Abstract
Objective
The effects of different exercise doses on motor function, balance, mobility, and quality of life (QOL) in patients with Parkinson's disease (PD) were evaluated.
Method
The exercise intervention dose was evaluated based on the recommendations of the American College of Sports Medicine (ACSM) for developing and maintaining cardiorespiratory health, muscle strength, and physical function for PD patients and classified into high ACSM compliance and low or uncertain ACSM compliance. The impact of ACSM compliance on Unified Parkinson's Disease Rating Scale, Part III (UPDRS-III), Berg Balance Scale (BBS), Timed Up and Go (TUG), and 39-item Parkinson's Disease Questionnaire (PDQ-39) in patients with PD was compared using the standardized mean difference (SMD) along with the corresponding 95% confidence interval (95% CI).
Results
A total of 26 articles were included, comprising 32 studies. Twenty-one studies were classified as high ACSM compliance, and 11 studies were classified as low or uncertain ACSM compliance. For the four outcome measures, the SMD ratio of exercise interventions with high ACSM compliance to those with low or uncertain ACSM compliance was as follows: UPDRS-III (− 0.74: − 0.17), TUG (− 0.62: − 0.17), PDQ-39 (− 0.58: − 0.31), and BBS (0.51: 0.52).
Conclusion
The results suggest that compared with exercise interventions with low or uncertain ACSM compliance, exercise interventions with high ACSM compliance had a more significant improvement effect on motor function, mobility, and QOL in PD patients. However, the effect on balance was not as pronounced, and further research is needed to validate these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson's disease (PD) is one of the most prevalent neurodegenerative disorders, characterized by motor and non-motor symptoms [1,2,3]. Due to the impairment in the motor system and motor function, PD is typically classified as a movement disorder [4]. The clinical features of PD mainly include resting tremors, bradykinesia, rigidity, postural instability, gait abnormalities, and gradually worsening symptoms over time [4,5,6,7]. In addition to motor symptoms, PD patients experience other non-motor symptoms such as cognitive and sensory impairments, insomnia, and depression [1, 8]. The World Health Organization (WHO) has stated that globally, the rate of disability and death attributed to PD is growing faster than any other neurological disorder. In 2015, there were over 6 million PD patients, with a mortality rate exceeding 100,000, representing a doubling since 1990, and this figure is projected to exceed 12 million in 2040 due to an aging population [9,10,11]. Therefore, we must strive to explore practical measures to alleviate the symptoms of PD patients.
The exact cause of Parkinson's disease is still unclear, but it is widely believed to be caused by genetic susceptibility, environmental factors, and abnormal immune system activity [2, 12]. The motor symptoms of PD are caused by damage to the nigrostriatal pathway in the midbrain, leading to a reduction in the neurotransmitter dopamine [12, 13]. PD prevalence leads to a significant decline in the quality of life for patients and their families, as well as an increased social burden. Currently, technological medical methods cannot cure PD, and we can only prevent or alleviate the clinical symptoms of PD through some therapeutic measures [3]. The treatment of PD includes drug therapy and non-pharmacological therapy. Levodopa is the most effective drug for treating PD, but its effect can only last for about 10 years [14]. Long-term use of levodopa or other treatment drugs by patients can lead to complications such as motor fluctuations and movement difficulties, as well as side effects such as insomnia and orthostatic hypotension [1]. Non-pharmacological treatments have received increased attention in recent years. In non-pharmacological therapy, exercise is an important auxiliary method for treating PD [15]. PD is a chronic progressive disease, and regular exercise can alleviate the skeletal muscle and cardiovascular problems that PD patients develop due to reduced physical activity [16, 17].
The previous studies have discussed the role of exercise as a neuroprotective intervention in PD and neurodegenerative disorders. Exercise has been found to directly or indirectly support synaptic health in the brain and can even have a direct impact on areas primarily affected by synapses in PD, providing neuroprotection. Furthermore, exercise may hinder neurodegeneration through various mechanisms such as improving serotonergic signaling, enhancing neurotrophic factor expression, and improving mitochondrial bioenergetics [18]. Therefore, theoretical research supports exercise intervention as a viable strategy for preventing and counteracting the progression of such diseases.
Practical studies have demonstrated that exercise intervention can improve gait, reduce the frequency of falls, and improve quality of life and aerobic capacity in PD patients [19, 20]. Currently, a large number of exercise intervention experiments have verified the preventive and therapeutic effects of different exercise programs on PD [21,22,23] as well as the effects of exercise on the clinical symptom manifestations of PD patients [22, 24, 25]. In recent years, multiple meta-analyses have compared the effects of different exercise interventions on the motor function of PD patients. Hao ZK and colleagues’ study showed that dance, yoga, virtual reality training, and resistance training have more advantages than other exercise modes [26]. Mustafaoglu R et al. pointed out that different exercise interventions have varying effects depending on functional performance areas, and that dance is an effective exercise to improve the quality of life of PD patients [27]. Zhou X et al. compared the effects of different intensities and cycles of aerobic and resistance training on PD patients [28]. Numerous studies have proven the preventive and therapeutic effects of exercise as a non-pharmacological therapy for PD patients, but research on the exercise dose during exercise intervention is relatively lacking. Therefore, the purpose of this paper is to explore the optimal exercise dose for treating and preventing PD.
The American College of Sports Medicine (ACSM) has developed recommended exercise prescriptions for PD patients, which involve aspects of flexibility, cardiovascular endurance, muscle strength, functional training, and motor control [29]. However, it is not currently clear whether exercise interventions based on ACSM guidelines have a greater impact on PD patients compared to interventions with lower compliance rates to these recommendations. The aim of this systematic review is to compare the effects of high compliance to ACSM guidelines versus low or uncertain compliance exercise interventions on PD patients.
Materials and methods
This systematic review and meta-analysis was reported following the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines and was registered in PROSPERO (CRD42023426987).
