Abstract
Background
Purpose
Although many studies have investigated the relationship between transient global amnesia (TGA) and migraine, to date, no meta-analysis has confirmed the existence and size of their association.
Methodology
Literature search involved MEDLINE, EMBASE, CENTRAL and PsycINFO. Observational controlled studies including TGA patients (Caplan, Hodges and Warlow) were retrieved. Quality evaluation was based on the Newcastle-Ottawa scale. The prevalence of migraine was compared in TGA patients vs. healthy controls (HC), as well as in TGA against TIA individuals. Data from case-control, cross-sectional and cohort studies were pooled separately.
Results
Literature search yielded 1178 articles, 12 of which were included in the present meta-analysis. Results from case-control (ten), cohort (one) and cross-sectional (one) studies were compatible with an association between TGA and migraine. The nationwide inpatient cross-sectional study was of lesser value due to its inpatient orientation. The high-quality, population-based, retrospective cohort (158,301 participants per group) determined a higher relative-risk (RR) of TGA for migraine vs. non-migraine individuals [RR = 2.48, 95%confidence-interval (95% CI) = (1.32, 4.87)]. Sensitivity testing based on stricter diagnostic criteria strengthened the estimated association [RR = 3.84, 95% CI = (1.57, 9.38)]. Additionally, pooled data from eight case–control studies (700 TGA, 746 HC) yielded similar results [Odds-Ratio, OR = 2.51, 95% CI = (1.85, 3.41)], with the association mainly driven by the three high-quality studies, rather than the five articles of moderate quality. Finally, pooled findings from four case–control studies of moderate-quality revealed a higher prevalence of migraine among TGA compared to TIA patients [OR = 1.82, 95% CI = (1.22, 2.73)].
Conclusions
A significant association between TGA and migraine was established. The underlying connecting mechanism remains undetermined, yet.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transient global amnesia (TGA) constitutes an enigmatic amnestic syndrome characterized by temporary memory dysfunction of abrupt onset and total resolution within 24 h from emergence [1, 2]. Anterograde memory is more severely affected, while retrograde memory presents a variable level of dysfunction [1, 2]. The clinical diagnosis of TGA is based on the criteria of Hodges and Warlow as follows [3, 4]: (1) attacks must be witnessed and information must be available from a capable observer who has to be present for most of the attacks; (2) there must be a clear-cut anterograde amnesia during the attack; (3) clouding of consciousness and loss of personal identity must be absent, and the cognitive impairment must be limited to amnesia (i.e., no aphasia, apraxia, etc.) (4) there should be no accompanying focal neurological symptom during the attack and no significant neurological sign afterward; (5) epileptic features must be absent; (6) attack must resolve within 24 h; and (7) patients with a recent head injury or active epilepsy (i.e., patients who have continued to receive medication or have had one seizure in the past 2 years) are excluded.
Although clinical and neuroimaging evidence is suggestive of an underlying hippocampal (and most notably cornu ammonis -CA1- located) dysfunction [5,6,7], the aetiology of TGA has yet to be determined. Soon after the introduction of the term TGA by Fisher and Adams, the hypothesis of an underlying epileptic mechanism was formulated [8, 9]. However, the performance of electroencephalographic studies on TGA individuals [10], as well as the low recurrence rate of the disease [11], led to the progressively declining popularity of this theory. Epileptic amnesia is now considered part of the differential diagnosis of TGA rather than a possible underlying mechanism [12].
The acute onset of the disease reasonably generated postulations about a vascular mechanism leading to focal ischemia [13]. In this context, the long-term risk of vascular events in TGA patients has been extensively assessed and compared to both healthy controls (HC) [14, 15] and individuals with transient ischemic attacks (TIA) [4, 16,17,18]. Results were indicative of a long-term vascular risk similar to HC and lower than patients with TIA. Apart from the good vascular-related prognosis of the syndrome, follow-up neuroimaging in TGA patients was compatible with a reversible nature of magnetic resonance imaging (MRI) lesions [6], limiting the possibility of an arterial-ischemic underlying mechanism. Additionally, a venous vascular mechanism involving internal jugular vein incompetence and congestion, ultimately leading to transient hippocampal ischemia, has been hypothesized. The existing evidence for the aforementioned theory [19,20,21] is complementarily supported by the multiple recordings of TGA cases triggered by Valsalva manoeuvre-related events [22]. However, the focal, hippocampal manifestations and imaging findings of TGA are difficult to associate with the global cerebral venous congestion.
Finally, the last among the most prevailing theories implicates migraine and the neurophysiologic substrate of aura, which is Cortical Spreading Depression (CSD) [7, 12]. CSD consists of a spreading neuronal depolarization followed by a suppression of the neuronal activity, with respect to the clinically (aura-wise) relevant cerebral areas. Based on experimental data, it has been hypothesized that CSD extending through the hippocampus may be accountable for the transient hippocampal dysfunction during TGA [23]. The possible relationship between migraine and TGA has been investigated by several authors, but to date the only published meta-analysis (2006) revealed no association between the two entities [24].
