Abstract
At present, the standard practices for home-based assessments of abnormal movements in Parkinson’s disease (PD) are based either on subjective tools or on objective measures that often fail to capture day-to-day fluctuations and long-term information in real-life conditions in a way that patient’s compliance and privacy are secured. The employment of wearable technologies in PD represents a great paradigm shift in healthcare remote diagnostics and therapeutics monitoring. However, their applicability in everyday clinical practice seems to be still limited. We carried out a systematic search across the Medline Database. In total, 246 publications, published until 1 June 2020, were identified. Among them, 26 reports met the inclusion criteria and were included in the present review. We focused more on clinically relevant aspects of wearables’ application including feasibility and efficacy of the assessment, the number, type and body position of the wearable devices, type of PD motor symptom, environment and duration of assessments and validation methodology. The aim of this review is to provide a systematic overview of the current knowledge and state-of-the-art of the home-based assessment of motor symptoms and fluctuations in PD patients using wearable technology, highlighting current problems and laying foundations for future works.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is the most common form of movement disorder, characterized by diverse day- and night-time motor and non-motor signs [1]. The cardinal daytime motor features are bradykinesia, rigidity, tremor, postural instability, and, in the case of advanced PD, levodopa-induced motor fluctuations and dyskinesias as well [2,3,4]. Levodopa-induced dyskinesias consist of abnormal involuntary movements appearing mostly as a consequence of the long-term levodopa treatment [5]. Night-time PD motor features include nocturnal hypokinesia or akinesia [6,7,8], restless legs syndrome (RLS), periodic limb movement in sleep (PLMS) and parasomnias (mainly the rapid eye movements sleep behaviour disorder, RBD) [9].
Due to diverse types and often fluctuating patterns of these movement abnormalities in PD and the difficulty in detecting their true nature in a laboratory/in-clinic setting [10, 11], several methods for the home-based assessments of abnormal movements in PD have been developed [12]. Symptom diaries edited by patients are typically used for the long-term assessments of daytime PD motor symptoms at home [13,14,15]. Other common tools are questionnaires, clinical rating scales (i.e. the MDS-UPDRS [16, 17]), clinicians’ phone calls and home visits, video-guided software technologies or telemedicine consultations. However, these tools are either based on subjective patient’s/caregiver’s perception or represent a snapshot of the symptoms’ character, often unable to provide the clinician with reliable overview of the symptoms’ characteristics under real-life conditions, in the long-term course, including the day-to-day fluctuations [13, 14, 18]. In addition, patients and caregivers are often unable to reliably communicate details on the type, frequency and intensity of sleep-related PD abnormal movements, despite their importance on the disease and quality-of-life outcome [14].
In recent years, technology is a unique driving force behind advances in healthcare and we now witness the advent of entirely new categories of interface mechanisms, including wearable devices. In the field of PD, there is an increasing number of studies using wearable technology for motor assessments in PD patients, aiming to overcome the above-mentioned limitations. Although results are promising, the use of wearable in everyday clinical practice has been quite limited. While we can debate the details of this trend in healthcare, it is essential to recognize, understand, and effectively leverage the growing landscape of wearables to increase their applicability in everyday clinical practice and ensure the best outcomes for PD patients, their caregivers, clinicians and society as a whole.
The aim of the current review is to provide a systematic overview of the current literature and state-of-the-art knowledge on the use of wearable technology to assess at home parkinsonian motor symptoms.
Methods
Extraction of the articles was performed by two authors (S.A., P.B.) and was evaluated by the rest of the authors.
Selection criteria
A systematic review of (1) articles in English, (2) published between 1 January 2008 and 1 June 2020 using the Medline database was performed. (3) We used the search terms listed in Supplemental Table 1, to identify articles addressing the application of wearable devices for the in-home monitoring of motor symptoms in PD patients. (4) The articles should contain an abstract. (5) In the study, we included only original articles. Case reports, reviews and editorials were excluded.
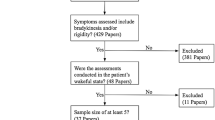
The initial search identified 237 records. We included additionally 9 relevant studies found either on the reference list of the retrieved articles, or on the ScienceDirect webpage as articles of possible interest or even in a parallel search in PubMed and Web Of Science using the terms: “Parkinson” in combination with “Sensors”. Twenty-six articles met all above-mentioned selection criteria and were included in the systematic review (Fig. 1).
Results
Among 26 studies, 24 studies assessed daytime PD symptoms and two studies assessed PD patients exclusively during the night. The results of the two latter studies are reported separately.
The structured reporting of the results includes the following sections: (1) feasibility and efficacy of a home-based assessment using wearables, (2) wearables: type, number and body location, (3) type of PD motor symptom, (4) assessment environment, (5) assessment activities, (6) assessment duration, (7) validations methods, (8) wearables during sleep. Since the use of wearables in PD has been previously reviewed highlighting the technology behind wearables [19], we preferred to focus on more clinically relevant aspects of the application of wearables.
Feasibility and efficacy
The vast majority of the included studies reported on technical efficacy (precision and reliability) of the used wearables and half of them [10, 13, 20,21,22,23,24,25,26,27,28,29,30,31] included feasibility and usability issues (wearable’s tolerability and participants’ compliance outside experimental conditions) as primary or secondary objectives.