Search strategy
We searched PubMed, Embase, Web of Science, and Cochrane databases from the date of establishment to March 12, 2023. The search strategy followed the PICOS principle and focused on research participants, interventions, and research methods. The search included the following subject headings and keywords: (“Parkinson Disease” or “Idiopathic Parkinson's Disease” or “Lewy Body Parkinson's Disease” or “Parkinson's Disease, Idiopathic” or “Parkinson's Disease, Lewy Body” or “Parkinson Disease, Idiopathic” or “Parkinson's Disease” or “Idiopathic Parkinson Disease” or “Lewy Body Parkinson Disease” or “Primary Parkinsonism” or “Parkinsonism, Primary” or “Paralysis Agitans”) AND (“Exercise” or “Exercises” or “Sports” or “Physical Activity” or “Motor Activity” or “Training” or “endurance training” or “Tai Chi” or “yoga” or “Balance” or “Resistance” or “Flexibility” or “Cardiovascular” or “Aerobic”) AND (“Randomized controlled trial” or “controlled clinical trial” or “randomized” or “placebo” or “randomly”). The specific search strategy is provided in Appendix 1. We also hand-searched the references of relevant review articles and retrieved articles for supplementary studies. If necessary, we contacted study authors to obtain additional information.
Criteria for selection of studies
If studies met the following criteria, they were included: (a) published randomized controlled trials; (b) study subjects were PD patients; (c) the experimental group intervention could be any type of exercise, such as resistance training, aerobic exercise, flexibility exercise, etc.; (d) control interventions could be no treatment or any treatment not related to exercise, such as conventional physical therapy, family education, psychotherapy, etc.; and (e) outcome measures in the study included Unified Parkinson's Disease Rating Scale, Part III (UPDRS-III) or Movement Disorder Society Unified Parkinson's Disease Rating Scale, Part III (MDS-UPDRS- III), Berg Balance Scale (BBS), Timed Up and Go (TUG) test, and 39-item Parkinson's Disease Questionnaire (PDQ-39).
The following studies were excluded: (a) Studies reported as conference abstracts, review articles, or editorials; (b) studies with exercise or no standard therapy as the control group; (c) studies involving patients with other cardiovascular or metabolic diseases; (d) studies that administered special drug treatment during the exercise intervention; and (e) duplicate publications reporting the same experimental data from a single study.
Two authors (WLC and DL) independently screened the titles and abstracts of the retrieved literature for eligibility. If either author deemed a study potentially eligible, the full text of the article was obtained. The two authors then independently assessed the full text for eligibility. In cases of disagreement, a third author (JX) provided a final decision through discussion until a consensus was reached. There were no restrictions on the age, sex, body mass index, publication date, or language of the study participants.
Data synthesis and analysis
The data extraction process was conducted independently by two authors (WLC and LJY). The primary outcomes considered in this study were UPDRS-III, BBS, TUG, and PDQ-39. An Excel spreadsheet was designed in advance to extract relevant data, including publication characteristics (title, author names, publication year, and country), methodological characteristics (number of study groups, group designs, interventions, and sample sizes), participant characteristics (age, sex ratio, disease duration, and Parkinson’s disease Hoehn and Yahr staging), exercise characteristics (intervention frequency, exercise intensity, exercise duration, repetition numbers, and set numbers), and risk assessment and outcome features.
When extracting outcome data, if the data were presented graphically without clear textual descriptions, Engauge Digitizer software was used for data extraction. For studies with multiple follow-ups, only the data immediately assessed after the intervention were extracted.
After data extraction, the exercise intervention dose and compliance were evaluated. The exercise intervention dose in the included studies was evaluated based on the recommendations of the American College of Sports Medicine for developing and maintaining cardiopulmonary and neuromotor function in PD patients [29]. Two authors (WLC and JX) independently scored each aspect (including frequency, intensity, duration, etc.) of the exercise intervention in each study according to the different criteria defined by the ACSM recommended dose, in order to assess exercise dose compliance (Table 1).
The scoring range for each exercise indicator was from 0 to 2 points. A score of 2 points indicated compliance with the criteria; a score of 1 point indicated uncertainty; and a score of 0 points indicated non-compliance. In cases of disagreement between the two authors, a discussion was held with the third author to reach a consensus. Based on this scoring system, we calculated the proportion of exercise dose compliance in each study according to the ACSM recommended dose. When the proportion was ≥ 70%, it was classified as high compliance to ACSM recommendations, and when the proportion was < 70%, it was classified as low or uncertain compliance to ACSM recommendations.
Statistical analysis
Meta-analysis was performed using STATA 16.0 to compare the results of the included studies. The studies were divided into two groups in the meta-analysis based on high and low or uncertain compliance to ACSM recommendations. The heterogeneity between studies of each subgroup was assessed using the Higgins I2 statistic and interpreted according to the recommendations of the Cochrane Handbook [30]. In the heterogeneity test, a fixed effect model was used to test the effect size if I2 ≤ 50%, while a random effect model was used if I2 > 50%, and the effect size was represented by the standardized mean difference (SMD) combined with a 95% confidence interval (95% CI). The possibility of publication bias was evaluated by constructing a funnel plot for each study’s effect size relative to standard error. The Begg’s rank correlation method and Egger’s linear regression method were used to test the asymmetry of the funnel plot, and P < 0.05 was considered statistically significant. Sensitivity analysis was also performed by iteratively excluding studies to test the robustness of the results.
Quality appraisal
The quality of the included studies was assessed by two pairs of authors (LCW and JX, DL and LJY), according to the Cochrane Collaboration’s recommended quality assessment criteria for randomized controlled trials [31]. All studies included in this review were randomized controlled trials. According to the Cochrane Handbook, when including randomized controlled trials, the recommended tool is the revised version of the Cochrane tool, called the risk of bias tool (Rob 2) [32]. The Rob 2 tool provides a framework for assessing the risk of bias in individual outcomes in any type of randomized trial. The evaluation indicators include random sequence generation, allocation concealment, blinding of participants and researchers, blinding of outcome assessments, incomplete outcomes, selective reporting, and other biases. Reviewers scored different studies based on the Cochrane Handbook, with the risk of bias in each domain classified into three levels: “low risk,” “some concerns,” and “high risk.” If the risk of bias evaluation results in all domains is low risk, then the overall risk of bias is low; if some domains are evaluated as “some concerns,” and there is no domain with high risk, then the overall risk of bias is “some concerns”; and if the risk of bias assessment result for any one domain is “high risk,” then the overall risk of bias is “high risk” [33].