On the grounds of the above-mentioned hypotheses and the vague pathophysiological background of TGA we decided to evaluate the existing clinical evidence supporting the possible underlying pathophysiological mechanisms of TGA. In this paper, we focused on one of the previously described hypotheses, the migraine-related theory. Observational (case–control, cross-sectional and cohort) controlled studies assessing the prevalence of a migraine history among TGA individuals and HC were retrieved for this purpose. In view of the association between migraine and TIA (as well as other cerebrovascular events) [25], it was additionally decided to retrieve observational controlled studies that investigate the prevalence of a migraine history in TGA vs. TIA patients (the main differential diagnosis of TGA). To the best of our knowledge, the present article is the first meta-analysis to systematically evaluate the association between migraine and TGA since the study of Quinette et al. 2006 [24].
Materials and methods
The present systematic review and meta-analysis adheres to the MOOSE reporting guidelines [26]. Each step of the review process was performed by two authors (unblinded to study information), independently (I.L., A.S.). Discrepancies were resolved by a third author (E. D.).
Search method
The search strategy is presented in the online resource. The structured search involved the following databases: MEDLINE (through PubMed), EMBASE (through Elsevier), CENTRAL (Cochrane Central Register of Controlled Trials, the Cochrane Library) and PsycINFO. An additional manual search involved the references included in the retrieved articles, as well as all articles that cited the papers retrieved by the structured literature search (through Google Scholar). Conference abstracts and abstracts in English from articles with full texts not published in English would be evaluated in case relevant information was provided. Titles and abstracts were manually screened for eligibility. Full texts were retrieved in case of inability to establish the eligibility of an article.
Eligibility criteria
Inclusion criteria were as follows:
-
Studies published between 1985 and Aug 7, 2020, that is following the introduction of Caplan’s diagnostic criteria [27], validated by Hodges and Warlow in 1990 [3]. Thereon, the clinical recognition of TGA was based on these criteria. Hence studies published before that period (1985) were not considered for inclusion.
-
Observational controlled studies (case–control, cross-sectional, cohort).
-
For case control and cross-sectional studies: inclusion of at least two groups of participants (outcome-wise). TGA patients constituted the first group and either HC (without a history of TGA) and/or individuals with TIA consisted the control group. Migraine was evaluated as part of the exposures. Any other CNS (Central Nervous System) disease-specific control group, e.g., patients with stroke, epilepsy, encephalitis and so on, was not considered as an appropriate control group.
-
For cohort studies: inclusion of at least two groups of participants (exposure-wise), subjects with migraine and HC (without a migraine history). TGA was assessed as part of the investigated outcomes.
Exclusion Criteria were as follows:
-
Studies implementing non-validated diagnostic criteria
-
Uncontrolled studies. Case–control and cross-sectional studies involving control groups other than HC and TIA were excluded. Cohort studies not assessing migraine vs. healthy individuals were excluded
-
Controlled studies not assessing the parameters of interest
-
Studies (all types) with equal or less than 10 participants per group
-
Studies other than observational, including Reviews, Meta-analyses, Case reports, Editorials-Commentaries-viewpoints, and so on
-
Study protocols
-
Book chapters-reviews
-
Studies not published in English. In case a study abstract was available in English it was evaluated for inclusion (as part of the grey literature)
Data extraction—quality assessment
The following data were extracted according to data extraction forms: first author, year of publication, study design and data collection process, country of origin and settings, set of diagnostic criteria and definitions for TGA, TIA and migraine, number of participants, age and sex distribution, as well as obtained results.
Case–control and cohort studies were assessed according to the Newcastle–Ottawa Scale (NOS) [28], while cross sectional studies were evaluated based on a modified version of the NOS, adapted to the context of our study (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp and online resource). NOS evaluates nine methodological items and their reporting (participant selection, comparability of groups and ascertainment of exposure/outcome), with values ≥ 7 compatible with a good study quality, between 2 and 7 with a moderate study quality and ≤ 2 with a poor study quality. Modified NOS evaluates eight methodological items and their reporting (adapted from the initial NOS), with values ≥ 6 consistent with a good study quality, between 2 and 6 with a moderate study quality and ≤ 2 with a poor study quality.
Statistical analysis
Statistical analyses were conducted using RevMan 5.4 statistical software [29]. A two-tailed p-value < 0.05 was used for the determination of statistical significance. Effect-sizes and their precision [95% confidence intervals (95% CIs)] were estimated using as weights the inverse variance of individual effects. Case–control (Odds-Ratio, OR), cross-sectional (OR) and cohort studies (Risk Ratio, RR, cumulative incidences would not be meta-analysed) investigating the association between migraine and TGA were separately analysed. Statistical heterogeneity was estimated by the calculation of the Q and I2 statistics (homogeneity accepted if both PQ > 0.1 and I2 < 30%). In the absence of statistical heterogeneity, fixed effects (FE) model was utilized, otherwise, random effects (RE) model was implemented. ORs and 95%CIs were illustrated with forest plots. In case of ten or more studies (rule of thumb) being combined, funnel plots were created for the determination of potential publication bias.
Two separate analyses were planned, TGA vs. HC and TGA vs. TIA. Subgroup analyses according to the type of migraine (with or without aura) were prespecified. Methodological flaws were statistically addressed with the stratification of the results according to the methodological quality of the retrieved studies based on the NOS (high, moderate, low), so that the quality of studies accountable for the existence and size of the association would be revealed.