Only six studies reported explicitly on practical feasibility [12, 14, 32,33,34,35]. In the study by De Lima et al. [33], primary objectives were recruitment success, compliance, attrition and system usability. The patient’s compliance was highly dependent on the degree of activity required; the more active the patient was requested to be, the less compliance was achieved, resulting in higher attrition. In the study by Fisher et al. [32], authors used a body-worn sensor system to assess movements in an unsupervised, undisturbed home environment. The patients were advised to remove the sensor if they became unduly burdensome. Although patient’s compliance was high, an accurate, real-time evaluation of parkinsonian symptoms was not achieved. The study by Bayés et al. [14] focused on practicality of the assessment method. It applied the Usability Scale (SUS) and the QUEST-questionnaire and participants’ satisfaction on the REMPARK system. They reported on their system as being user-friendly and satisfying study participants. Similarly, the system (brand not mentioned) used by Pastorino et al. [35] was reported by participants as comfortable and 65% could consider using it in real-life conditions as well. The study by Boroojerdi et al. [34] questioned participants about practicality of patch sensors (use difficulty, patch usage training, removal pain, interference with daily activities, interference with sleep, embarrassment from patch in public) and found their system being generally well tolerated.
Finally, four studies focused almost exclusively on technical efficacy of wearables without reporting on feasibility issues [36,37,38,39].
Wearables
A wearable device can integrate multiple sensors and can be worn on various body parts, such as hips, wrist or waist, feet and ankles, or can be integrated in body equipment or clothing [40]. For the evaluation of human movement, the most common wearable sensor type is a six-axis Inertial Motion Unit (IMU) [40], composed of a three-axis accelerometer and a three-axis gyroscope. It can also include an additional three-axis magnetometer, which would be considered a nine-axis IMU. Magnetometer sensor is used to add information about 3D space orientation. The typical sampling frequency adopted for movement studies is 100 Hz.
Available studies used very different types and number of wearables, placed in different body locations and therefore a general consensus on the best wearable type and body location and the optimal sensor number is lacking.
Type of sensors
Most of the reported wearables include accelerometric and/or gyroscopic measures. Wearable sensors can be sold as a stand-alone body-fixed electronic device or can be integrated into a whole system. Hybrid systems are defined as the blend of technologies that combined wearable and non-wearable devices [40]. The latest wearables have the capacity of being connected to other devices wirelessly, via Wi-Fi or Bluetooth Low Energy (BLE). Many vendors offer proprietary software for data download and analysis. The majority of the wearables system offer the option of saving the data locally, i.e. on a SD card, often data can be downloaded wirelessly in real time for immediate visualization [19].
Most of the studies describe in details the applied sensor system. In 23 studies, the exact type and label of the applied system was clearly mentioned (Table 1), whereas in the study by Das et al. [37], the brand of the sensors was omitted. The latter used a set of tri-axial accelerometers located on the four limbs and on the waist.
Five studies [23,24,25, 28, 29] utilized the six-axial IMU Dynaport Hybrid (McRoberts, The Hague, Netherlands), that can be used either as a stand-alone device, thanks to an SD card, or can be connected via Bluetooth communication with a personal computer [19]. Two studies [12, 27] utilized the Kinesia™ (Cleveland Medical Devices Inc., Cleveland, OH), which is a hybrid system composed of a finger-worn device, made up of 6-axis IMU, and operates using a real-time wireless communication with software application installed in a computer device/tablet. The software application guides the patient through the automated motor assessment. Two studies [38, 39] used the SHIMMER IMUs and the MercuryLive platform. The system consists of wearable 9-axis inertial measurement unit sensors, which can be used as a standalone system or can send real-time information to a web-based remote monitoring platform. The MercuryLive software aims on remote interactions between clinicians at hospital and patients at home. Pastorino et al. [35, 36] employed the PERFORM, which is a hybrid system consisting of a set of tri-axial accelerometers for the extremities, a belt-sensor, composed of an accelerometer and a gyroscope, to record proximal body movements and a data acquisition unit. Data recorded are then sampled and processed by a software, enabling medical professionals the remote monitoring of patients. A recent study [34] used the NIMBLE biosensor patch (MC10, Inc., Lexington, MA, USA), composed of an accelerometer and an EMG enclosed in a flexible patch, that attaches to skin via an adhesive sticker. This hybrid system can transmit information wirelessly to a smartphone or a tablet as well as to a cloud server.
Three studies [10, 14, 21] used the so-called 9 × 2 inertial measurement unit, developed in the frame of the REMPARK European project. This is a waist device composed of an accelerometer, a gyroscope, a magnetometer, a microcontroller, a Bluetooth communication module and a memory storage unit. This hybrid system relies on especially developed applications, enabling, i.e. disease management and diary fulfilment by patients. The collected information is sent to a cloud server, thus enabling the online manage of the disease.
Actigraphy is the monitoring of human rest/activities, usually done with a watch-like package worn on the wrist. The sensor adopted is usually a uniaxial accelerometer, low energy, which can measure motor activity for one or more weeks. After the measurement, data are usually downloaded and analyzed.
Pan et al. [26] used the wrist actigraph from MicroMini-Motionlogger (Ambulatory Monitoring, Inc.) aggregating daytime data into a 1-min epoch whilst Binder et al. [22] used the Actiwatch (Cambridge Neurotechnology Ltd., UK). Both actigraphy systems include a uniaxial accelerometer.
De Lima et al. [33] gathered accelerometric data using the Pebble smartwatch (Intel Pharma Analytics Platform) connected to the patients’ smartphone. In this hybrid system, the motion information is transferred from the watch to the phone via the Fox Wearable Companion App and after an estimated analysis, is transmitted to a cloud environment. The app allows the patients to estimate their motor states or to report the time of medication intake. Lipsmeier et al. [30] employed a Samsung smartphone (Galaxy S3 mini; Samsung, Seoul, South Korea) with a preinstalled Roche PD Mobile Application v1 (Roche, Basel, Switzerland). The Smartphone-incorporated IMU served the detection of body movements during the execution of motor tasks guided by the Roche PD Mobile Application.