Results
Study selection
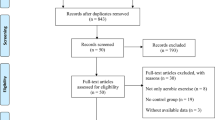
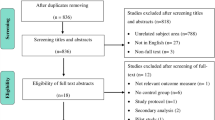
A total of 9300 literature were retrieved from four databases, PubMed (n = 1386), Embase (n = 1728), Web of Science (n = 2532), and Cochrane (n = 3654). After removal of duplicates, 6342 records remained. Following a thorough review of the titles and abstracts, 158 articles were considered as potential candidates for inclusion. Finally, after a comprehensive reading of the full texts, 26 relevant articles were incorporated [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59] (Fig. 1).
Study characteristics
The 26 included articles covered 32 comparative research studies, with six articles reporting on two exercise intervention groups. A total of 1177 participants were included in the 32 studies, with 607 participants in the intervention group and 570 participants in the control group. In terms of gender ratio, except for three studies that did not report the proportion of gender, the intervention group included 341 males and 239 females, while the control group included 345 males and 206 females. The age of the participants ranged from 46 to 83 years old, with the intervention group ranging from 49 to 79 years old and the control group ranging from 46 to 83 years old. Two studies did not report the duration of the disease among the participants. After excluding these two studies, the disease duration of the intervention group ranged from 0.59 to 24.6 years, and that of the control group ranged from 0.58 to 31 years. Eleven studies did not report Hoehn and Yahr stage scores, with the Hoehn and Yahr stage scores of the intervention group ranging from 0.5 to 4 and those of the control group from 1 to 4. With regard to geographic distribution, eight studies were from Brazil, five from the US, four from China, three from Turkey, and two each from Sweden, Belgium, India, and South Korea, while Thailand, the UK, Iran, and Italy each had one study. In terms of participant recruitment, the participants were primarily recruited through hospital clinics, communities, and advertising media (Table 2).
Looking at the outcome measures included in the studies, UPDRS III was included in 16 studies, involving 731 participants, including 375 in the intervention group and 356 in the control group. BBS was included in 12 studies, involving 357 participants, including 180 in the intervention group and 177 in the control group. TUG was included in 18 studies, involving 631 participants, including 323 in the intervention group and 308 in the control group. PDQ-39 measures were included in 14 studies, involving 507 participants, including 258 in the intervention group and 249 in the control group.
The duration of the interventions in the 32 studies ranged from 6 weeks to 16 months, with exercise frequency ranging from 2 times per week to 7 times per week. All studies included supervised exercise or home-based exercise interventions. Among the 32 studies, six interventions involved resistance training, two involved aerobic exercise, and three each involved balance training, treadmill training, and Tai Chi exercise. One study involved aquatic-based exercise, while other interventions included exercise based on virtual reality, Pilates, Nordic walking, and more. Based on the ACSM recommendations, 25 studies involved aerobic exercise dose, 12 studies involved resistance exercise dose, and 16 studies involved flexibility exercise dose (Table 3).
Risk of bias
All studies found that the risk of bias in random sequence generation was low. Among the included 26 studies, 12 were considered to have low risk of allocation concealment bias, 14 did not report the allocation method, and were, therefore, considered to have an uncertain risk. The blinding of both researchers and participants was associated with a higher risk of bias because exercise interventions were difficult to implement in a double-blind manner. Therefore, the overall risk of bias in this category was relatively high. Regarding outcome assessment blinding, 15 studies used random testing or blinded assessors, resulting in low risk; 10 studies did not mention the outcome assessment method and had some concerns; and one study did not guarantee outcome blinding, thus considered to have high risk. Among the 15 studies with incomplete outcome reporting, the number of subjects after the intervention was consistent or mostly consistent with baseline, so they were considered to have low risk. In five studies, the number of dropouts was small (5–10 individuals), resulting in some concerns, while four studies had a significant difference in the number of subjects before and after the intervention (≥ 10 individuals), resulting in high risk. The risk of selective reporting bias was low in 20 studies, and there were some concerns in six studies due to failure to report pre-registered plans or provide detailed explanations for subject dropouts. Five studies were at high risk of other biases (Fig. 2).
Compliance with the ACSM recommendations
Compliance with the ACSM recommendations was ≥ 70% in 21 of the 32 studies, while in 11 studies, compliance with ACSM was less than 70%. The reasons for low compliance were the mismatch between exercise intervention dose and the ACSM recommendations, as well as insufficient information on exercise prescription for appropriate evaluation.
From the perspective of outcome measures, compliance proportions were analyzed as follows: For studies with UPDRS-III as the outcome measure, 12 studies had high ACSM compliance, while four studies had low or uncertain ACSM compliance. For studies with BBS as the outcome measure, seven studies had high ACSM compliance, while five studies had low or uncertain ACSM compliance. For studies with TUG as the outcome measure, 12 studies had high ACSM compliance, while six studies had low or uncertain ACSM compliance. For studies with PDQ-39 as the outcome measure, 11 studies had high ACSM compliance, while three studies had low or uncertain ACSM compliance.
Meta-analysis
Motor function
In our analysis of 16 studies involving 731 participants with UPDRS-III as the outcome measure, we first performed a heterogeneity test and found an I2 greater than 50% (I2 = 87.4%, P = 0.000); thus, we used a random effects model for statistical analysis. Our analysis found a total combined SMD of − 0.6 (95% CI − 1.05, − 0.15), indicating the beneficial effect of exercise intervention on UPDRS-III in PD patients. In subgroup analysis, we grouped studies according to the proportion of compliance with ACSM recommendations. The combined SMD for the subgroup with high ACSM compliance was -0.74 (95% CI − 1.26, − 0.22). For the subgroup with low or uncertain ACSM compliance, the combined SMD was − 0.17 (95% CI − 1.16, 0.82). Subgroup difference analysis showed a significant difference between exercise interventions with high ACSM compliance and those with low or uncertain ACSM compliance (Fig. 3). Therefore, we conclude that exercise interventions with high ACSM compliance have better therapeutic effects on UPDRS-III in PD patients than those with low or uncertain ACSM compliance.
