Abstract
Background
Pick’s disease (PiD) is a unique subtype of frontotemporal lobar degeneration characterized pathologically by aggregates of 3-Repeat tau. Few studies have examined the clinical variability and disease progression in PiD. We describe the clinical features, neuropsychological profiles and coexistent pathologies in 21 cases of autopsy-confirmed PiD.
Methods
This study was a retrospective analysis of patients with Pick’s disease evaluated at Mayo Clinic, Rochester or Jacksonville (1995–2018), and identified through an existing database.
Results
Twenty-one cases with sufficient clinical data were identified. Behavioral variant FTD (bvFTD; 12/21) was the most common phenotype, followed by primary progressive aphasia (PPA; 7/21), corticobasal syndrome (CBS; 1/21) and amnestic dementia (1/21). Median age at disease onset was 54 years, with PPA cases (median = 52 years) presenting earlier than bvFTD (median = 59). Median disease duration (onset–death) overall was 10 years and did not differ significantly between bvFTD (median = 9.5 years) and PPA (median = 13). Age at death was not significantly different in PPA (median = 66) compared to bvFTD (median = 68.5). A third of the cases (n = 7/21) demonstrated pure PiD pathology, while the remainder showed co-existent other pathologies including Alzheimer’s type (n = 6), cerebral amyloid angiopathy (n = 3), combined Alzheimer’s and amyloid angiopathy (n = 4), and Lewy body disease (n = 1).
Conclusions
Our study shows that bvFTD and PPA are the most common clinical phenotypes associated with PiD, although rare presentations such as CBS were also seen. Coexisting non-Pick’s pathology was also present in many cases. Our study highlights the clinical and pathologic heterogeneity in PiD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Frontotemporal dementia (FTD) refers to a spectrum of clinical syndromes in which there is striking degeneration of the frontal and temporal lobes. Frontotemporal lobar degeneration (FTLD) is an overarching term for the spectrum of FTD-related pathologies. Pick’s disease (PiD) is a rare pathologic subtype of FTLD-tau characterized by severe frontotemporal, knife-edge like cortical atrophy on gross examination. Histologically, PiD is characterized by rounded, circumscribed argyrophilic neuronal cytoplasmic inclusions called Pick bodies that stain positive with Bielschowsky, but not with Gallyas silver stain [1]. Among FTLDs associated with tau pathology, PiD pathology accounts for approximately 30% cases [2]. PiD is unique among tau pathologies associated with FTLD-tau, because it has three conserved 30–33 amino acid repeats in the microtubule binding domain of the tau (known as a 3R tauopathy). Select few studies have examined the clinical features of patients with PiD. The largest clinical series of autopsy-confirmed PiD to date reported that the most common clinical phenotypes of PiD were behavioral variant FTD (bvFTD) and language variant FTD [3, 4], but the small number of well-studied autopsied cases leaves uncertainty with respect to the clinical presentations and clinical course of PiD. Since FTLD-motor neuron disease (MND), which is characterized by FTD-TDP-43, and a language predominant phenotype predicts shorter survival than behavioral predominant phenotype [5], we aimed to investigate differences in disease duration in PiD. The objective of our study was to describe the clinical phenotypes, neuropsychologic profiles, and co-existent pathology in autopsy-confirmed cases of PiD.
Methods
Study patients
This study, which was a retrospective review of the Mayo Clinic, Rochester, MN and Jacksonville, FL pathology database, identified 21 cases of PiD from 1995 to 2018. The clinical notes were reviewed, and the following data were extracted for each case: sex, age at disease onset (estimated based on the patient’s medical records), age at clinical presentation, disease duration, and age at death. Current diagnostic criteria for bvFTD and primary progressive aphasia (PPA) [6, 7] were applied to the clinical evaluations retrospectively. Presence or absence of core and supporting features (bvFTD or PPA presentation) were determined based on available clinical information. Patients with PPA presentation were further categorized into one of the established PPA subtypes [7, 8].