One study by Fisher et al. [32] used the Axivity AX-3, a stand-alone small tri-axial accelerometer, which can sample continuously 14 days at 100 Hz, and has a USB port for data access. A study by Griffiths et al. [20] used the wrist-worn Parkinson’s Kinetigraph (PKG; Global Kinetics Corporation), a tri-axial integrated microelectromechanical System (iMEMS) accelerometer. Finally, Ramsperger et al. [13] exploited for the in-lab initial measurements the Mobility Lab system (APDM, Portland, USA), which included inertial measurement units on the lateral part of each ankle and afterwards for the 12-week home-based sub-study, a six-axis IMU (Rehacom®, Hasomed, Magdeburg, Germany).
Number of sensors
Most of the studies used a small number of body-fixed sensors. Seventeen studies [10, 12,13,14, 20,21,22,23,24,25,26,27,28,29,30,31, 33] used only one sensor, one study [32] used two sensors, one study [34] used four sensors, three studies [35,36,37] used five sensors, one study [39] used eight sensors and one study [38] used nine body-worn sensors.
Body-location of the sensors
In the reviewed studies, wearable sensors were placed on the trunk (waist, lower back or sternum), the extremities (bilateral or unilateral, dominant or non-dominant limb), the upper limbs (forearm, wrist, fingers or back-of-hand), or on the lower limbs (ankle or shin). Few studies placed sensors in multiple body locations [34,35,36,37,38]. The body localizations of sensors in the reviewed studies are presented in Table 2.
Eight of the reviewed studies [10, 14, 21, 23,24,25, 28, 29] placed the single wearable device using a belt. In three studies [10, 14, 21], the wearable was attached on the waist near the iliac crest, and in the other five [23,24,25, 28, 29], the wearable device was fixed on the lower back. Two studies [12, 27] applied the KinesiaTM system at the index finger of the more affected hand of the patient. Six studies [20, 22, 26, 31,32,33] fixed the wearable on the wrist, among them five [20, 22, 26, 31, 33] only on one wrist (of the most affected [20, 22, 31] and/or the dominant hand [20, 26, 31]), and one [32] on both wrists through a Velcro-strap. In four other studies [35,36,37,38], the monitoring devices were attached both on the trunk and on the four limbs. In the study by Ramsperger et al. [13], a single sensor was placed at the ankle of the most dyskinetic leg. Patel et al. [39] placed eight body-fixed devices on extremities, more precisely bilaterally at two different levels (proximal and distal). Boroojerdi et al. [34] attached patch sensors to chest and the more affected side of shin, forearm and back-of-hand. In the study by Lipsmeier et al. [30], location changed depending on the performed motor task; during free daily activities, the smartphone had to be worn in a belt pouch or in the trouser pocket, whereas during the performance of standardized motor tasks, it could be asked to be hold it in the palm of the hand.
Only few studies justified their selection regarding the body part to apply the wearables. Weiss et al. [24] chose the lower back because of its proximity to the centre of mass of the body and the comfortability of the location, thus avoiding interference with physical activity. In another study by the same group [23], wearables were placed at the lower back to monitor gait and to estimate fall risk. Pérez-Lopez et al. [10] placed a single body-fixed sensor on the waist and noticed the high sensitivity of the position in detecting mild trunk dyskinesias, in contrast to the low sensitivity of the position in detecting weak dyskinesias on distal limbs. Boroojerdi el al. [34] chose to localize the sensors on the chest and the more affected side of shin, forearm and back-of-hand, because of prior results, indicating these locations as being more accurate in detecting body movements.
Type of PD motor symptom
PD patients suffer from a wide spectrum of motor symptoms and different wearables have been applied for home-based monitoring of motor symptoms in PD. Among the reviewed studies, seven studies assessed bradykinesia [12, 21, 27, 30, 31, 36, 38], seven studies assessed tremor [12, 22, 27, 30, 34, 38], ten studies assessed motor fluctuations/dyskinesias [10, 13, 14, 20, 27, 31, 32, 34, 35, 38], nine studies assessed postural instability and gait disturbance [14, 21, 23,24,25, 28,29,30, 34] and only one study assessed dysarthria [30]. In three studies, authors did not clearly state the type of motor symptom that was assessed by wearables [26, 37, 39]. Usually, the decision on the target symptom defined the type and body placement of the wearables (see Table 1). The Dynaport Hybrid (McRoberts, The Hague, Netherlands) has been typically used for the assessment of axial symptoms and gait in PD patients [23,24,25]. Dijkstra et al. [25] placed the DynaPort Hybrid system at the lower back of PD patients and successfully assessed objective features of gait (walking with 81.7% sensitivity and 76.4% specificity, shuffling with only 6.4% sensitivity) and postures abnormalities (lying with 99.3% sensitivity and 76.4% specificity, sitting with 85.4% sensitivity and 75.7% specificity, standing with 74.4% sensitivity and 80.5% specificity) in patients with mild to moderate PD. In addition, the DynaPort Hybrid system was reported to be able to discriminate PD patients from controls as well as PD fallers from PD non-fallers. PD patients had significantly slower gait amplitude and slope, but higher gait width while undergoing ADLs compared to controls [24]. In addition, PD fallers showed higher gait variability in the vertical and anterior–posterior directions, less consistency in the vertical direction and less smooth gait pattern in the vertical and anterior–posterior directions compared to PD non-fallers [23].