Results
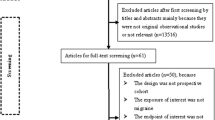
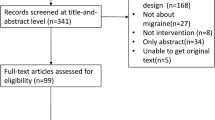
The structured literature search provided 1178 studies (MEDLINE; 901, EMBASE; 243, CENTRAL; 25 and PsycINFO; 9), while the manual search retrieved seven additional articles. After the manual screening of titles and abstracts, 97 full texts were evaluated for inclusion and, finally, 12 papers were involved in the present systematic review and meta-analysis [4, 14, 17, 18, 30,31,32,33,34,35,36,37,38]. Among the retrieved studies one was a retrospective cross-sectional study based on an inpatient database [30], one was designed as a retrospective cohort study based on a National Health Insurance database [32] and 10 were case–control studies with retrospective, prospective or mixed data collection. Among conference abstracts and abstracts in English from articles not published in English, none presented adequate reporting to be included in the present systematic review and meta-analysis (as part of the grey literature). The literature search is depicted in Fig. 1. Excluded studies with corresponding reasons are presented in the online resource. Table 1 summarizes the characteristics of the retrieved articles, while Table 2 summarizes the quality evaluation based on the NOS. For the comparison of TGA vs. HC, among the included papers, five recorded a moderate quality [4, 31, 33, 35, 36], whereas the rest registered a good methodological quality [14, 18, 30, 32, 34, 37]. For the comparison of TGA vs. TIA, all of the retrieved papers (n = 4) were appraised as of moderate quality [4, 17, 18, 34].
TGA vs. HC
Yi et al. performed the only cross-sectional study using data from the Nationwide Inpatient Sample (NIS), which represents 20% of the U.S. community hospitals [30]. A fraction of the participants in the control group (inpatient database) corresponded to patients with other CNS diseases. Moreover, the data collection process was based on record linkage (it was not reported if diagnoses established by non-specialists, that is non-neurologists, were considered acceptable, claims data are generally susceptible to coding deviations) and each patient discharge was considered as the unit of analysis (it was assumed that each discharge appeared once per patient, probable overlap was not addressed). Furthermore, the demographic characteristics of the two groups presented significant differences both in terms of sex and age (Table 1, the majority of the controls belonged to a younger age group in which TGA is extremely rare, future appearance of the disease cannot be ruled out). Despite the aforementioned very serious limitations, in view of the unprecedented numerical power of the study we decided to present obtained results. It was determined that individuals with TGA headache presented with six-fold greater odds [OR = 5.98, 95% CI = (5.42, 6.60)] of migraine headache compared with non-TGA patients (socio-demographic factors along with comorbidities were adjusted in the context of the statistical analysis). Nevertheless, the prevalence of migraine in both groups (0.76% in the non-TGA group and 4.94% in the TGA group) is much lower than the established prevalence in the general population [38] indicating a substantial underestimation in both groups (probably due to the focus on inpatient evaluations). Therefore, although the overall study quality was good (Table 2), the innate inpatient orientation of the NIS database substantially limited its value in the investigation of the association between migraine and TGA.
Lin et al. conducted the only cohort study (of high quality) using data from Taiwan’s National Health Insurance program which covers approximately 98% of Taiwan’s residents [32]. The data collection process was based on record linkage. Migraine diagnosis was ascertained if coded by a neurologist during the study period (treatment-seeking individuals were included in the migraine group, parallel misclassification of non-treatment seeking individuals with migraine in the control group was possible). Antecedent TGA, cerebrovascular disease or epilepsy diagnoses led to the exclusion of candidates from both groups. Two different approaches were implemented for the diagnosis of TGA. A primary approach included all TGA cases coded by neurologists, as well as non-specialists, and a sensitivity approach involved only cases diagnosed by neurologists and having undergone neuroimaging within 1 month from coding (coding deviations were less probable with the latter approach). Despite the relatively young mean age of the participants and the short follow-up period, the migraine cohort presented a significantly higher TGA risk than the (age, sex, vascular comorbidity and Charlson score matched) HC cohort [primary analysis: RR = 2.48, 95% CI = (1.32, 4.87), sensitivity analysis: RR = 3.84, 95% CI = (1.57, 9.38)]. Notably the above-mentioned effect was driven by the subgroup of 40–60-year-old female individuals. Similarly, adjusted cumulative rates of TGA were determined higher in the migraine cohort (relevant figures were not provided), with no apparent difference in the TGA incidence between aura and non-aura individuals.
The majority of the retrieved articles followed a case–control design. For the comparison of TGA cases with HC, the results from eight articles were pooled (700 TGA patients and 746 HC). Retrospective, prospective and mixed data collection strategies were implemented (Table 1). Claims data were not used in any of the studies; therefore, an accurate diagnosis is more probable even in the context of retrospective studies (direct evaluation of medical records). Only the paper of Akkawi et al. (high-quality) did not match cases with controls for sex (age-matching was performed, the rest of the studies reported both age and sex matching) during the study design (statistical analysis was adjusted) [33]. Pooled results were indicative of a significantly higher migraine history in the TGA group [OR = 2.51, 95% CI = (1.85, 3.41), PQ = 0.33, I2 = 13%] (Fig. 2). A stronger association between migraine and TGA was determined by the high-quality case control studies [OR = 3.48, 95% CI = (2.13, 5.71), PQ = 0.32, I2 = 11%] [14, 18, 34] in comparison with the moderate-quality articles [OR = 2.06, 95%CI = (1.40, 3.03), PQ = 0.54, I2 = 0%] [4, 31, 33, 35, 36]. The study of Melo et al. (high quality) provided results indicative of a more common history of migraine in TGA patients compared to HC [OR = 8.67, 95% CI = (2.66, 44.6)], but absolute values were not provided for both groups, impeding its inclusion in the meta-analysis. Finally, the association of migraine according to the presence of aura or not was examined only in the classic case–control study of Hodges et al. [4], with obtained results suggestive of an increased prevalence of a migraine history among TGA individuals (Fig. 3).