Neuropsychological analysis
Neuropsychological profiles at presentation were obtained from the chart where available. Global cognitive function was assessed with the Mattis Dementia Rating Scale [9]. Executive function was measured with the Controlled Oral Word Association Test (COWAT) [10] and Trail Making Test part B [11]. Language function was assessed with the Boston Naming Test [12] and Category Fluency [13]. Learning and memory were assessed with Auditory–Verbal Learning Test (AVLT) [14] learning over trials and delayed recall. Visuospatial functioning was assessed with the Judgement of Line Orientation [15]. All scores were converted to scaled scores based (mean = 10; SD 3) on the Mayo Older Adult Normative Studies (MOANS) [16]. For participants below the MOANS age range (56 and above), the lowest age group was used to determine their scaled score. Cognitive impairment was defined as greater than one standard deviation below the mean, which equates to a scaled score of ≤ 6. In some cases, neuropsychological testing was not reported, because either it could not be completed due to the severity of cognitive impairment upon presentation or it was unavailable in the medical record.
Pathology protocols
Postmortem investigations adhered to established institutional laboratory and pathology protocols for brain banking and neuropathological investigation. All pathological analysis was completed by neuropathologists. Brains were collected at autopsy, weighed and fixed in 10% formalin, before dissection. Tissue samples were taken from a variety of neocortical areas including the superior frontal gyrus, motor cortex, temporal cortex, parietal and calcarine cortex, as well as anterior cingulate gyrus, hippocampus, amygdala, entorhinal cortex, nucleus basalis of Meynert, and basal ganglia. Pick bodies were detected with immunohistochemistry, with antibodies to tau (AT8), 3R-tau, 4R-tau or silver stains (Bielschowsky and Gallyas silver stains). Tissue samples were also studied with hematoxylin/eosin stain, alpha-synuclein and, TDP-43. Presence of co-pathologies was abstracted from pathology reports.
Statistical methods
Group differences for continuous clinical and demographic data were compared using nonparametric t tests including Wilcoxon rank sum test. Binary data were analyzed using Chi-square tests (Fisher’s exact for cells if there were cells with small numbers). Significance was set at p < 0.05. Statistical analyses were performed using JMP software (version 14.1.0; SAS Institute Inc., Cary, NC). Given small numbers, results are reported as median, minimum, and maximum.
Results
Frequency of phenotypes
Behavioral variant frontotemporal dementia: Behavioral variant FTD was diagnosed in 12 of 21 (57%) cases at presentation. Ten cases (48%) met criteria for probable bvFTD, retrospectively. Two cases had no neuroimaging data available, and they both met criteria for possible bvFTD by clinical criteria. Behavioral disinhibition was present in 11 of 12 cases on presentation. Stereotyped or compulsive behaviors were present in 7 of 12 cases. Early apathy was present in 8 of 12 cases. Five of 12 cases had loss of empathy upon presentation, and hyperorality was present in 6 cases. An overall dysexecutive neuropsychological profile was present in 8 of the 12 cases on presentation. Five of 12 cases also developed aphasia features over the course of their disease. Primary progressive aphasia: Primary progressive aphasia was diagnosed in 7 of the 21 cases of Pick’s disease (33%). Three cases met diagnostic criteria for semantic variant PPA (svPPA) and 3 for agrammatic PPA (agPPA). All three agPPA cases had apraxia of speech (AOS). One of the seven cases did not have sufficient clinical information to identify a subtype and was PPA-unclassified (PPA-U). Three of the seven cases developed behavioral changes over years of follow-up and 1 case developed progressive motor features resembling a progressive supranuclear palsy phenotype during later stages. CBS phenotype: One patient presented with corticobasal syndrome (CBS). Age of onset in this patient was 69 years. This patient had asymmetric postural tremor, limb apraxia, and limb rigidity. There was also vertical supranuclear gaze palsy in this patient. Amnestic phenotype: One patient presented with progressive amnesia clinically thought to be Alzheimer’s dementia. Visuospatial and language impairment appeared later. Behavioral deficits were not present.
Demographic data
The median age of disease onset was 54 years (range 46, 77). Age at symptom onset was earlier in patients with PPA compared to bvFTD presentation (52 vs 59; p = 0.03). Age at presentation was also noted to be slightly earlier in PPA cases, but this did not reach statistical significance (p = 0.19). Median disease duration overall was 10 years and did not differ significantly between bvFTD and PPA cases. Age at death was not significantly different between bvFTD and PPA (Table 1). There was no family history of dementia in the 11 bvFTD cases, while 2 of the 7 PPA cases had a family history of dementia. There was no family history of motor neuron disease or parkinsonism in any case. None had mutations in common FTLD genes (MAPT, GRN and C9orf72).