The KinesiaTM system (Cleveland Medical Devices Inc., Cleveland, OH) was typically employed to detect cardinal PD symptoms, mainly tremor as well as bradykinesia and dyskinesia [12, 27]. In the study by Mera et al. [12], the KinesiaTM system showed satisfactory capability in capturing the positive or negative response of several symptoms to parkinsonian medication, highlighting its potential utility as long-term objective monitoring of PD symptoms. Indeed, medication significantly improved rest and postural tremor and average speed and amplitude scores while kinetic tremor and average rhythm scores did not significantly improve.
Among the reviewed studies, two studies [20, 31] used the Parkinson’s Kinetigraph (PKG; Global Kinetics Corporation) device to assess bradykinesia, dyskinesia and motor fluctuations. The system was reported to be reliable in distinguishing between early and troublesome motor fluctuations as well as dyskinetic and non-dyskinetic patterns, in patients with PD. The bradykinesia and dyskinesia scores assessed by PKG were significantly correlated with the UPDRS part III and part IV, respectively [20].
A single 9 × 2 IMU located on the waist was employed in two of the reviewed studies [10, 21] and showed good validity in detecting bradykinesia and bradykinetic patterns in gait (92.52% sensitivity, 89.07% specificity, 91.81% accuracy) [21] and in identifying choreic dyskinesias (93% sensitivity and 95% specificity for any strong dyskinesia and mild trunk dyskinesia) in PD patients [10]. However, single 9 × 2 IMU was not sensitive enough to detect mild dyskinesias appearing on the limbs (39% sensitivity) [10].
Bayés et al. [14] applied the same type of IMU located on the waist (part of the REMPARK system) and reported satisfactory capability (97% sensitivity and 88% specificity) in discriminating medication-associated ON vs OFF states based on gait analysis and the identification of dyskinetic patterns. Similarly, the single Rehacom IMU device (Hasomed, Magdeburg, Germany) located at the most affected side, could discriminate PD patients with and without dyskinesias with a satisfactory 85% sensitivity and 98% specificity [13].
Lipsmeier et al. [30] also used an IMU sensor and demonstrated the reliability and validity of a mobile application (Samsung Galaxy S3 together with the Roche PD Mobile Application v1) in detecting tremor, bradykinesia, postural instability, balance and gait and therefore discriminating PD patients from controls. The test–retest reliability was found to range from 0.64 for finger tapping to 0.98 for postural tremor. Most of the active tests and passive monitoring features significant discriminated between PD patients and control subjects and significant correlated with their corresponding MDS-UPDRS items or subscale scores.
The NIMBLE biosensor patch (MC10, Inc., Lexington, MA, USA) was used to record abnormal movements from PD patients (including tremor, gait and motor fluctuations) and transform this information in meaningful symptom severity scores. Accuracy for each activity ranged from 32% (pronation/supination) to 67% (rest-tremor-amplitude) [34].
Finally, the PERFORM system employed by Pastorino et al. [35, 36] was showed to be able in differentiating between ON vs OFF states with a good correspondence (88%) between data achieved from wearable sensors and data collected on motor symptoms’ diaries [35]. In addition, the system was able (with an accuracy of about 70%) to identify and classify bradykinesia severity compared to UPDRS-based clinical evaluation in PD patients [36].
Assessment environment
Despite some similarities, study designs and methodology varied among the reviewed studies. Most of the studies assessed patients both at in-clinic and at-home environment, using a two-step approach [12, 13, 20, 23,24,25,26, 28, 29, 32, 34, 36, 39]. Data from the in-clinic assessment were used to create and train the detection algorithm under controlled conditions, whereas data from the in-home part served the application and validation of the algorithm under real-life conditions [13, 20, 24, 25, 32, 34, 36, 39]. A typical example of this dual method is the study by Ramsperger et al. [13]. In this study, an algorithm for the detection of leg dyskinesias and the prevention of falling in PD patients was created under controlled conditions, and then validated in real-life conditions at home.
Seven studies [14, 21, 22, 30, 31, 33, 35, 37, 38] assessed participants only at home without previous training of the algorithm in controlled conditions. In the work of Bayés et al. [14], the study design included two steps, excluding an in-clinic assessment and including a day where participants and their caregivers were trained in operating the system and filling the diaries, without any data collection. This was followed by a three-day period of movement recordings and data collection under uncontrolled conditions at home.
Two studies [10, 27] divided PD patients into two groups subjected to different study protocols. In the study by Heldman et al. [27], patients were randomized to either a traditional management in-clinic (controls) or to an in-home sensor-based assessment. Study objective was to assess the impact of using at-home wearables on the advanced therapy referral rate. Authors found a significantly higher referral rate by patients assessed with body sensors and concluded that wearables could help identify patients who could potentially take advantage of an advanced Parkinson’s therapy. In the study by Pérez-Lopez et al. [10], participants were assessed either in-clinic, to train a part of the detection algorithm, or at home, to train another part of the algorithm and to evaluate its validity in real-life conditions.
Assessment activities
The degree of freedom during the execution of activities is crucial and can be classified in three categories: completely free (participants carried sensors and were able to move freely during their activities of daily living (ADL)), restricted (participants were assessed by performing default motor tasks, such as tasks based on the MDS-UPDRS clinical rating scale) and partially free or, respectively, partially restricted (participants had to perform scripted daily activities in a free or guided manner).