TGA vs. TIA
Among the retrieved papers, only four case–control studies of moderate quality evaluated the prevalence of a positive migraine history between TGA and TIA individuals [4, 17, 33, 36]. The article of Melo et al. [37] contained a TIA group, but relevant figures (Effect size with 95% CI) were provided only for the TGA and HC groups. Vascular comorbidities were generally more common (significantly in the case of two studies [4, 17]) in the TIA groups. Despite the long-established relationship between migraine (especially with aura) and vascular comorbidities (TIA included) [25, 39, 40], pooled results were compatible with a higher prevalence of migraine in TGA individuals compared to TIA patients [PQ = 0.71, I2 = 0%, OR = 1.82, 95% CI = (1.22, 2.73)] (Fig. 4). The studies of Zorzon et al. and Hodges et al. recruited matched cases and controls for the parameters of age and sex [4, 36] (the other two studies addressed demographical differences during statistical analyses). Finally, the association of migraine according to the presence of aura or not was examined only in two case–control studies [4, 17], with obtained evidence suggestive of no difference in the prevalence of migraine history between the TGA and TIA groups (Fig. 3).
Discussion
The purpose of the present systematic review and meta-analysis was to evaluate the clinical association between migraine and TGA. Results from case–control (ten), cohort (one) and cross-sectional (one) studies were in accordance with an existing relationship between the two entities. The previous findings of the only other published meta-analysis (2006), involving only five of the twelve retrieved articles failed to reach similar conclusions [24]. In the present meta-analysis, migraine was determined more common among TGA patients in comparison with HC (2 to 3.5-fold, according to both case–control and cohort studies with higher figures generated in the context of higher-quality studies), as well as TIA individuals (less than two-fold, based on case–control studies), a group of patients that have already been associated with a personal history of migraine (therefore, an attenuated association was anticipated) [25]. High-quality evidence originated principally from case–control and cohort studies, whereas the single cross-sectional study was appraised as of limited value due to its inpatient orientation. Unfortunately, the association between TGA and the presence of aura was poorly studied; therefore, a safe conclusion cannot be reached.
Migraine has been, additionally, investigated as a potential risk factor contributing to the recurrence of TGA. Four recent articles focused on the examination of the parameters conferring a risk for recurrence. The study of Morris et al., a retrospective cohort based on the medical records of the Mayo Clinic (Rochester, Minnesota), reviewed a total of 1044 cases among which 143 suffered from recurrent TGA [11]. Recurrent episodes were significantly associated with both a personal and a family history of migraine headache. Similarly, the retrospective cohort of Alessandro et al. (203 TGA individuals, 16 with recurrent episodes) obtained results suggestive of a significantly increased tendency for recurrence in migraine patients compared to individuals free of migraine [41], whereas the prospective cohort of Tynas et al. (93 cases, 15 with recurrent disease), as well as the retrospective cohort of Oliveira et al. (70 patients with TGA, 19 with recurrent disease) did not provide evidence indicative of an association between migraine and recurrence (although absolute numbers for migraine were higher in the recurrent TGA groups) [42, 43]. Despite the non-significant results of the latter studies it is apparent that a history of migraine may assume a role in the recurrence of the disease, which is of probably affinity to our results suggesting an association between migraine and TGA in general (unique as well as recurrent).
The retrospective cohort of Lin et al. provided complementary evidence for the relationship between migraine and TGA, by examining the effect of a positive migraine history on the age of TGA onset [31]. Among individuals developing TGA after the age of 40, those with a migraine history presented a significantly younger age of onset (mean age 56.6 years) against those without a history of the disease (mean age 61.4 years). In addition to that, the association between migraine and TGA was mainly driven by the group of 40–60-year-old female individuals. Morris et al. supported these results with similar findings (mean age of onset for migraine patients 61.1 years vs. 65.4 years for non-migraine controls) [11]. Finally, a previous hierarchical clustering analysis of TGA cases classified the characteristics of a younger age of TGA onset (< 56 years) and a history of migraine together, proposing that migraine may represent a risk factor for TGA in younger individuals [24]. Taking all the aforementioned evidence into consideration, it is probable that migraine is not only associated with an overall elevated risk of TGA (and probably of the recurrent form of the disease as well), but also with an earlier age of TGA onset. Given this background, the latent effect of migraine might be accountable for the association between a younger age of onset with recurrent episodes of TGA (mean age of onset for recurrent TGA 58.8 years vs. mean age for unique TGA 65.2 years [11]), a theory which is based on the relationship of migraine with both of the aforementioned parameters. Overall, it could be argued that in a hypothetical continuum of clinical severity, a positive history of migraine appears to be associated with a more severe form of TGA (younger onset, recurrent disease).