Neuropsychological profile
The neuropsychological results at the time of initial clinical presentation for 15 of the 21 cases are summarized in Table 2. Of participants for whom neuropsychological testing was available, five individuals were below the MOANS age range (1 bvFTD, 4 PPA). There was large variability in time from symptom onset to neuropsychological testing. Formal neuropsychological testing could not be completed in 2 of 12 bvFTD and 3 of 7 PPA cases (2 agPPA and 1 svPPA) due to the severity of cognitive impairment.
One bvFTD and one PPA patient showed intact functioning on a global measure of cognition (DRS-2) during baseline testing. On measures of executive functioning, five patients were impaired on a test of attention and set shifting (Trails B), and eight demonstrated impaired lexical fluency (COWAT). Both PPA cases who completed language testing were impaired on measures of semantic fluency and auditory comprehension, and one was impaired on confrontation naming. Five bvFTD patients had impaired semantic fluency, three had impaired naming, and one had impaired auditory comprehension. Of those tested for whom AVLT data were available, four bvFTD cases showed impaired learning and five had impaired recall (with four unable to recall any of the words). One PPA patient who completed the AVLT had average performance. The single cases of CBS and AD both demonstrated impaired memory. Performance on a measure of visuospatial abilities (line orientation) was intact in all cases.
Pathological subtypes
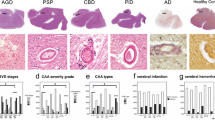
Of the 21 cases with Pick’s disease, 7 (33%) cases had pure Pick’s pathology, while the other 14 cases had additional pathologies (Fig. 1). The most common co-existent pathology was Alzheimer disease type pathology. Four cases with Alzheimer’s-type pathology also had cerebral amyloid angiopathy (CAA). Three cases had CAA without Alzheimer’s type pathology coexisting with Pick’s disease. Most cases had low levels of Alzheimer-type pathology, while only two cases showed intermediate level of Alzheimer-type pathology (CBS and amnestic phenotype). The individual with the amnestic phenotype had both severe Pick’s pathology combined with Alzheimer-type pathology (Alzheimer disease neuropathologic changes: A2, B3, C3) at autopsy. The CBS case had coexistent limbic Lewy body pathology in addition to Alzheimer’s disease pathology (Alzheimer disease neuropathologic changes: A2, B3, C3). There was no statistical difference in disease duration between cases with pure PiD and those with co-pathology.
Coexistence of other neurodegenerative pathologies in primary pathological Pick’s disease cases (N = 21). Co-pathology frequencies are depicted in numbers (percentage in parenthesis) for all 21 cases where Pick’s disease was the primary diagnosis. Amyloid was the most common secondary pathology. Abbreviations: AD = Alzheimer’s disease, CAA = cerebral amyloid angiopathy, LBD = Lewy body disease
Table 3 summarizes the clinical phenotypes of autopsy-confirmed Pick’s disease described in the literature. So far, there have been two other case series dedicated to describing phenotypes in Pick’s disease [3, 4]. Other studies have described Pick’s disease as part of pathological studies of language disorders, hippocampal atrophy or case studies [17, 18].
Discussion
This study highlights the phenotypic diversity of PiD. This series also highlights coexistent pathologies associated with clinical phenotypes in autopsy-confirmed PiD, a rare neurodegenerative condition. The most common clinical phenotypes were bvFTD and PPA, although one case of CBS and an amnestic phenotype were also observed. Predicting pathology with antemortem clinical syndromes can be challenging in rare conditions. Overall, the spectrum of clinical syndromes observed in this autopsy series was similar to those reported by Piguet et al. [3]. CBS phenotype, although rare, has been reported previously, present in conjunction with a language variant PPA [4, 19]. Interestingly, in one previous study and ours, the CBS phenotype was accompanied by a secondary pathologic finding of cortical Lewy bodies. Although this is a rare presentation of PiD, it may have treatment implications such as response to levodopa or donepezil, which should be explored in the future. Early memory complaint as a presenting feature of PiD was noted in a large series in Europe that also examined hippocampal pathology exclusively [18].