Among the included studies, 14 studies [13, 14, 20, 22,23,24, 26, 28, 29, 31,32,33, 36, 37] assessed participants wearing sensors continuously during the day while performing unconstrained everyday activities, three studies [12, 27, 38] monitored patients exclusively while performing guided default motor tasks, and two studies [25, 35] gave participants a partial freedom, requiring the execution of scripted daily activities executed in a free or guided manner. In five studies [21, 30, 34, 35, 39], participants had to perform both constrained and unconstrained activities. In the study by Samà et al. [21], patients had to execute, in a free manner, scripted ADL. However, they could also execute non-scripted unexpected activities, such as the answer to a phone call. In the study by Pastorino et al. [35], patients had to perform initially a pre-scheduled assessment session and then were left free to undergo their normal daily activities. In the study by Patel et al. [39], patients had to perform UPDRS-specific tasks as well as daily living activities; however, it was not clearly mentioned if these activities were constrained or unconstrained. In the study by Boroojerdi et al. [34], participants had to wear body-fixed sensors during a whole day both, while performing free their ADL, as well as while undergoing motor assessments (at least one set of motor tasks in the morning before levodopa intake and at least two sessions of motor tasks after the medication intake).
Assessment duration
The duration of wearables assessment might depend on several factors, such as the study design, the study objectives, the type of parkinsonian symptoms (tremor, rigor, dyskinesias etc.), the amount of obtained data and the battery capacity.
Among the included studies, a wide spectrum of assessment duration was observed. The duration ranged from minutes (i.e. monitoring patients only during the execution of default motor tasks or standardized mobility-related activities [25]) to several days while patients had to perform their normal activities. Among 24 studies, 15 studies [13, 20, 22,23,24, 26,27,28,29, 31, 32, 34, 35, 37, 39] applied the wearables for ≥ 24 consecutive hours, six studies [12, 14, 21, 25, 30, 36] assessed PD patients for < 24 h, whereas two of them [10, 29] did not specify the duration of the assessment. The longest assessment was reported by De Lima et al. [33] were patients of the Netherlands cohort were assessed continuously for 13 consecutive weeks, 7/7 days a week, 24 hours a day.
Validation method
The use of valid comparison methods to properly evaluate and validate home-obtained data is important. Comparison systems presented by the different studies ranged from symptom’s diaries, to clinical rating scales or questionnaires and video-recordings, labelled by movement disorder experts. However, several of the reviewed studies do not describe any comparison methods [12, 13, 20, 24, 28, 29, 39].
In six out of 24 studies [14, 22, 32, 33, 35, 37], patients had to fill out symptom’s diaries, most of them with the aim of comparing and validating sensor-derived measures with diaries’ annotations.
Other studies not including an in-clinic part [14, 37] used symptoms’ diaries for creating algorithms and validating the home-based sensor-gathered data. Bayés et al. [14] employed different strategies to improve the validity of diaries: (1) their comparison with daily variations in the UPDRS part III (assessed once a day by a researcher) and with motor states’ estimations (performed during telephone counseling every 2 hours), and (2) the presence of a motor state for a minimum of two consecutive diary’s annotations for validation.
Seven of the reviewed studies [22, 23, 26, 30, 31, 34, 36, 38] used clinical rating scales or questionnaires as comparison method.
The most common employed clinical rating scale is the MDS-UPDRS, particularly its parts II (activities of daily living) and III (motor signs). Chen et al. [38] assessed UPDRS III via a webcam-based motor assessment as a validation method for movements recorded by the wearable devices.
Weiss et al. [23], assessed the falling risk in PD patients, by employing many different fall-risk traditional assessment means, e.g. fall history during the previous year, fall calendar filled out once a month and the New Freezing of Gait Questionnaire (NFOG-Q).
Four studies [10, 21, 25, 36] used video recordings during home-based assessments, which were then reviewed by two movement disorder specialists. Samà et al. [21] applied video recordings of patients while undergoing constrained daily living activities as gold standard for bradykinesia detection.
Wearables during sleep
Feasibility and efficacy
Data on the feasibility and comfortability of wearables during night-time assessments are very limited. None of the reviewed studies illustrated explicit interest in practical feasibility. The choice of number and body location of the sensors seemed not to depend on patients’ compliance. In fact Bhidayasiri et al. [7] chose to use only one sensor and to localize it at the sternum, due to the proximity of sternum with the centre of body-mass and to minimize artefacts associated with arm movements.
Concerning the type of wearables used, both sleep-studies [6, 7] employed the Night-Recorder system (Table 1). It consisted of body-fixed sensors composed of triaxial iMEMS accelerometers and gyroscope and it was employed for the monitoring of episodes of rolling over and getting out of bed during sleep (as surrogate markers of nocturnal hypokinesia) [7].
The study by Bhidayasiri et al. used only one sensor at the sternum, whereas the study by Sringean et al. [6] used five wearables attached both on the trunk and on the four limbs.
Assessment environment
Both sleep-studies [6, 7] assessed PD patients directly at home, applying for one night (< 24 consecutive hours) an unconstrained program of activities, without any changes at the home setting and without having previously trained the algorithm in controlled conditions.
Validation method
In both night-studies [6, 7], comparison methods were employed. Bhidayasiri et al. [7] compared the wearables’ measurements with sleep diaries annotations, filled out by PD patients and reporting getting out of bed episodes as well as sleep time periods. In the study by Sringean et al. [6] clinical scales, video recordings and sleep diaries were applied, where patients had to document sleep periods in sleep diaries and events of getting out of bed.
Discussion
In the last years, an increasing number of studies reported on the use of wearables for the home-based assessment and monitoring of parkinsonian abnormal movements. The studies often address feasibility issues, technical efficiency and reliability of wearables, sensor types (mechanism, number of sensors and body localization), and validation methodology. The aim of this article was to systematically review recent studies that assessed abnormal movements in patients with PD using wearable technology. We primarily focused on presenting clinically relevant aspects of wearables’ application and less on technical issues.