As mentioned earlier, migraine-related theories regarding the underlying pathophysiological mechanisms of TGA are among the most prevalent ones. CSD extending through the hippocampus has been proposed as the missing link that unifies the two entities [44]. CSD has been associated with the presence of aura [45, 46] (despite a relatively few exceptions for migraine without aura [47, 48]). Therefore, the limited evidence with respect to the association of TGA with the presence of aura comes in contradiction with this theory. Intriguingly, our findings demonstrated that migraine is even more common among TGA individuals compared to TIA patients. TIAs [25], as well as vascular events in general, have been associated with a history of migraine, an association which is stronger in the presence of aura [39, 40]. Additionally, individuals with aura demonstrate a more prominent prothrombotic predisposition compared to those without aura [49, 50]. Given the above-mentioned knowledge, as well as the benign vascular sequelae after a TGA episode, the association of TGA with migraine might lie in aura-irrelevant parameters. In addition to the above, hippocampal CSD is triggered at a higher threshold compared to other cortical areas suggesting that concomitant aura manifestations ought to be present during a TGA episode [7]. However, although migraine episodes have been recorded as possible triggers of TGA [51], TGA episodes are not consistently accompanied by aura or headache manifestations, while a positive history of migraine appears to contribute a risk towards TGA regardless of the status of the disease: active or inactive migraine [7].
At this point, it is prudent to point-out the complex neurohormonal-metabolic background of migraine [52,53,54] and subsequently the multiple possible pathophysiological pathways that may be implicated and shared in both entities. Inferentially, migraine and TGA may be linked by multiple underlying mechanisms other than CSD. Of note, there is accumulating evidence indicative of an association between migraine and cerebral energy, as well as oxidative mismatch [52]. Interventions that ameliorate the mitochondrial function and present anti-oxidative properties appear to exert a prophylactic effect against migraine [49, 54]. Considering the selective sensitivity of the CA-1 region of the hippocampus to oxidative and metabolic stress, migraine could create a local energy and oxidative disequilibrium that facilitates the emergence of TGA [55,56,57]. The alternative scenario that the relationship between migraine and TGA is no more than an indirect association between the two entities may, also, be the case. For example, a relatively more recent TGA theory suggests that psychological disorders could lead to the induction of brain metabolism disturbances, which in turn may lead to transient amnesia [58]. The psychological burden of migraine individuals is well recognized [59]. Herein the shared affinity of both diseases with psychological disorders, may provide the missing link between the two entities. Finally, a positive family history of migraine has been associated with recurrences, a finding that might imply the existence of a genetic predisposition towards TGA [11].
Intriguingly, TGA presents many similarities with a migrainous syndrome defined as late onset migraine accompaniments (LOMAs). Epidemiological evidence suggests that TGA does not affect individuals younger than 50 years [60]. On the other hand, migraine attacks tend to decrease in frequency and severity among patients older than 50 years; headaches are generally milder with less important functional consequences [61]. However, typical aura without headache and even without a prior history of migraine can occur at any age and are relatively more common among patients over the age of 50 (mainly with visual manifestations that last for several minutes) [62, 63]. These symptoms, termed as LOMAs, mimic the presentation of transient ischemic phenomena and seizures, but their prognosis is considered benign [61]. Consequently, LOMAs and TGA present several similarities, regarding their demographics, as well as their transient-reversible nature and benign prognosis. Nonetheless, the stereotypic recurrences, along with the focal neurological (or retinal) deficits accompanying the former offer a clear distinction between the two entities.
The present study has several limitations. First of all, the presence of aura was poorly studied by the retrieved articles and, therefore, concluding evidence for an existing association was not obtained. Secondly, TGA was diagnosed according to the long-established clinical criteria of Caplan and Hodges and Warlow. Published evidence suggests that a clinical diagnosis may not always be accurate (ischemic amnesia could mimic this condition [64, 65]) and, occasionally, might be compromised even after neuroimaging investigations [66]. Therefore, the presence of ascertainment bias cannot be ruled out, especially in the context of retrospective data collection that additionally includes a risk for information bias (TGA diagnosis based on medical records). On the grounds of the recognized increased prevalence of migraine in stroke patients, the misclassification of individuals with stroke in the TGA group may be accountable for the higher frequency of migraine compared to HC [39, 40]. However, sensitivity analysis based on stricter diagnostic criteria (involving neuroimaging), performed in the retrospective cohort of Lin et al., strengthened the estimated association between TGA and migraine [32]. Finally, the majority of the retrieved case–control studies were performed in tertiary neurological department settings (except for Arena et al.) and, therefore, are prone to referral and Berkson’s (hospitalization) biases, with cases presenting more important comorbidities being involved in these studies. Nevertheless, the population-based retrospective cohort of Lin et al. determined a similar size of association with the pooled results of the retrieved case–control studies [32].
In conclusion, existing evidence is suggestive of a potential association between TGA and migraine. Additional high-quality studies are warranted for the acquisition of more robust conclusions with respect to the relative importance of aura in this association. To delve into the relationship between TGA and migraine, it would be of value if future research applied clustering approaches that will give prominence to the parameters closely related with migraine in TGA individuals. In this way the latent underlying component of migraine headache and TGA may be, ultimately, revealed. Future studies should always conform to the reporting guidelines to ensure the capitalization of the obtained results [67, 68].
Data availability
Data sharing is not applicable–no new data generated.