Among the PPA subtypes, both semantic variant PPA and agPPA were observed which is consistent with previous studies [3]. Our data in combination with previously reported series of PiD suggest there is no clear association of Pick’s pathology with specific subtypes of primary progressive aphasia. In a study of 69 PPA patients, 2 semantic PPA cases, 4 agPPA cases, and 2 mixed PPA cases had a pathological diagnosis of Pick’s disease [20]. While svPPA is most commonly associated with FTLD-TDP-43 Type C pathology [20], our study shows that PiD pathology may produce this phenotype of PPA. Prior studies have shown that the presence of AOS is a strong predictor of tau pathology [21]. In this study, AOS was found to be present in all three patients with agPPA. While AOS is typically seen with 4R tauopathies such as corticobasal degeneration or progressive supranuclear palsy, 3R Pick’s pathology has been reported [21] and should be considered as an additional etiology in the differential diagnosis. Therefore, while clinical diagnosis can suggest a pathologic substrate, biomarkers which are highly specific for tauopathy subtypes may be necessary adjuncts when considering FTD patients for proteinopathy-specific interventions.
In this series, age of onset was earlier in the PPA cases compared to bvFTD cases. Conversely, a previous study assessing the incidence and epidemiology of a heterogenous group of FTLD syndromes and pathologies found that PPA cases had the oldest age at diagnosis [22]. Other series specific to PiD found no difference in the age of onset. This could be due to the retrospective nature of our study where symptom onset was estimated from chart review or the wide phenotypic variability of a rare disease. No difference in age at death and disease duration was detected between bvFTD and PPA cases. This finding contrasts with previous reports which indicate bvFTD survival is significantly shorter than PPA [3]. In our series, both behavioral and language phenotypes of PiD revealed a wide range of disease duration (Table 1). Whether pathology or phenotype predicts survival in PiD is still unclear, but other studies have provided support to the hypothesis that underlying pathology predicts survival in neurodegenerative conditions [23]. Mean survival time in sporadic FTLD-TDP has been reported at 7.9 years, but with a wide range of 2–18 years [24]. Mean survival of FTLD-tau has been reported to be approximately 9 years [25]. Another study previously studying survival differences between tau-positive and tau-negative FTLD showed decreased survival in tau-positive pathology [26]. A recent meta-analysis of survival in FTD showed no difference between phenotypes except FTD-MND, which had shorter survival although pathologies were heterogenous [27]. Based on available literature, there may be no survival difference among the common clinical subtypes of PiD. Survival data in rare causes of neurodegeneration are weakened by confounding variables and small sample sizes. Specifically, in this study we are unable to risk adjust patients for medical comorbidities that may have affected disease duration.
The neuropsychological profiles of PiD patient subtypes (PPA and bvFTD) were supportive of the clinical diagnosis and quite variable in severity. In cases of bvFTD, neuropsychological testing generally showed deficits in executive and memory functions. In the PPA cases, neuropsychological testing demonstrated difficulty with naming and category fluency. Overall, interpretation of neuropsychological data and defining patterns in these cases is limited by the variability in the number of years from onset to testing as well as smaller subset of patients with available data.
We found co-pathology existed in up to 67% of our cases. Other studies have examined and reported the existence of Alzheimer-type pathology in PiD in the hippocampus, but not in similar proportions [18]. Another autopsy series examining concomitant pathologies in neurodegenerative conditions reported that pure PiD is more common among tauopathies [28]. We did not observe any cases with concomitant TDP-43 pathology in our series as reported previously [29, 30]. Cortical Lewy body pathology was observed in one case, and it has previously been reported in PiD with bvFTD, PPA and pure parkinsonian phenotypic presentations [4, 31]. Our case with Lewy body pathology presented with a CBS phenotype and also had Alzheimer-type pathology. Previously reported CBS phenotypes in PiD had shown pure Pick’s disease and with Lewy bodies [4, 19]. Our case series suggests that complex neurodegenerative dementias can have variable phenotypes when multiple pathologies are present. Our observations are similar to larger studies on clinicopathological specificity of FTLD [28].