In the subgroup with high ACSM compliance, individual study heterogeneity in the outcome measure UPDRS-III was 88.5%. In the subgroup with low or uncertain ACSM compliance, the heterogeneity was 85.3%. Visual inspection of the funnel plot (Fig. 7A) showed approximate symmetry on both sides, indicating no obvious publication bias. Furthermore, Begger’s test (P = 0.719) and Egger’s test (P = 0.484) confirmed the absence of significant publication bias. In sensitivity analysis (Fig. 8A), we found that no single study had a significant impact on the overall results, demonstrating the robustness of our findings.
Balance
In the 12 studies with 357 participants that used BBS as the outcome measure, we first performed a heterogeneity test and found an I2 less than 50% (I2 = 30.4%, P = 0.149); thus, we used a fixed effects model for statistical analysis. Our analysis found a total combined SMD of 0.51 (95% CI 0.30, 0.73), indicating the beneficial effect of exercise intervention on BBS in PD patients. In subgroup analysis, we grouped studies according to the proportion of compliance with ACSM recommendations. The combined SMD for the subgroup with high ACSM compliance was 0.51 (95% CI 0.26, 0.76), while for the subgroup with low or uncertain ACSM compliance, the combined SMD was 0.52 (95% CI 0.12, 0.92). Subgroup difference analysis showed no significant difference between exercise interventions with high ACSM compliance and those with low or uncertain ACSM compliance (Fig. 4). Therefore, we conclude that exercise interventions with high ACSM compliance are not superior to those with low or uncertain ACSM compliance in terms of the therapeutic effect on BBS in PD patients.
In the subgroup with high ACSM compliance, the individual study heterogeneity for BBS was 56.3%. In the subgroup with low or uncertain ACSM compliance, the individual study heterogeneity was 0.0%. The funnel plot analysis (Fig. 7B) showed approximate symmetry on both sides, indicating no obvious publication bias. Furthermore, Begger’s test (P = 0.217) and Egger’s test (P = 0.102) confirmed the absence of significant publication bias. In sensitivity analysis (Fig. 8B), we found that no single study had a significant impact on the overall results, demonstrating the robustness of our findings.
Mobility
When analyzing the results for the Timed Up and Go test, we included a total of 631 participants from 18 studies. We first performed a heterogeneity test and found I2 to be less than 50% (I2 = 29.2%, P = 0.120); thus, we used a fixed effects model for statistical analysis. Our analysis found a total combined SMD of − 0.44 (95% CI − 0.60, − 0.28), indicating the beneficial effect of exercise intervention on TUG in PD patients. In subgroup analysis, we grouped studies according to the proportion of compliance with ACSM recommendations. The combined SMD for the subgroup with high ACSM compliance was − 0.62 (95% CI − 0.82, − 0.41), while for the subgroup with low or uncertain ACSM compliance, the combined SMD was − 0.17 (95% CI − 0.43, − 0.08). Subgroup difference analysis showed a significant difference between exercise interventions with high ACSM compliance and those with low or uncertain ACSM compliance (Fig. 5). Therefore, we conclude that exercise interventions with high ACSM compliance have better therapeutic effects on TUG in PD patients than those with low or uncertain ACSM compliance.
In the subgroup with high compliance, the individual studies measuring TUG showed a heterogeneity of 17.7%. In the low or uncertain compliance subgroup, heterogeneity was 0.0%. Funnel plot inspection (Fig. 7C) showed approximate symmetry on both sides, indicating no obvious publication bias. Furthermore, Begger’s test (P = 0.677) and Egger’s test (P = 0.649) confirmed the absence of significant publication bias. In sensitivity analysis (Fig. 8C), we found that no single study had a significant impact on the overall results, demonstrating the robustness of our findings.
Quality of life
When the outcome was PDQ-39, we analyzed 507 participants from 14 studies. First, we found significant heterogeneity (I2 = 80.0%, P = 0.000) through heterogeneity testing, and thus, a random effects model was adopted for statistical analysis. Our analysis found a total combined SMD of − 0.54 (95% CI − 0.96, − 0.12), indicating the beneficial effect of exercise intervention on the PDQ-39 of PD patients. In subgroup analysis, we grouped studies according to the proportion of compliance with ACSM recommendations. The combined SMD for the subgroup with high ACSM compliance was -0.58 (95% CI − 0.99, − 0.18), while for the subgroup with low or uncertain ACSM compliance, the combined SMD was -0.31 (95% CI − 1.83, 1.21). Subgroup difference analysis showed a significant difference between exercise interventions with high ACSM compliance and those with low or uncertain ACSM compliance (Fig. 6). Therefore, we conclude that exercise interventions with high ACSM compliance have better therapeutic effects on the PDQ-39 of PD patients than those with low or uncertain ACSM compliance.
In the subgroup with high ACSM compliance, the individual study heterogeneity in measuring PDQ-39 was 71.8%. In the subgroup with low or uncertain ACSM compliance, the heterogeneity was 93.2%. Inspection of the funnel plot (Fig. 7D) revealed approximate symmetry on both sides, indicating no obvious publication bias. Furthermore, Begger’s test (P = 0.870) and Egger’s test (P = 0.913) confirmed the absence of significant publication bias. In sensitivity analysis (Fig. 8D), we found that no single study had a significant impact on the overall results, demonstrating the robustness of our findings.
Discussion
This system review and meta-analysis synthesize various exercise modes, intensity levels, exercise duration, and other indicators used in the previous research to verify the influence of exercise dose on improving PD patients grouped according to ACSM compliance. To our knowledge, no other reviews currently ascertain the influence of exercise dose on PD patients using ACSM compliance as a standard.
In the previous studies, LO Lima et al. [60] and Lamotte G et al. [61] researched on the effects of progressive resistance and endurance exercise on PD patients, analyzing strength, fitness, and physical condition (maximum oxygen uptake and gait) as outcome measures. The authors concluded that resistance exercise could improve strength performance and physical condition. Still, both review analyses had limitations of limited data, and the objectivity of the results needed to be verified. Therefore, based on many randomized controlled trials, this study used UPDRS-III, BBS, TUG, and PDQ-39 as outcome measures to ensure the objectivity of the results as much as possible. Yang Y et al.'s meta-analysis also found that Tai Chi improved motor function and balance [62], consistent with our conclusions. We also found that the SMD of UPDRS-III was slightly higher than that reported in this study's review (SMD: -0.74 vs. -0.57) in the meta-analysis of exercise interventions that highly adhered to ACSM recommendations, which may have benefited our review results, indicating that an appropriate exercise dose would have more favorable effects on participants. After reviewing previous meta-analyses and relevant studies, we found that meta-analyses on PD patients focused more on a specific exercise program (Tai Chi [63], dance [64], yoga [65], treadmill [66], etc.) or a comparison between different types of exercise (aerobic exercise [67], resistance exercise [68], endurance exercise [69], etc.) and a network meta-analyses [26, 70]. Therefore, we can only infer that exercise can improve UPDRS-III, BBS, TUG, and PDQ-39 in PD patients, and no specific exercise program has been proven superior to others.