References
Bartsch T, Butler C (2013) Transient amnesic syndromes. Nat Rev Neurol 9(2):86–97. https://doi.org/10.1038/nrneurol.2012.264
Kirshner HS (2011) Transient global amnesia: a brief review and update. Curr Neurol Neurosci Rep 11(6):578–582. https://doi.org/10.1007/s11910-011-0224-9
Hodges JR, Warlow CP (1990) Syndromes of transient amnesia: towards a classification: a study of 153 cases. J Neurol Neurosurg Psychiatry 53(10):834–843
Hodges JR, Warlow CP (1990) The aetiology of transient global amnesia. A case-control study of 114 cases with prospective follow-up. Brain 113(Pt 3):639–657. https://doi.org/10.1093/brain/113.3.639
Förster A, Griebe M, Gass A, Kern R, Hennerici MG, Szabo K (2012) Diffusion-weighted imaging for the differential diagnosis of disorders affecting the hippocampus. Cerebrovasc Dis 33(2):104–115. https://doi.org/10.1159/000332036
Bartsch T, Alfke K, Stingele R et al (2006) Selective affection of hippocampal CA-1 neurons in patients with transient global amnesia without long-term sequelae. Brain 129(Pt 11):2874–2884. https://doi.org/10.1093/brain/awl248
Bartsch T, Deuschl G (2010) Transient global amnesia: functional anatomy and clinical implications. Lancet Neurol 9(2):205–214. https://doi.org/10.1016/S1474-4422(09)70344-8
Fisher CM, Adams RD (1958) Transient global amnesia. Trans Am Neurol Assoc 83(143–6):5
Fisher CM, Adams RD (1964) Transient global amnesia. Acta Neurol Scand 40(Suppl 9):1–83
Jaffe R, Bender MB (1996) E.E.G. studies in the syndrome of isolated episodes of confusion with amnesia “transient global amnesia.” J Neurol Neurosurg Psychiatr. 29:472–474
Morris KA, Rabinstein AA, Young NP (2020) Factors associated with risk of recurrent transient global amnesia. JAMA Neurol. 77(12):1551. https://doi.org/10.1001/jamaneurol.2020.2943 ([published online ahead of print, 2020 Aug 31])
Quinette P, Constans JM, Hainselin M, Desgranges B, Eustache F, Viader F (2015) Hippocampal modifications in transient global amnesia. Rev Neurol (Paris) 171(3):282–288. https://doi.org/10.1016/j.neurol.2015.01.003
Mathew NT, Meyer JS (1974) Pathogenesis and natural history of transient global amnesia. Stroke 5:303–311
Arena JE, Brown RD, Mandrekar J, Rabinstein AA (2017) Long-term outcome in patients with transient global amnesia: a population-based study. Mayo Clin Proc 92(3):399–405. https://doi.org/10.1016/j.mayocp.2016.11.015
Romero JR, Mercado M, Beiser AS et al (2013) Transient global amnesia and neurological events: the framingham heart study. Front Neurol. 4:47. https://doi.org/10.3389/fneur.2013.00047 (Published 2013 May 14)
Mangla A, Navi BB, Layton K, Kamel H (2014) Transient global amnesia and the risk of ischemic stroke. Stroke 45(2):389–393. https://doi.org/10.1161/STROKEAHA.113.003916
Pantoni L, Bertini E, Lamassa M, Pracucci G, Inzitari D (2005) Clinical features, risk factors, and prognosis in transient global amnesia: a follow-up study. Eur J Neurol 12(5):350–356. https://doi.org/10.1111/j.1468-1331.2004.00982.x
Zorzon M, Antonutti L, Masè G, Biasutti E, Vitrani B, Cazzato G (1995) Transient global amnesia and transient ischemic attack. Natural history, vascular risk factors, and associated conditions. Stroke 26(9):1536–1542. https://doi.org/10.1161/01.str.26.9.1536
Cejas C, Cisneros LF, Lagos R et al (2010) Internal jugular vein valve incompetence is highly prevalent in transient global amnesia. Stroke 41:67–71
Altamura C, Vernieri F (2010) Internal jugular vein valve incompetence in transient global amnesia. More circumstantial evidence or the proof solving the mystery? Stroke 41:1–2
Modabbernia A, Taslimi S, Ashrafi M, Modabbernia MJ, Hu HH (2012) Internal jugular vein reflux in patients with transient global amnesia: a meta-analysis of case-control studies. Acta Neurol Belg 112(3):237–244. https://doi.org/10.1007/s13760-012-0072-7
Agosti C, Borroni B, Akkawi NM, Padovani A (2010) Cerebrovascular risk factors and triggers in transient global amnesia patients with and without jugular valve incompetence: results from a sample of 243 patients. Eur Neurol 63(5):291–294. https://doi.org/10.1159/000292502