Conclusion
In summary, our series underscores both clinical and pathological heterogeneity in a rare neurodegenerative condition such as PiD. Although PiD most commonly presents with bvFTD and PPA phenotypes, CBS and amnestic syndromes can also be observed, and PiD should be considered in the differential diagnosis of these syndromes. Unlike FTLD-MND, disease duration did not appear to be different between the various phenotypes in our series and a larger series looking at survival is warranted. Coexistence of Alzheimer’s type pathology in PiD was common in our series, highlighting the need for specific biomarkers in neurodegenerative conditions that increase with age.
References
Murray ME, Kouri N, Lin WL, Jack CR Jr, Dickson DW, Vemuri P (2014) Clinicopathologic assessment and imaging of tauopathies in neurodegenerative dementias. Alzheimers Res Ther 6(1):1. https://doi.org/10.1186/alzrt231
Dickson DW, Kouri N, Murray ME, Josephs KA (2011) Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau). J Mol Neurosci 45(3):384–389. https://doi.org/10.1007/s12031-011-9589-0
Piguet O, Halliday GM, Reid WG, Casey B, Carman R, Huang Y, Xuereb JH, Hodges JR, Kril JJ (2011) Clinical phenotypes in autopsy-confirmed Pick disease. Neurology 76(3):253–259. https://doi.org/10.1212/WNL.0b013e318207b1ce
Irwin DJ, Brettschneider J, McMillan CT, Cooper F, Olm C, Arnold SE, Van Deerlin VM, Seeley WW, Miller BL, Lee EB, Lee VM, Grossman M, Trojanowski JQ (2016) Deep clinical and neuropathological phenotyping of Pick disease. Ann Neurol 79(2):272–287. https://doi.org/10.1002/ana.24559
Coon EA, Sorenson EJ, Whitwell JL, Knopman DS, Josephs KA (2011) Predicting survival in frontotemporal dementia with motor neuron disease. Neurology 76(22):1886–1893. https://doi.org/10.1212/WNL.0b013e31821d767b
Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134(Pt 9):2456–2477. https://doi.org/10.1093/brain/awr179
Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, Grossman M (2011) Classification of primary progressive aphasia and its variants. Neurology 76(11):1006–1014. https://doi.org/10.1212/WNL.0b013e31821103e6
Botha H, Duffy JR, Whitwell JL, Strand EA, Machulda MM, Schwarz CG, Reid RI, Spychalla AJ, Senjem ML, Jones DT, Lowe V, Jack CR, Josephs KA (2015) Classification and clinicoradiologic features of primary progressive aphasia (PPA) and apraxia of speech. Cortex 69:220–236. https://doi.org/10.1016/j.cortex.2015.05.013
Mattis S (1988) Dementia rating scale: professional manual. Psychological Assessment Resources, Odessa
Benton A, Hamsher KD (1978) Multilingual aphasia examination: manual. University of Iowa, Iowa City
Reitan RM, Wolfson D (1993) The Halstead–Reitan neuropsychological test battery: theory and clinical interpretation. Neuropsychology Press, Tucson
Kaplan E, Goodglass H, Weintraub S (1983) The Boston naming test. Lea & Febiger, Philadelphia
Strauss E, Sherman EMS, Spreen O (2006) A compendium of neuropsychological tests. Oxford University Press, New York
Rey A (1964) L'examen clinique en psychologie. Presses Universitaires de France, Paris
Benton AL, Hamsher Kd, Varney NR, Spreen O (1983) Contributions to neuropsychological assessment. Oxford University Press, New York
Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC (1996) Neuropsychological tests' norms above age 55: COWAT, BNT, MAE token, WRAT-R reading, AMNART, STROOP, TMT, and JLO. Clin Neuropsychol 10(3):262–278. https://doi.org/10.1080/13854049608406689
Rankin KP, Mayo MC, Seeley WW, Lee S, Rabinovici G, Gorno-Tempini ML, Boxer AL, Weiner MW, Trojanowski JQ, DeArmond SJ, Miller BL (2011) Behavioral variant frontotemporal dementia with corticobasal degeneration pathology: phenotypic comparison to bvFTD with Pick's disease. J Mol Neurosci 45(3):594–608. https://doi.org/10.1007/s12031-011-9615-2