Currently, clinical physical interventions for the prevention and treatment of PD include deep brain stimulation, transcranial direct current stimulation, whole-body vibration, VR-assisted training, and interventions using wearable devices. These interventions have been applied in PD rehabilitation or are being investigated in clinical trials. Due to variations in intervention dosage and participant characteristics, experimental results have shown some differences, but the majority of published intervention studies have demonstrated positive outcomes [71,72,73]. Furthermore, through summarizing published experiments and unpublished clinical trials registered in multiple national clinical trial registries, it has been observed that there is diversity in the interventions used for PD treatment, but the extracted outcome measures are highly similar. Non-pharmacological interventions primarily focus on outcome measures related to motor function, gait, and quality of life in PD patients. Accordingly, our study also focuses on outcome measures related to motor function and quality of life in PD patients. Lastly, in addition to outcome measure extraction, we have also noted the combination of exercise interventions with the aforementioned physical treatment methods, such as utilizing VR-assisted technology for exercise [40]. From the currently published experimental results, the combination of these interventions has shown greater effectiveness compared to traditional single-mode exercise interventions or physical treatments alone [40, 49]. Comprehensive rehabilitation approaches such as intensive rehabilitation therapy and multimodal rehabilitation therapy [74, 75] that combine pharmacological and non-pharmacological treatments are also crucial. Existing research has demonstrated positive effects of comprehensive rehabilitation therapy on various aspects of PD patients, such as improving bradykinesia and motor learning abilities. However, it should be noted that different patients may benefit from different treatments depending on their physical condition, age, and comorbidities.
Our research has found that exercise interventions could improve the scores of UPDRS-III (SMD = − 0.6; 95% CI − 1.05, − 0.15), BBS (SMD = 0.51; 95% CI 0.30, 0.73), TUG (SMD = − 0.44; 95% CI − 0.60, − 0.28), and PDQ-39 (SMD = − 0.54; 95% CI − 0.96, − 0.12) in PD patients, which is consistent with common knowledge and previous research conclusions [15, 76, 77] that exercise is an effective non-pharmacological treatment for PD patients. From the results of subgroup analysis, compared with exercise interventions with low or uncertain ACSM compliance, exercise interventions with high ACSM compliance had a better improvement effect on UPDRS-III (SMD − 0.74 vs. − 0.17), TUG (SMD − 0.62 vs. − 0.17), and PDQ-39 (SMD − 0.58 vs. − 0.31) in PD patients, but the improvement effect on BBS (SMD: 0.51 vs. 0.52) was not obvious. From the difference in effect size (SMD) comparison, exercise with high ACSM compliance had the most significant improvement effect on UPDRS-III (0.57), followed by TUG (0.45), and PDQ-39 (0.27), respectively.
One key point of this study is the interpretation of ACSM compliance. Exercise interventions recommended by ACSM include aerobic exercise, resistance exercise, and flexibility exercise, each with detailed descriptions of the recommended exercise dose. However, descriptions of exercise dose in randomized controlled trials for PD patients are not comprehensive or can only be attributed to one type of exercise intervention. For example, 11 studies only reported the exercise dose of aerobic exercise, four studies only reported the exercise dose of resistance exercise, and only five studies reported exercise dose that met the full classification recommended by ACSM. Additionally, some studies failed to report or inadequately reported the exercise intervention dose, such as only describing the dose as “individualized.” This means that even if the exercise intervention dose is highly compliant with ACSM recommendations, it may be incorrectly classified as a low or uncertain compliance group. Similar to pharmacological treatments, detailed descriptions of the exercise prescription in intervention are essential for pinpointing the reasonable range of the exercise dose. Although we need to differentiate treatment for individuals during the specific implementation process, we should also adjust within the range of reasonable exercise prescriptions.
This study also has certain limitations that may lead to bias in the results. The 21 highly ACSM-compliant exercise interventions included various types of exercise, such as virtual reality training, balance training, resistance training, flexibility training, water-based exercise, mind–body exercise, etc., which may have relatively high heterogeneity among studies. Secondly, the interventions provided varied in frequency, intensity, time, and so on, so it is difficult to compare and recommend general standards for the best exercise intervention. Previous meta-analysis and randomized controlled trials lacked comparative studies of exercise intensity and frequency, which made it difficult to determine and design the best exercise program for PD patients in terms of type, dose, and duration. Furthermore, there is a risk of potential bias in every study. Any unclear or high risk of bias factors in each study will increase the final estimate of the intervention effect. The overall bias of the results in this review may be more related to blinding of the interveners and participants, followed by blinding of allocation and output of results. Lastly, although the extraction of data from the figures and tables was minimized to reduce errors, it is inevitable (Table 4).
Conclusion
This review supports the recommendation that exercise is an effective measure for improving clinical symptoms in PD patients, and our results confirm this conclusion once again. In the process of analyzing the best exercise dose for PD patients, we found that compared with exercise interventions with low or uncertain ACSM compliance, exercise interventions with high ACSM compliance had a more significant improvement effect on motor function, mobility, and QOL, but not on balance. Additionally, some studies did not provide detailed exercise intervention plans, so this needs to be further validated in the future research.