Olesen J, Jørgensen MB (1986) Leao’s spreading depression in the hippocampus explains transient global amnesia. A hypothesis Acta Neurol Scand 73(2):219–220
Quinette P, Guillery-Girard B, Dayan J et al (2006) What does transient global amnesia really mean? Review of the literature and thorough study of 142 cases. Brain 129(Pt 7):1640–1658. https://doi.org/10.1093/brain/awl105
Magalhães JE, Sampaio Rocha-Filho PA (2018) Migraine and cerebrovascular diseases: epidemiology, pathophysiological, and clinical considerations. Headache 58(8):1277–1286. https://doi.org/10.1111/head.13378
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 283(15):2008–2012. https://doi.org/10.1001/jama.283.15.2008 (PMID: 10789670)
Caplan L (1985) Transient global amnesia. In: Vinken PJ, Gruyn GW, Klawans HL (eds) Handbook of Clinical Neurology, Vol 1(45). Elsevier, The Netherlands, pp 205–218
Liampas I, Siokas V, Brotis A, Aloizou AM, Mentis AA, Vikelis M, Dardiotis E (2020) Meta-analysis of melatonin levels in cluster headache-review of clinical implications. Acta Neurol Scand. 142(4):356–367. https://doi.org/10.1111/ane.13317 (Epub ahead of print. PMID: 32677039)
Review Manager (RevMan) [Computer program]. Version 5.4. Copenhagen: The Nordic cochrane centre, The cochrane collaboration, build date 26/05/2020
Yi M, Sherzai AZ, Ani C et al (2019) Strong association between migraine and transient global amnesia: a National inpatient sample analysis. J Neuropsychiatry Clin Neurosci 31(1):43–48. https://doi.org/10.1176/appi.neuropsych.17120353
Jovanovic ZB, Pavlovic AM, Vujisic Tesic BP et al (2018) Comprehensive ultrasound assessment of the craniocervical circulation in transient global amnesia. J Ultrasound Med 37(2):479–486. https://doi.org/10.1002/jum.14355
Lin KH, Chen YT, Fuh JL et al (2014) Migraine is associated with a higher risk of transient global amnesia: a nationwide cohort study. Eur J Neurol 21(5):718–724
Baracchini C, Farina F, Ballotta E, Meneghetti G, Manara R (2015) No signs of intracranial arterial vasoconstriction in transient global amnesia. J Neuroimaging 25(1):92–96. https://doi.org/10.1111/jon.12090
Maalikjy Akkawi N, Agosti C, Anzola GP et al (2003) Transient global amnesia: a clinical and sonographic study. Eur Neurol 49(2):67–71. https://doi.org/10.1159/000068501
Sander D, Winbeck K, Etgen T, Knapp R, Klingelhöfer J, Conrad B (2000) Disturbance of venous flow patterns in patients with transient global amnesia. Lancet 356(9246):1982–1984. https://doi.org/10.1016/S0140-6736(00)03313-4
Schmidtke K, Ehmsen L (1998) Transient global amnesia and migraine. A case control study. Eur Neurol 40(1):9–14. https://doi.org/10.1159/000007948
Melo TP, Ferro JM, Ferro H (1992) Transient global amnesia. A case control study. Brain 115(Pt 1):261–270. https://doi.org/10.1093/brain/115.1.261
GBD 2016 Headache Collaborators (2018) Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 17(11):954–976. https://doi.org/10.1016/S1474-4422(18)30322-3
Mahmoud AN, Mentias A, Elgendy AY et al (2018) Migraine and the risk of cardiovascular and cerebrovascular events: a meta-analysis of 16 cohort studies including 1 152 407 subjects. BMJ Open 8(3):e020498. https://doi.org/10.1136/bmjopen-2017-020498 (Published 2018 Mar 27)
Sacco S, Ornello R, Ripa P et al (2015) Migraine and risk of ischaemic heart disease: a systematic review and meta-analysis of observational studies. Eur J Neurol 22(6):1001–1011. https://doi.org/10.1111/ene.12701
Alessandro L, Calandri IL, Suarez MF et al (2019) Transient global amnesia: clinical features and prognostic factors suggesting recurrence. Arq Neuropsiquiatr 77(1):3–9. https://doi.org/10.1590/0004-282X20180157
Oliveira R, Teodoro T, Marques IB (2020). Risk factors predicting recurrence of transient global amnesia. Neurol Sci. doi: https://doi.org/10.1007/s10072-020-04788-6. Epub ahead of print. PMID: 33033897.
