resolution of vascular engorgement and tortuosity from as early as postoperative day 1 suggests a possible adjunct effect of intravitreal bevacizumab. Significantly, we did not observe any serious adverse events attributable to the drug, and there were no signs of any negative effects on normal retinal vessel growth at 3 months postoperatively.
In conclusion, combined laser photocoagulation and intravitreal bevacizumab treatment resulted in prompt resolution of vascular proliferation and a favorable anatomical outcome in a premature newborn with zone I ROP and was not associated with any serious ocular or systemic adverse events. These findings indicate that controlled studies with long-term follow-ups are warranted to determine the potential safety and benefits of VEGF inhibitors for treating zone I ROP.
References
Stenkuller PG, Du L, Gilbert C et al (1999) Childhood blindness. J AAPOS 3:26–32
Cryotherapy for Retinopathy of Prematurity Cooperative Group (1990) Multicenter trial of cryotherapy for retinopathy of prematurity. Three-month outcome. Arch Ophthalmol 108:195–204
Early Treatment for Retinopathy of Prematurity Cooperative Group (2003) Revised indication for the treatment of retinopathy of prematurity: results of early treatment for ROP randomized trial. Arch Ophthalmol 121:1684–1696
Flynn JT, Chan-Ling T (2006) Retinopathy of prematurity: Two distinct mechanisms that underlie zone 1 and zone 2 disease. Am J Ophthalmol 142:46–59
Katz X, Kychenthal A, Dorta P (2000) Zone I retinopathy of prematurity. J AAPOS 4:373–376
O’Keefe M, Lanigan B, Long VW (2003) Outcome of zone I retinopathy of prematurity. Acta Ophthalmol Scand 81:614–616
Kychenthal A, Dorta P, Katz X (2006) Zone I retinopathy of prematurity: clinical characteristics and treatment outcomes. Retina 26:S11–S15
Pierce EA, Avery RL, Foley ED et al (1995) Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA 92:905–909
Aiello LP, Avery RL, Arrigg PG et al (1994) Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 331:1480–1487
Pierce EA, Foley ED, Smith LE (1996) Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch Ophthalmol 114:1219–1228
Okamoto N, Tobe T, Hackett SF et al (1997) Animal model. Transgenic mice with increased expression of vascular endothelial growth factor in the retina: a new model of intraretinal and subretinal neovascularization. Am J Pathol 151:281–291
Brady-McCreery KM, McCreery CJ, Sriram V et al (2001) Serum vascular endothelial growth factor and threshold retinopathy of prematurity [abstract]. In: Annual meeting of the American Academy of Ophthalmology; 4th–8th November 2001. American Academy of Ophthalmology, vol 3. Abstract, New Orleans, San Francisco, p 113
Lynch SS, Cheng CM (2007) Bevacizumab for neovascular ocular diseases. Ann Pharmacother 41:614–625
Fung AE, Rosenfeld PJ, Reichel E (2006) The International Intravitreal Bevacizumab Safety Survey: using the internet to assess drug safety worldwide. Br J Ophthalmol 90:1344–1349
Naug H, Browning J, Gole G, Gobe G (2000) Vitreal macrophages express VEGF165 in oxygen-induced retinopathy. Clin Exp Optom 28:48–52
Yourey PA, Gohari S, Su JL, Alderson RF (2000) Vascular endothelial cell growth factors promote the in vitro development of rat photoreceptor cells. J Neurosci 15:6781–6788
Murata T, Nakagawa K, Khalil A, Ishibashi T, Inomata H, Sueishi K (1996) The temporal and spatial vascular endothelial growth factor expression in retinal vasculogenesis of rat neonates. Lab Invest 74:68–79
Shahar J, Avery RL, Heilweil G et al (2006) Electrophysiologic and retinal penetration studies following intravitreal injection of bevacizumab (Avastin). Retina 26:262–269
Manzano RP, Peyman GA, Khan P, Kivilcim M (2006) Testing intravitreal toxicity of bevacizumab (Avastin). Retina 26:257–261
Bakri SJ, Cameron JD, McCannel CA, Pulido JS, Marler RJ (2006) Absence of histologic retinal toxicity of intravitreal bevacizumab in a rabbit model. Am J Ophthalmol 142:162–164
Shah PK, Narendran V, Tawansy KA, Raghuram A, Narendran K (2007) Intravitreal bevacizumab (Avastin) for post laser anterior segment ischemia in aggressive posterior retinopathy of prematurity. Indian J Ophthalmol 55:75–76
Travassos A, Teixeira S, Ferreira P et al (2007) Intravitreal bevacizumab in aggressive posterior retinopathy of prematurity. Ophthalmic Surg Lasers Imaging 38:233–237
Avery RL, Pearlman J, Pieramici DJ et al (2006) Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology 113:1695–1705
Beer PM, Wong SJ, Hammad AM, Falk NS, O’Malley MR, Khan S (2006) Vitreous levels of unbound bevacizumab and unbound vascular endothelial growth factor in two patients. Retina 26:871–876
Repka MX, Palmer EA, Tung B (2000) Involution of retinopathy of prematurity. Arch Ophthalmol 118:645–649
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chung, E.J., Kim, J.H., Ahn, H.S. et al. Combination of laser photocoagulation and intravitreal bevacizumab (Avastin®) for aggressive zone I retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 245, 1727–1730 (2007). https://doi.org/10.1007/s00417-007-0661-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-007-0661-y