Data Availability
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
References
Samii A, Nutt JG, Ransom BR (2004) Parkinson’s disease. Lancet 363:1783–1793. https://doi.org/10.1016/S0140-6736(04)16305-8
Lew M (2007) Overview of Parkinson’s disease. Pharmacotherapy 27:155S-160S. https://doi.org/10.1592/phco.27.12part2.155S
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386:896–912. https://doi.org/10.1016/S0140-6736(14)61393-3
Tysnes O-B, Storstein A (2017) Epidemiology of Parkinson’s disease. J Neural Transm 124:901–905. https://doi.org/10.1007/s00702-017-1686-y
Sveinbjornsdottir S (2016) The clinical symptoms of Parkinson’s disease. J Neurochem 139:318–324. https://doi.org/10.1111/jnc.13691
Antony PMA, Diederich NJ, Krüger R, Balling R (2013) The hallmarks of Parkinson’s disease. FEBS J 280:5981–5993. https://doi.org/10.1111/febs.12335
de Rijk MC, Tzourio C, Breteler MM et al (1997) Prevalence of parkinsonism and Parkinson’s disease in Europe: the EUROPARKINSON Collaborative Study. European Community Concerted Action on the Epidemiology of Parkinson’s disease. J Neurol Neurosurg Psychiatry 62:10–15. https://doi.org/10.1136/jnnp.62.1.10
Barnett-Cowan M, Dyde RT, Fox SH et al (2010) Multisensory determinants of orientation perception in Parkinson’s disease. Neuroscience 167:1138–1150. https://doi.org/10.1016/j.neuroscience.2010.02.065
Vos T, Allen C, Arora M et al (2016) Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet 388:1545–1602. https://doi.org/10.1016/S0140-6736(16)31678-6
Wang H, Naghavi M, Allen C et al (2016) Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388:1459–1544. https://doi.org/10.1016/S0140-6736(16)31012-1
Dorsey ER, Sherer T, Okun MS, Bloem BR (2018) The emerging evidence of the Parkinson pandemic. J Park Dis 8:S3–S8. https://doi.org/10.3233/JPD-181474
Reich SG, Savitt JM (2019) Parkinson’s disease. Med Clin N Am 103:337–350. https://doi.org/10.1016/j.mcna.2018.10.014
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184. https://doi.org/10.1136/jnnp.55.3.181
Jankovic J, Poewe W (2012) Therapies in Parkinson’s disease. Curr Opin Neurol 25:433–447. https://doi.org/10.1097/WCO.0b013e3283542fc2
Bloem BR, de Vries NM, Ebersbach G (2015) Nonpharmacological treatments for patients with Parkinson’s disease. Mov Disord 30:1504–1520. https://doi.org/10.1002/mds.26363
Yu W-Y, Yang Q-H, Wang X-Q (2022) The mechanism of exercise for pain management in Parkinson’s disease. Front Mol Neurosci 15:1039302. https://doi.org/10.3389/fnmol.2022.1039302
Ascherio A, Schwarzschild MA (2016) The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol 15:1257–1272. https://doi.org/10.1016/S1474-4422(16)30230-7
Schirinzi T, Canevelli M, Suppa A et al (2020) The continuum between neurodegeneration, brain plasticity, and movement: a critical appraisal. Rev Neurosci 31:723–742. https://doi.org/10.1515/revneuro-2020-0011
Crizzle AM, Newhouse IJ (2006) Is physical exercise beneficial for persons with Parkinson’s disease? Clin J Sport Med 16:422–425. https://doi.org/10.1097/01.jsm.0000244612.55550.7d
Ahlskog JE (2011) Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology 77:288–294. https://doi.org/10.1212/WNL.0b013e318225ab66
Dos Santos DM, Komeroski IG, Monteiro EP et al (2018) Effects of dance practice on functional mobility, motor symptoms and quality of life in people with Parkinson’s disease: a systematic review with meta-analysis. Aging Clin Exp Res 30:727–735. https://doi.org/10.1007/s40520-017-0836-2
Liu H-H, Yeh N-C, Wu Y-F et al (2019) Effects of Tai Chi exercise on reducing falls and improving balance performance in Parkinson’s disease: a meta-analysis. Park Dis 2019:1–8. https://doi.org/10.1155/2019/9626934
Perry SIB, Nelissen PM, Siemonsma P, Lucas C (2019) The effect of functional-task training on activities of daily living for people with Parkinson`s disease, a systematic review with meta-analysis. Complement Ther Med 42:312–321. https://doi.org/10.1016/j.ctim.2018.12.008
Lauze M, Daneault JF, Duval C (2016) The effects of physical activity in Parkinson’s disease: a review. J Park Dis 6:685–698. https://doi.org/10.3233/jpd-160790
Stuckenschneider T, Askew CD, Meneses AL et al (2019) The effect of different exercise modes on domain-specific cognitive function in patients suffering from Parkinson’s disease: a systematic review of randomized controlled trials. J Park Dis 9:73–95. https://doi.org/10.3233/jpd-181484
Hao Z, Zhang X, Chen P (2022) Effects of ten different exercise interventions on motor function in Parkinson’s disease patients-a network meta-analysis of randomized controlled trials. Brain Sci. https://doi.org/10.3390/brainsci12060698
Mustafaoglu R, Ahmed I, Pang MYC (2022) Which type of mind-body exercise is most effective in improving functional performance and quality of life in patients with Parkinson’s disease? A systematic review with network meta-analysis. Acta Neurol Belg 122:1433–1446. https://doi.org/10.1007/s13760-022-02070-4
Zhou X, Zhao P, Guo X et al (2022) Effectiveness of aerobic and resistance training on the motor symptoms in Parkinson’s disease: systematic review and network meta-analysis. Front Aging Neurosci 14:935176. https://doi.org/10.3389/fnagi.2022.935176
Garber CE, Blissmer B, Deschenes MR et al (2011) Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults. Med Sci Sports Amp Exerc 43:1334–1359. https://doi.org/10.1249/MSS.0b013e318213fefb
Deeks JJ, Higgins JP, Altman DG, Group on behalf of the CSM (2019) Analysing data and undertaking meta-analyses. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons Ltd, pp 241–284
Higgins JPT, Altman DG, Gotzsche PC et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928–d5928. https://doi.org/10.1136/bmj.d5928
Sterne JAC, Savović J, Page MJ et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898. https://doi.org/10.1136/bmj.l4898
Cumpston M, Li T, Pagre MJ et al (2019) Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 10:ED000142. https://doi.org/10.1002/14651858.ED000142