Tynas R, Panegyres PK (2020) Factors determining recurrence in transient global amnesia. BMC Neurol 20(1):83. https://doi.org/10.1186/s12883-020-01658-8 (Published 2020 Mar 6)
Owen D, Paranandi B, Sivakumar R, Seevaratnam M (2007) Classical diseases revisited: transient global amnesia. Postgrad Med J 83(978):236–239
Goadsby PJ (2007) Cortical spreading depression—better understanding and more questions. Focus on “distinct vascular conduction with cortical spreading depression.” J Neurophysiol 97:3827
Charles AC, Baca SM (2013) Cortical spreading depression and migraine. Nat Rev Neurol 9:637–644
Woods RP, Iacoboni M, Mazziotta JC (1994) Brief report: Bilateral spreading cerebral hypoperfusion during spontaneous migraine headache. N Engl J Med 331:1689–1692
Gelmers HJ (1982) Common migraine attacks preceded by focal hyperemia and parietal oligemia in the rCBF pattern. Cephalalgia 2:29–32
Liampas IN, Siokas V, Aloizou AM et al (2020) Pyridoxine, folate and cobalamin for migraine: A systematic review. Acta Neurol Scand 142(2):108–120. https://doi.org/10.1111/ane.13251
Liampas I, Siokas V, Mentis AA et al (2020) Serum homocysteine, pyridoxine, folate, and vitamin B12 levels in migraine: systematic review and meta-analysis. Headache 60(8):1508–1534. https://doi.org/10.1111/head.13892 ([published online ahead of print, 2020 Jul 2])
Donnet A (2015) Transient global amnesia triggered by migraine in a French Tertiary-care center: an 11-year retrospective analysis. Headache 55(6):853–859. https://doi.org/10.1111/head.12545
Gross EC, Lisicki M, Fischer D, Sándor PS, Schoenen J (2019) The metabolic face of migraine - from pathophysiology to treatment. Nat Rev Neurol 15(11):627–643. https://doi.org/10.1038/s41582-019-0255-4
Liampas I, Siokas V, Brotis A, Dardiotis E. Vitamin D serum levels in patients with migraine: A meta-analysis [published online ahead of print, 2020 Mar 30]. Rev Neurol (Paris). 2020;S0035–3787(20)30464–1. doi:https://doi.org/10.1016/j.neurol.2019.12.008
Liampas I, Siokas V, Brotis A, Vikelis M, Dardiotis E (2020) Endogenous melatonin levels and therapeutic use of exogenous melatonin in migraine: systematic review and meta-analysis. Headache 60(7):1273–1299. https://doi.org/10.1111/head.13828 ([published online ahead of print, 2020 Apr 30])
Wang X, Michaelis EK (2010) Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci 30(2):12. https://doi.org/10.3389/fnagi.2010.00012 (PMID: 20552050; PMCID: PMC2874397)
Wang X, Pal R, Chen XW et al (2005) High intrinsic oxidative stress may underlie selective vulnerability of the hippocampal CA1 region. Brain Res Mol Brain Res 140:120–126. https://doi.org/10.1016/j.molbrainres.2005.07.018
Bartsch T, Döhring J, Reuter S, Finke C, Rohr A, Brauer H, Deuschl G, Jansen O (2015) Selective neuronal vulnerability of human hippocampal CA1 neurons: lesion evolution, temporal course, and pattern of hippocampal damage in diffusion-weighted MR imaging. J Cereb Blood Flow Metab 35(11):1836–1845. https://doi.org/10.1038/jcbfm.2015.137
Noël A, Quinette P, Guillery-Girard B et al (2008) Psychopathological factors, memory disorders and transient global amnesia. Br J Psychiatry 193:145–151
Peres MFP, Mercante JPP, Tobo PR, Kamei H, Bigal ME (2017) Anxiety and depression symptoms and migraine: a symptom-based approach research. J Headache Pain 18:37
Spiegel DR, Smith J, Wade RR, Cherukuru N, Ursani A, Dobruskina Y, Crist T, Busch RF, Dhanani RM, Dreyer N (2017) Transient global amnesia: current perspectives. Neuropsychiatr Dis Treat 24(13):2691–2703. https://doi.org/10.2147/NDT.S130710.PMID:29123402;PMCID:PMC5661450
Vongvaivanich K, Lertakyamanee P, Silberstein SD, Dodick DW (2015) Late-life migraine accompaniments: a narrative review. Cephalalgia 35(10):894–911. https://doi.org/10.1177/0333102414560635 (Epub 2014 Dec 12 PMID: 25505036)
Wijman CA, Wolf PA, Kase CS, Kelly-Hayes M, Beiser AS (1998) Migrainous visual accompaniments are not rare in late life: the Framingham study. Stroke 29(8):1539–1543. https://doi.org/10.1161/01.str.29.8.1539 (PMID: 9707189)
Donnet A, Daniel C, Milandre L, Berbis J, Auquier P (2012) Migraine with aura in patients over 50 years of age: the Marseille’s registry. J Neurol 259(9):1868–1873. https://doi.org/10.1007/s00415-012-6423-8 (PMID: 22302276)
Gupta M, Kantor MA, Tung CE, Zhang N, Albers GW (2015) Transient global amnesia associated with a unilateral infarction of the fornix: case report and review of the literature. Front Neurol. 5:291. https://doi.org/10.3389/fneur.2014.00291 (Published 2015 Jan 12)
Naldi F, Baiardi S, Guarino M, Spinardi L, Cirignotta F, Stracciari A (2017) Posterior hippocampal stroke presenting with transient global amnesia. Neurocase 23(1):22–25. https://doi.org/10.1080/13554794.2016.1270329
Förster A, Al-Zghloul M, Wenz H, Böhme J, Groden C, Neumaier-Probst E (2017) Isolated punctuate hippocampal infarction and transient global amnesia are indistinguishable by means of MRI. Int J Stroke 12(3):292–296. https://doi.org/10.1177/1747493016676613
Liampas I, Chlinos A, Siokas V, Brotis A, Dardiotis E (2019) Assessment of the reporting quality of RCTs for novel oral anticoagulants in venous thromboembolic disease based on the CONSORT statement. J Thromb Thrombolysis 48(4):542–553. https://doi.org/10.1007/s11239-019-01931-9
Kodounis M, Liampas IN, Constantinidis TS et al (2020) Assessment of the reporting quality of double-blind RCTs for ischemic stroke based on the CONSORT statement. J Neurol Sci 415:116938. https://doi.org/10.1016/j.jns.2020.116938
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval
The manuscript does not contain clinical studies or patient data.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liampas, I., Siouras, A.S., Siokas, V. et al. Migraine in transient global amnesia: a meta-analysis of observational studies. J Neurol 269, 184–196 (2022). https://doi.org/10.1007/s00415-020-10363-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-10363-y