Most of the reviewed studies showed positive results regarding accuracy and reliability of wearables in detecting abnormal movements. However, practicality of sensors’ systems was not always reported and it seems that in many cases, it remains an issue that might limit the applicability of the systems. Indeed, in the few cases where comfort and tolerability and therefore satisfactory compliance from participants were the primary outcome, the sensitivity and sensibility of the system did not always reach satisfactory levels. A compromise between battery life, sampling rate, data size and measurement precision is needed. Privacy and data protection are not always properly addressed.
Different sensor types have been used and due to the different objectives and study groups, a direct comparison between these systems is not possible. Clinimetric properties of each device have to be tested in laboratory as well as in real-life conditions. In the choice of adequate sensors for a study, their reliability, validity and sensitivity to change for specific clinical parameters, as well as their ecological validity when employed outside controlled conditions have to be considered [19, 40].
The type of motor symptom to be assessed is crucial when choosing the appropriate wearable device and various aspects of the motor symptoms that are displayed by subjects with PD have been investigated in the reviewed studies. In most of these studies, algorithms were developed either to detect the presence of a specific motor feature or to differentiate the ON from the OFF condition in a subject with PD. However, very often patients with PD suffer from a wide spectrum of abnormal movements (tremor, bradykinesia, gait abnormalities, medication-induced dyskinesias, sleep-related abnormal movements) which can fluctuate concurrently and their discrimination is crucial for the treatment decision-making. Therefore, it is important for the ideal home-based monitoring and management system for PD to be able to monitor motor states in real-life conditions allowing the discrimination between normal and abnormal movements during normal quotidian activities, between motor state variations and between different types of motor symptoms. Currently, none of the available system is able to provide such a holistic approach on the home-based monitoring of motor symptoms in PD.
In respect to body localization, different placements and various number of sensors were selected, based on the movements to be monitored and the main aim of the study. However, we found scarcely any report [34] on a system where practicality and tolerability were well balanced with an extended motor assessment of multiple systems (axial, head, posture, bilaterally limbs, etc.), which in the case of PD seems to be essential. Sensor integrated clothing might be a solution, which future studies on PD should certainly consider.
In respect to validation methodology, most of the studies used the two-steps approach, an in-clinic step for algorithm creation and an in-home step where the algorithm was applied and tested in “real-life” conditions. Although this approach offers many advantages, still the constrained conditions at the laboratory applied for the creation of algorithms cannot be considered as completely bias-free. Moreover, the choice between the execution of unconstrained daily activities, established daily activities or partially constrained daily activities, as well as the choice to use comparison methods (i.e. symptoms’ diaries) and the decision about the frequency of their employment, seems to be an important decision for the study design. In fact, an excessive request of activity might negatively impact compliance and, therefore, a compromise between assessment precision and minimization of the potential burden (e.g. study by Fisher et al. [32]) is often necessary.
Different monitoring durations were reported; however, the majority of studies applied wearables for more than 24 h. Indeed, in PD, a continuous 24 h monitoring for consecutive days offers a more precise view on disease-related and medication-related fluctuations and therefore valuable information for the clinician in respect to disease staging and treatment decision. Unfortunately, data on accuracy of detecting sleep-related movements and practicality during sleep have only been scarcely reported, even in those studies that collected data longer than 24 h.
In general, it could be affirmed that minimization of obtrusion, the use of small number of comfortable sensors, requiring a low degree of activity from participants [33] and intervening as less as possible in the daily proceedings of the in-home activities, seems to be an important factor influencing compliance and adherence of study participants.
Some of the reviewed studies shared limitations. The majority of studies reported only on a small number of participants and therefore, interpretation of the results should be done with caution. In addition, studies often included PD patients with very different clinical profiles and disease stages and therefore their results were not always comparable.
The limited timeframe of 12 years poses a limitation for our systematic review. However, since the in-home wearables-based clinical approaches gained greatest interest during the last decade, and taking into account the speed of new scientific discoveries and technological changes, we considered it appropriate to review devices of recent generation. Even under this limited timeframe, technology and the precision of the performed techniques have markedly changed, limiting comparability between studies. Another limitation of our review is the possible selection bias based on language criteria due to the exclusion of articles that were not written in English.
Conclusion
The use of wearable devices represents a paradigm shift in healthcare remote diagnostics and therapeutics monitoring in PD and has been made possible by the technological revolution in these systems. Compact-sized, low-cost and reliable devices are becoming widely available and offer great opportunities to overcome past limitations, such as subjectivity and lack of home-based continuous long-term monitoring of daytime cardinal symptoms in PD.
However, the current technical challenges, especially related to long-term practicality and accuracy, need to be addressed in larger cohort studies, fully understood and resolved before wearable technology can be successfully integrated in the routine management of PD.