Kovacs GG, Rozemuller AJ, van Swieten JC, Gelpi E, Majtenyi K, Al-Sarraj S, Troakes C, Bodi I, King A, Hortobagyi T, Esiri MM, Ansorge O, Giaccone G, Ferrer I, Arzberger T, Bogdanovic N, Nilsson T, Leisser I, Alafuzoff I, Ironside JW, Kretzschmar H, Budka H (2013) Neuropathology of the hippocampus in FTLD-Tau with Pick bodies: a study of the BrainNet Europe Consortium. Neuropathol Appl Neurobiol 39(2):166–178. https://doi.org/10.1111/j.1365-2990.2012.01272.x
Boeve BF, Maraganore DM, Parisi JE, Ahlskog JE, Graff-Radford N, Caselli RJ, Dickson DW, Kokmen E, Petersen RC (1999) Pathologic heterogeneity in clinically diagnosed corticobasal degeneration. Neurology 53(4):795–800. https://doi.org/10.1212/wnl.53.4.795
Spinelli EG, Mandelli ML, Miller ZA, Santos-Santos MA, Wilson SM, Agosta F, Grinberg LT, Huang EJ, Trojanowski JQ, Meyer M, Henry ML, Comi G, Rabinovici G, Rosen HJ, Filippi M, Miller BL, Seeley WW, Gorno-Tempini ML (2017) Typical and atypical pathology in primary progressive aphasia variants. Ann Neurol 81(3):430–443. https://doi.org/10.1002/ana.24885
Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, Hauser MF, Witte RJ, Boeve BF, Knopman DS, Dickson DW, Jack CR Jr, Petersen RC (2006) Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain 129(Pt 6):1385–1398. https://doi.org/10.1093/brain/awl078
Coyle-Gilchrist IT, Dick KM, Patterson K, Vazquez Rodriquez P, Wehmann E, Wilcox A, Lansdall CJ, Dawson KE, Wiggins J, Mead S, Brayne C, Rowe JB (2016) Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology 86(18):1736–1743. https://doi.org/10.1212/WNL.0000000000002638
Rascovsky K, Salmon DP, Lipton AM, Leverenz JB, DeCarli C, Jagust WJ, Clark CM, Mendez MF, Tang-Wai DF, Graff-Radford NR, Galasko D (2005) Rate of progression differs in frontotemporal dementia and Alzheimer disease. Neurology 65(3):397–403. https://doi.org/10.1212/01.wnl.0000171343.43314.6e
Armstrong RA (2016) Survival in the pre-senile dementia frontotemporal lobar degeneration with TDP-43 proteinopathy: effects of genetic, demographic and neuropathological variables. Folia Neuropathol 54(2):137–148. https://doi.org/10.5114/fn.2016.60391
Chiu WZ, Kaat LD, Seelaar H, Rosso SM, Boon AJ, Kamphorst W, van Swieten JC (2010) Survival in progressive supranuclear palsy and frontotemporal dementia. J Neurol Neurosurg Psychiatry 81(4):441–445. https://doi.org/10.1136/jnnp.2009.195719
Xie SX, Forman MS, Farmer J, Moore P, Wang Y, Wang X, Clark CM, Coslett HB, Chatterjee A, Arnold SE, Rosen H, Karlawish JH, Van Deerlin VM, Lee VM, Trojanowski JQ, Grossman M (2008) Factors associated with survival probability in autopsy-proven frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatry 79(2):126–129. https://doi.org/10.1136/jnnp.2006.110288
Kansal K, Mareddy M, Sloane KL, Minc AA, Rabins PV, McGready JB, Onyike CU (2016) Survival in frontotemporal dementia phenotypes: a meta-analysis. Dement Geriatr Cogn Disord 41(1–2):109–122. https://doi.org/10.1159/000443205
Robinson JL, Lee EB, Xie SX, Rennert L, Suh E, Bredenberg C, Caswell C, Van Deerlin VM, Yan N, Yousef A, Hurtig HI, Siderowf A, Grossman M, McMillan CT, Miller B, Duda JE, Irwin DJ, Wolk D, Elman L, McCluskey L, Chen-Plotkin A, Weintraub D, Arnold SE, Brettschneider J, Lee VM, Trojanowski JQ (2018) Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain 141(7):2181–2193. https://doi.org/10.1093/brain/awy146
Irwin DJ, Byrne MD, McMillan CT, Cooper F, Arnold SE, Lee EB, Van Deerlin VM, Xie SX, Lee VM, Grossman M, Trojanowski JQ (2016) Semi-automated digital image analysis of Pick's disease and TDP-43 proteinopathy. J Histochem Cytochem 64(1):54–66. https://doi.org/10.1369/0022155415614303