Fisher BE, Wu AD, Salem GJ et al (2008) The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson’s disease. Arch Phys Med Rehabil 89:1221–1229. https://doi.org/10.1016/j.apmr.2008.01.013
Schenkman M, Hall DA, Barón AE et al (2012) Exercise for people in early- or mid-stage Parkinson disease: a 16-month randomized controlled trial. Phys Ther 92:1395–1410
Amano S, Nocera JR, Vallabhajosula S et al (2013) The effect of Tai Chi exercise on gait initiation and gait performance inpersons with Parkinson’s disease. Parkinsonism Relat Disord 19:955–960. https://doi.org/10.1016/j.parkreldis.2013.06.007
Gao Q, Leung A, Yang Y et al (2014) Effects of Tai Chi on balance and fall prevention in Parkinson’s disease: a randomized controlled trial. Clin Rehabil 28:748–753. https://doi.org/10.1177/0269215514521044
Conradsson D, Löfgren N, Nero H et al (2015) The effects of highly challenging balance training in elderly with Parkinson’s disease: a randomized controlled trial. Neurorehabil Neural Repair 29:827–836. https://doi.org/10.1177/1545968314567150
Cugusi L, Solla P, Serpe R et al (2015) Effects of a Nordic Walking program on motor and non-motor symptoms, functional performance and body composition in patients with Parkinson’s disease. NeuroRehabilitation 37:245–254. https://doi.org/10.3233/NRE-151257
Liao YY, Yang YR, Cheng SJ et al (2015) Virtual reality-based training to improve obstacle-crossing performance and dynamic balance in patients with Parkinson’s disease. Neurorehabil Neural Repair 29:658–667. https://doi.org/10.1177/1545968314562111
Ni M, Signorile JF, Balachandran A, Potiaumpai M (2016) Power training induced change in bradykinesia and muscle power in Parkinson’s disease. Parkinsonism Relat Disord 23:37–44. https://doi.org/10.1016/j.parkreldis.2015.11.028
Collett J, Franssen M, Meaney A et al (2017) Phase II randomised controlled trial of a 6-month self-managed community exercise programme for people with Parkinson’s disease. J Neurol Neurosurg Psychiatry 88:204–211. https://doi.org/10.1136/jnnp-2016-314508
Demonceau M, Maquet D, Jidovtseff B et al (2017) Effects of twelve weeks of aerobic or strength training in addition to standard care in Parkinson’s disease: a controlled study. Eur J Phys Rehabil Med 53:184–200. https://doi.org/10.23736/S1973-9087.16.04272-6
Ribas CG, Alves da Silva L, Corrêa MR et al (2017) Effectiveness of exergaming in improving functional balance, fatigue and quality of life in Parkinson’s disease: a pilot randomized controlled trial. Parkinsonism Relat Disord 38:13–18. https://doi.org/10.1016/j.parkreldis.2017.02.006
Ferreira RM, Alves WMGC, Lima TA et al (2018) The effect of resistance training on the anxiety symptoms and quality of life in elderly people with parkinson’s disease: a randomized controlled trial. Arq Neuropsiquiatr 76:499–506. https://doi.org/10.1590/0004-282x20180071
Peloggia Cursino M, Raquel DF, Zamfolini Hallal C, Faganello Navega FR (2018) Kinematic variables of gait and quality of life in Parkinsonians after different treadmill trainings: a randomized control trial. Motricidade 14:29–39. https://doi.org/10.6063/motricidade.10809
Arfa-Fatollahkhani P, Safar Cherati A, Habibi SAH et al (2019) Effects of treadmill training on the balance, functional capacity and quality of life in Parkinson’s disease: a randomized clinical trial. J Complement Integr Med. https://doi.org/10.1515/jcim-2018-0245
de Lima TA, Ferreira-Moraes R, Alves WMGDC et al (2019) Resistance training reduces depressive symptoms in elderly people with Parkinson disease: a controlled randomized study. Scand J Med Sci Sports 29:1957–1967. https://doi.org/10.1111/sms.13528
Santos P, Machado T, Santos L et al (2019) Efficacy of the Nintendo Wii combination with Conventional Exercises in the rehabilitation of individuals with Parkinson’s disease: a randomized clinical trial. NeuroRehabilitation 45:255–263. https://doi.org/10.3233/NRE-192771
Silva AZD, Israel VL (2019) Effects of dual-task aquatic exercises on functional mobility, balance and gait of individuals with Parkinson’s disease: a randomized clinical trial with a 3-month follow-up. Complement Ther Med 42:119–124. https://doi.org/10.1016/j.ctim.2018.10.023
Khuzema A, Brammatha A, Arul Selvan V (2020) Effect of home-based Tai Chi, Yoga or conventional balance exercise on functional balance and mobility among persons with idiopathic Parkinson’s disease: an experimental study. Hong Kong Physiother J 40:39–49. https://doi.org/10.1142/S1013702520500055
Moon JH, Jung JH, Cho HY (2020) Effects of balance training using a wii fit balance board on balance, gait and activities of daily living in patients with parkinson disease: a pilot, randomized controlled trial. Medico-Leg Update 20:1799–1803. https://doi.org/10.37506/v20/il/2020/mlu/194564
Leavy B, Joseph C, Lofgren N et al (2020) Outcome evaluation of highly challenging balance training for people with Parkinson disease: a multicenter effectiveness-implementation study. J Neurol Phys Ther 44:15–22. https://doi.org/10.1097/npt.0000000000000298
Youm C, Kim Y, Noh B et al (2020) Impact of trunk resistance and stretching exercise on fall-related factors in patients with Parkinson’s disease: a randomized controlled pilot study. Sensors 20:4106. https://doi.org/10.3390/s20154106
Chen J, Chien HF, Francato DCV et al (2021) Effects of resistance training on postural control in Parkinson’s disease: a randomized controlled trial. Arq Neuropsiquiatr 79:511–520. https://doi.org/10.1590/0004-282X-ANP-2020-0285
Çoban F, Belgen Kaygısız B, Selcuk F (2021) Effect of clinical pilates training on balance and postural control in patients with Parkinson’s disease: a randomized controlled trial. J Comp Eff Res 10:1373–1383. https://doi.org/10.2217/cer-2021-0091
Göz E, Çolakoğlu BD, Çakmur R, Balci B (2021) Effects of pilates and elastic taping on balance and postural control in early stage Parkinson’s disease patients: a pilot randomised controlled trial. Noro Psikiyatr Ars 58:308–313. https://doi.org/10.29399/npa.24935