References
Tysnes OB, Storstein A (2017) Epidemiology of Parkinson’s disease. J Neural Transm 124:901–905. https://doi.org/10.1007/s00702-017-1686-y
Kalia LV, Lang AE (2015) Seminar Parkinson’s disease. Lancet 386:896–912
Williams-gray CH (2016) Parkinson’s disease key points. Medicine 44:542–546. https://doi.org/10.1016/j.mpmed.2016.06.001
Xia R, Mao Z-H (2012) Progression of motor symptoms in Parkinson’s disease. Neurosci Bull 28:39–48. https://doi.org/10.1007/s12264-012-1050-z
Manson A, Stirpe P, Schrag A (2012) Levodopa-induced-dyskinesias clinical features, incidence, risk factors, management and impact on quality of life. J Parkinson’s Dis 2:189–198. https://doi.org/10.3233/JPD-2012-120103
Sringean J, Taechalertpaisarn P, Thanawattano C, Bhidayasiri R (2016) How well do Parkinson’s disease patients turn in bed? Quantitative analysis of nocturnal hypokinesia using multisite wearable inertial sensors. Parkinsonism Relat Disord 23:10–16. https://doi.org/10.1016/j.parkreldis.2015.11.003
Bhidayasiri R, Sringean J, Taechalertpaisarn P, Thanawattano C (2016) Capturing nighttime symptoms in Parkinson disease: technical development and experimental verification of inertial sensors for nocturnal hypokinesia. J Rehabil Res Dev 53:487–498. https://doi.org/10.1682/JRRD.2015.04.0062
Stack EL, Ashburn AM (2006) Impaired bed mobility and disordered sleep in Parkinson’s disease. Mov Disord 21:1340–1342. https://doi.org/10.1002/mds.20944
Bargiotas P, Bassetti CL (2017). Sleep-related movement disorders and disturbances of motor control. https://doi.org/10.1097/WCO.0000000000000466
Pérez-lópez C, Samà A, Rodríguez-martín D, Moreno-aróstegui JM, Cabestany J, Bayes A, Mestre B, Alcaine S, Quispe P, Laighin GÓ, Sweeney D, Quinlan LR, Counihan TJ, Browne P, Annicchiarico R, Costa A, Lewy H, Rodríguez-molinero A (2016) Dopaminergic-induced dyskinesia assessment based on a single belt-worn accelerometer. Artif Intell Med 67:47–56
Nieuwboer A, de Weerdt W, Dom R, Lesaffre E (1998) A frequency and correlation analysis of motor deficits in Parkinson patients. Disabil Rehabil 20:142–150. https://doi.org/10.3109/09638289809166074
Mera TO, Heldman DA, Espay AJ, Payne M, Giuffrida JP (2012) Feasibility of home-based automated Parkinson’s disease motor assessment. J Neurosci Methods 203:152–156. https://doi.org/10.1016/j.jneumeth.2011.09.019
Ramsperger R, Meckler S, Heger T, Van Uem J, Hucker S, Braatz U, Graessner H, Berg D, Manoli Y (2016) Parkinsonism and Related Disorders Continuous leg dyskinesia assessment in Parkinson’s disease e clinical validity and ecological effect Lab-based Investigation only on medication ON. Parkinsonism Relat Disord 26:41–46
Bayés À, Samá A, Prats A, Pérez-López C, Crespo-Maraver M, Moreno JM, Alcaine S, Rodriguez-Molinero A, Mestre B, Quispe P, de Barros AC, Castro R, Costa A, Annicchiarico R, Browne P, Counihan T, Lewy H, Vainstein G, Quinlan LR, Sweeney D, G. ÓLaighin, J. Rovira, D. Rodrigue z-Martin, J. Cabestany, (2008) A “HOLTER” for Parkinson’s disease: validation of the ability to detect on-off states using the REMPARK system. Gait Posture 59:1–6. https://doi.org/10.1016/j.gaitpost.2017.09.031
Reimer J, Grabowski M, Lindvall O, Hagell P (2004) Use and interpretation of on/off diaries in Parkinson’s disease. J Neurol Neurosurg Psychiatry 75:396–400. https://doi.org/10.1136/jnnp.2003.022780
Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT (2003) State of the art review the unified parkinson’s disease rating scale (updrs): status and recommendations. Mov Disord Soc 18:738–750. https://doi.org/10.1002/mds.10473
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30:1591–1601. https://doi.org/10.1002/mds.26424
Pietracupa S, Latorre A, Berardelli A, Fabbrini G (2014) Parkinsonian patients and poor awareness of dyskinesias. Mov Disord. https://doi.org/10.1002/mds.24017
Godinho C, Domingos J, Cunha G, Santos AT, Fernandes RM, Abreu D, Gonçalves N, Matthews H, Isaacs T, Duffen J, Al-jawad A, Larsen F, Serrano A, Weber P, Thoms A, Sollinger S, Graessner H, Maetzler W, Ferreira JJ (2016) A systematic review of the characteristics and validity of monitoring technologies to assess Parkinson’s disease. J NeuroEng Rehabil. https://doi.org/10.1186/s12984-016-0136-7
Griffiths RI, Kotschet K, Arfon S, Ming Z, Johnson W (2012) Automated Assessment of Bradykinesia and Dyskinesia in Parkinson’s disease. J Parkinson’s Dis 2:47–55. https://doi.org/10.3233/JPD-2012-11071
Samà A, Pérez-López C, Rodríguez-Martín D, Català A, Moreno-Aróstegui JM, Cabestany J, de Mingo E, Rodríguez-Molinero A (2017) Estimating bradykinesia severity in Parkinson’s disease by analysing gait through a waist-worn sensor. Comput Biol Med 84:114–123. https://doi.org/10.1016/j.compbiomed.2017.03.020
Binder S, Deuschl G, Volkmann J (2009) Effect of cabergoline on Parkinsonian tremor assessed by long-term actigraphy. Eur Neurol 61:149–153. https://doi.org/10.1159/000186505
Weiss A, Herman T, Giladi N, Hausdorff JM (2014) Objective assessment of fall risk in Parkinson’s disease using a body-fixed sensor worn for 3 days. PLoS ONE. https://doi.org/10.1371/journal.pone.0096675