Freeman SH, Spires-Jones T, Hyman BT, Growdon JH, Frosch MP (2008) TAR-DNA binding protein 43 in Pick disease. J Neuropathol Exp Neurol 67(1):62–67. https://doi.org/10.1097/nen.0b013e3181609361
Forrest SL, Crockford DR, Sizemova A, McCann H, Shepherd CE, McGeachie AB, Affleck AJ, Carew-Jones F, Bartley L, Kwok JB, Kim WS, Jary E, Tan RH, McGinley CV, Piguet O, Hodges JR, Kril JJ, Halliday GM (2019) Coexisting Lewy body disease and clinical parkinsonism in frontotemporal lobar degeneration. Neurology 92(21):e2472–e2482. https://doi.org/10.1212/wnl.0000000000007530
Funding
This study was funded by Alzheimer Disease Research Center (ADRC) (Grant Numbers: P30 AG016574 and P50 AG062677).
Author information
Authors and Affiliations
Contributions
Study conception and design was by JG-R, KJ, ES and BB and all authors contributed appropriately. Material preparation, data collection and analysis were performed by PC, MP, EA, EC and ES. The first draft of the manuscript was written by PC and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
Drs. Choudhury, Scharf, Jones, Duffy, Reichard, Alden, Paolini, Murray, Dickson and Parisi have no conflicts of interests or disclosures. Dr. J Graff-Radford receives research support from National Institute of Health. Dr. Duffy has no conflicts of interest. Ms. Constantopolous has no disclosures. Dr. Fields has no conflicts of interests. She receives research support from NIH. Dr. Machulda received research support from National Institute of Health and National Institute of Deafness and Other Communication Disorders. Dr. Josephs has no conflicts of interest. He receives research support from the National Institute of Health. Dr. Knopman served on a Data Safety Monitoring Board for the DIAN study. He serves on a Data Safety monitoring Board for a tau therapeutic for Biogen, but receives no personal compensation. He is an investigator in clinical trials sponsored by Biogen, Lilly Pharmaceuticals and the University of Southern California. He serves as a consultant for Samus Therapeutics, Third Rock and Alzeca Biosciences, but receives no personal compensation. He receives research support from the NIH. Dr. Petersen has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Roche, Inc. Merck, Inc. Genentech, Inc. Biogen, Inc. Eisai, Inc. GE Healthcare. Dr. Petersen has received personal compensation in an editorial capacity for UpToDate. Dr. N Graff-Radford has received research support from Eli Lilly, Novartis, Biogen, and AbbVie. Dr. Boeve serves as an investigator for clinical trials sponsored by Biogen, Alector, and EIP Pharma. He receives royalties from the publication of a book entitled Behavioral Neurology of Dementia (Cambridge Medicine, 2009, 2017). He serves on the Scientific Advisory Board of the Tau Consortium. He receives research support from the NIH, the Mayo Clinic Dorothy and Harry T. Mangurian Jr. Lewy Body Dementia Program, the Little Family Foundation, and the Turner Family Foundation.
Ethics approval
The present study was approved by the Mayo Clinic Institutional Review Board.
Consent to participate
All patients and/or their proxies had provided written consent to the use of their medical records and tissue for research.
Rights and permissions
About this article
Cite this article
Choudhury, P., Scharf, E.L., Paolini, M.A. et al. Pick’s disease: clinicopathologic characterization of 21 cases. J Neurol 267, 2697–2704 (2020). https://doi.org/10.1007/s00415-020-09927-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-09927-9