Khobkhun F, Suwannarat J, Pheungphrarattanatrai A et al (2021) The effects of a 10-week home-based exercise programme in individuals with Parkinson’s disease during the COVID-19 pandemic: a pilot study. Appl Sci 11:4518. https://doi.org/10.3390/app11104518
Li Z, Wang T, Shen M et al (2022) Comparison of Wuqinxi Qigong with stretching on single- and dual-task gait, motor symptoms and quality of life in Parkinson’s disease: a preliminary randomized control study. Int J Environ Res Public Health 19:8042. https://doi.org/10.3390/ijerph19138042
Lima LO, Scianni A, Rodrigues-de-Paula F (2013) Progressive resistance exercise improves strength and physical performance in people with mild to moderate Parkinson’s disease: a systematic review. J Physiother 59:7–13. https://doi.org/10.1016/S1836-9553(13)70141-3
Lamotte G, Rafferty MR, Prodoehl J et al (2015) Effects of endurance exercise training on the motor and non-motor features of parkinson’s disease: a review. J Park Dis 5:21–41. https://doi.org/10.3233/jpd-140425
Yang Y, Li X-Y, Gong L et al (2014) Tai Chi for improvement of motor function, balance and gait in Parkinson’s disease: a systematic review and meta-analysis. PLoS ONE 9:e102942. https://doi.org/10.1371/journal.pone.0102942
Yu X, Wu X, Hou G et al (2021) The impact of Tai Chi on motor function, balance, and quality of life in Parkinson’s disease: a systematic review and meta-analysis. Evid Based Complement Alternat Med 2021:1–10. https://doi.org/10.1155/2021/6637612
Carapellotti AM, Stevenson R, Doumas M (2020) The efficacy of dance for improving motor impairments, non-motor symptoms, and quality of life in Parkinson’s disease: a systematic review and meta-analysis. PLoS ONE 15:e0236820. https://doi.org/10.1371/journal.pone.0236820
Ban M, Yue X, Dou P, Zhang P (2021) The effects of yoga on patients with Parkinson’s Disease: a meta-analysis of randomized controlled trials. Behav Neurol 2021:5582488. https://doi.org/10.1155/2021/5582488
Luna NMS, Brech GC, Canonica A et al (2020) Effects of treadmill training on gait of elders with Parkinson’s disease: a literature review. Einstein Sao Paulo 18:eRW5233. https://doi.org/10.31744/einstein_journal/2020RW5233
Li Y, Song H, Shen L, Wang Y (2021) The efficacy and safety of moderate aerobic exercise for patients with Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. Ann Palliat Med 10:2638–2649. https://doi.org/10.21037/apm-20-1661
Gollan R, Ernst M, Lieker E et al (2022) Effects of resistance training on motor- and non-motor symptoms in patients with parkinson’s disease: a systematic review and meta-analysis. J Park Dis 12:1783–1806. https://doi.org/10.3233/jpd-223252
de Almeida FO, Santana V, Corcos DM et al (2022) Effects of endurance training on motor signs of Parkinson’s disease: a systematic review and meta-analysis. Sports Med 52:1789–1815. https://doi.org/10.1007/s40279-022-01650-x
Lei H, Ma Z, Tian K et al (2022) The effects of different types of Tai Chi exercises on motor function in patients with Parkinson’s disease: a network meta-analysis. Front Aging Neurosci 14:936027. https://doi.org/10.3389/fnagi.2022.936027
Broeder S, Vandendoorent B, Hermans P et al (2023) Transcranial direct current stimulation enhances motor learning in Parkinson’s disease: a randomized controlled trial. J Neurol 270:3442–3450. https://doi.org/10.1007/s00415-023-11669-3
Mazhar T, Jameel A, Sharif F, Asghar M (2023) Effects of conventional physical therapy with and without proprioceptive neuromuscular facilitation on balance, gait, and function in patients with Parkinson’s disease. J Pak Med Assoc 73:1280–1283. https://doi.org/10.47391/JPMA.6710
Jiménez-Barrios M, González-Bernal J, Cubo E et al (2023) Functionality and quality of life with Parkinson’s disease after use of a dynamic upper limb orthosis: a pilot study. Int J Environ Res Public Health 20:4995. https://doi.org/10.3390/ijerph20064995
Dhamija R, Saluja A, Goyal V (2022) Multi-Modal rehabilitation therapy in Parkinson’s disease and related disorders. Ann Indian Acad Neurol. https://doi.org/10.4103/aian.aian_164_22
Frazzitta G, Bertotti G, Riboldazzi G et al (2012) Effectiveness of intensive inpatient rehabilitation treatment on disease progression in parkinsonian patients. Neurorehabil Neural Repair 26:144–150. https://doi.org/10.1177/1545968311416990
Gronek P, Haas AN, Czarny W et al (2021) The mechanism of physical activity-induced amelioration of parkinson’s disease: a narrative review. Aging Dis 12:192–202. https://doi.org/10.14336/ad.2020.0407
Jin X, Wang L, Liu S et al (2019) The impact of mind-body exercises on motor function, depressive symptoms, and quality of life in Parkinson’s disease: a systematic review and meta-analysis. Int J Environ Res Public Health 17:31. https://doi.org/10.3390/ijerph17010031
Acknowledgements
All authors contributed to this study.
Funding
This research was supported by “China Association for Science and Technology project: Research on Health Science Popularization Content Guidelines”(number: kpbwh-2021-2-6).
Author information
Authors and Affiliations
Contributions
WLC conceived and designed the study, and the screening of titles and abstracts was completed by WLC and DL, with disputes resolved by JX. Data inclusion was completed by WLC and LJY. WLC and JX independently scored the compliance of each exercise intervention with ACSM recommended dose. All authors participated in the quality assessment of the included literature. The initial draft of the manuscript was completed by WLC and DL, and all authors provided comments on the first few versions of the manuscript and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Dong Li is co-first author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cui, W., Li, D., Yue, L. et al. The effects of exercise dose on patients with Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. J Neurol 270, 5327–5343 (2023). https://doi.org/10.1007/s00415-023-11887-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11887-9