Weiss A, Sharifi S, Plotnik M, Van Vugt JPP, Giladi N, Hausdorff JM (2011) Toward automated, at-home assessment of mobility among patients with Parkinson disease, using a body-worn accelerometer. Neurorehabil Neural Repair 25:810–818. https://doi.org/10.1177/1545968311424869
Dijkstra B, Kamsma YP, Zijlstra W (2010) Detection of gait and postures using a miniaturized triaxial accelerometer-based system: Accuracy in patients with mild to moderate Parkinson’s disease. Arch Phys Med Rehabil 91:1272–1277. https://doi.org/10.1016/j.apmr.2010.05.004
Pan W, Kwak S, Li F, Wu C, Chen Y, Yamamoto Y, Cai D (2013) Actigraphy monitoring of symptoms in patients with Parkinson’s disease. Physiol Behav 119:156–160. https://doi.org/10.1016/j.physbeh.2013.05.044
Heldman DA, Giuffrida JP, Cubo E (2016) Wearable sensors for advanced therapy referral in Parkinson’s disease. J Parkinson’s Dis 6:631–638. https://doi.org/10.3233/JPD-160830
Herman T, Weiss A, Brozgol M, Giladi N, Hausdorff JM (2014) Gait and balance in Parkinson’s disease subtypes: objective measures and classification considerations. J Neurol 261:2401–2410. https://doi.org/10.1007/s00415-014-7513-6
Bernad-Elazari H, Herman T, Mirelman A, Gazit E, Giladi N, Hausdorff JM (2016) Objective characterization of daily living transitions in patients with Parkinson’s disease using a single body-fixed sensor. J Neurol 263:1544–1551. https://doi.org/10.1007/s00415-016-8164-6
Lipsmeier F, Taylor KI, Kilchenmann T, Wolf D, Scotland A, Schjodt-Eriksen J, Cheng WY, Fernandez-Garcia I, Siebourg-Polster J, Jin L, Soto J, Verselis L, Boess F, Koller M, Grundman M, Monsch AU, Postuma RB, Ghosh A, Kremer T, Czech C, Gossens C, Lindemann M (2018) Evaluation of smartphone-based testing to generate exploratory outcome measures in a phase 1 Parkinson’s disease clinical trial. Mov Disord 33:1287–1297. https://doi.org/10.1002/mds.27376
Tan EE, Hogg EJ, Tagliati M (2019) The role of personal kinetigraphTM fluctuator score in quantifying the progression of motor fluctuations in Parkinson’s disease. Funct Neurol 34:21–28
Fisher JM, Hammerla NY, Ploetz T, Andras P, Rochester L, Walker RW (2016) Unsupervised home monitoring of Parkinson’s disease motor symptoms using body-worn accelerometers. Parkinsonism Relat Disord 33:44–50. https://doi.org/10.1016/j.parkreldis.2016.09.009
De Lima ALS, Hahn T, Evers LJW, De Vries NM, Cohen E, Afek M, Bataille L, Daeschler M, Claes K, Boroojerdi B, Terricabras D, Little MA, Baldus H, Bloem BR, Faber MJ (2017) Feasibility of large-scale deployment of multiple wearable sensors in Parkinson’s disease. PLoS ONE 12:1–15. https://doi.org/10.1371/journal.pone.0189161
Boroojerdi B, Ghaffari R, Mahadevan N, Markowitz M, Melton K, Morey B, Otoul C, Patel S, Phillips J, Sen-Gupta E, Stumpp O, Tatla D, Terricabras D, Claes K, Wright JA, Sheth N (2019) Clinical feasibility of a wearable, conformable sensor patch to monitor motor symptoms in Parkinson’s disease. Parkinsonism Related Disord 61:70–76. https://doi.org/10.1016/j.parkreldis.2018.11.024
M. Pastorino, J. Cancela, M.T. Arredondo, L. Pastor-Sanz, S. Contardi, F. Valzania (2013) Preliminary results of ON/OFF detection using an integrated system for Parkinson’s disease monitoring. In: 35th Annual international conference of the IEEE Engineering in Medicine and Biology Society (EMBC), pp 941–944. Doi: https://doi.org/10.1109/EMBC.2013.6609657.
M. Pastorino, J. Cancela, M.T. Arredondo, M. Pansera, L. Pastor-Sanz, F. Villagra, M.A. Pastor, J.A. Martín (2011) Assessment of bradykinesia in Parkinson’s disease patients through a multi-parametric system. In: 33rd Annual international conference of the IEEE Engineering in Medicine and Biology Society (EMBC), pp 1810–1813. doi: https://doi.org/10.1109/IEMBS.2011.6090516.
S. Das, B. Amoedo, F.D. la Torre, J. Hodgins (2012) Detecting parkinson’s symptoms in uncontrolled home environments : a multiple instance learning approach. In: 34th Annual international conference of the IEEE Engineering in Medicine and Biology Society (EMBC), pp 3688–3691
Chen B, Patel S, Buckley T, Rednic R, Mcclure DJ, Shih L, Tarsy D, Welsh M, Bonato P (2011) A web-based system for home monitoring of patients with Parkinson’s disease using wearable sensors. IEE Trans Biomed Eng 58:831–836
Patel S, Member S, Chen B, Mancinelli C, Paganoni S, Shih L, Welsh M, Dy J, Bonato P, Member S (2011) Wearable sensor technology in the home setting. In: Annual international conference of the IEEE Engineering in Medicine and |Biological Society, pp 1552–1555.
Tarnita D (2016) Wearable sensors used for human gait analysis. Rom J Morphol Embryol 57:373–382
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ancona, S., Faraci, F.D., Khatab, E. et al. Wearables in the home-based assessment of abnormal movements in Parkinson’s disease: a systematic review of the literature. J Neurol 269, 100–110 (2022). https://doi.org/10.1007/s00415-020-10350-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-10350-3