Abstract
Sensory trick may relieve dystonic symptoms in patients with idiopathic cervical dystonia (CD). We investigated the patterns of brain functional MRI (fMRI) during resting state, sensory trick simulation and sensory trick imagination in CD patients both with and without an effective sensory trick. We recruited 17 CD patients and 15 healthy controls. Nine patients (CD-trick) had an effective sensory trick, while 8 patients (CD-no-trick) did not. Cervical range of motion validated instrument assessed dystonic posture and sensory trick effect. Participants underwent resting state fMRI, which was repeated by patients while executing the sensory trick. Patients also performed an fMRI task in which they were asked to imagine a sensory trick execution. CD-trick and CD-no-trick patients were comparable in terms of CD severity. Applying the sensory trick, CD-trick patients significantly improved dystonic posture. CD-no-trick patients showed an increased functional connectivity of sensorimotor network relative to controls during classic resting state fMRI. During resting state fMRI with sensory trick, CD-trick patients showed a decrease of sensorimotor network connectivity. During the sensory trick imagination fMRI task, CD-trick relative to CD-no-trick patients increased the recruitment of cerebellum bilaterally. This study suggests a hyper-connectivity of sensorimotor areas during resting state in CD-no-trick subjects. In CD-trick patients, the sensory trick performance was associated with a decreased connectivity of the sensorimotor network. The increased activation of cerebellum in CD-trick patients during the sensory trick imagination suggests a possible role of this area in modulating cortical activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cervical dystonia (CD) is a common form of focal dystonia characterized by involuntary twisting or turning movements of the head leading to intermittent or constant abnormal postures [1]. The sustained co-contraction of agonist and antagonist muscles such as the sternocleidomastoid and the splenius capitis interferes with voluntary movements with a consequent impact on patients’ quality of life [2]. Working capacity, working productivity and sleeping quality are often severely affected [3, 4].
The first-line treatment of CD is the botulinum toxin type A (BoNT-A) injection into the involved muscles [1, 5]. Pallidal deep brain stimulation or selective denervation are secondary treatment options when medications or botulinum fail to improve symptoms [1]. Physiotherapy can be added [1, 6]. A multimodal approach including intensive movement practice, neuromodulation combined with motor training, electromyography biofeedback training, muscular elongation and relaxation exercises, postural exercises and electrotherapy has been reported to improve head position, pain, and quality of life [7].
Up to 70–80% of CD patients have the possibility to reduce dystonic symptoms using spontaneous alleviating gestures, usually called “sensory tricks” [8]. These maneuvers consist in touching the face with fingertips to temporary ameliorate the dystonic posture or to reduce the abnormal movements causing discomfort [9]. A slight touch of the chin, cheek or neck is usually sufficient to relieve symptoms [10]. Sometimes, even the beginning or the imagination of the gesture without touching the face are described as useful to improve symptoms [11, 12].
Both the pathophysiology of CD and the mechanisms underlying sensory trick phenomenon are still debated. Several neuroimaging and neurophysiological studies have investigated the pathophysiology of primary dystonia suggesting a structural and functional alteration of the cerebello-thalamo-cortical circuit [13,14,15,16,17]. An altered cerebello-thalamo-cortical connectivity may cause a loss of inhibition at the cortical level and an excessive motor output [18]. Particularly, basal ganglia damage and their altered connections with the thalamus and brainstem can cause hyperactivity of the direct pathways and a consequent loss of inhibition of the motor cortex [19, 20]. Recent evidence confirmed the presence of abnormal patterns of cortical sensorimotor inhibitory functions in CD [21]. The presence of proprioceptive deficits in CD patients has been suggested by the correlation between the severity of the symptoms and the altered activity of the primary sensory cortex [22]. Cerebellum might also play a key role in this complex scenario because of its importance in the modulation of the primary motor cortex output. Cerebellar alterations have been reported in dystonia patients [23, 24]. A subsequent abnormal sensorimotor cortex plasticity may occur in dystonia patients [25].
A few studies using positron emission tomography (PET) and transcranial magnetic stimulation (TMS) suggested the possible role of sensory trick in modulating the increased sensorimotor cortex excitability in CD patients [26, 27]. The efficacy of sensory trick could be due to an integration of both motor and sensory inputs which together may balance the abnormal inhibition to facilitation ratio at multiple levels of the central nervous system [8, 26]. However, the functional brain networks involved in the case of an effective sensory trick are still under investigation. A better knowledge of the mechanisms underlying alleviating maneuvers could help to better understand the pathophysiology of CD and to individuate new targets of treatment.
The aims of this study were to investigate the patterns of resting state fMRI connectivity in patients with CD with and without an effective sensory trick (CD-trick and CD-no-trick), and to assess the patterns of brain fMRI activation during the imagination of sensory trick in CD-trick and CD-no-trick patients.
Methods
Participants and clinical assessment
Seventeen patients with CD (9 CD-trick and 8 CD-no-trick) and 15 matched healthy controls were enrolled. CD patients with rotational torticollis and laterocollis were included. We excluded cases with severe antero-retrocollis and head/upper limb tremor interfering with the possibility to perform MRI. Neurological evaluation included the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) (Table 1) [28]. In addition, CD patients performed a specific assessment of the head position to assess the severity of the dystonic posture using a cervical range of motion (CROM) validated instrument (Fig. 1, Table 1) [29]. CD-trick patients underwent the CROM evaluation also with the application of their own sensory trick to measure the improvement of dystonic posture [30]. Patients were asked to touch the chin, cheek or neck with the finger-tip according to their most used and effective sensory trick maneuver. Subjects were asked to perform the trick as they were used to do, without voluntary activation of antagonistic muscles and avoiding counterpressure. Both the maximal excursion of dystonia and the posture achieved with sensory trick were assessed 5 times each and a mean value was calculated to take into account the possible dystonia variability during the assessment. Through the CROM device, three dial angle meters are used to take most of the measurements: the sagittal plane meter and the lateral flexion meter are gravity meters; the rotation meter is magnetic and responds quickly to the shoulder-mounted magnetic yoke, accurately measuring cervical rotation. The CROM device permitted to precisely assess the severity of dystonia by measuring cervical rotation, lateral flexion, flexion and extension degrees and to detect the effect of sensory trick on the head position. All measures were acquired ~ 3 months after BoNT-A injection, immediately before the next injection (patients underwent BoNT-A injections every 3 months).
MRI acquisition
Structural and functional MRI scans were obtained on a 3.0 Tesla system (Intera, Philips). The following sequences were acquired:
Structural MR sequences
(1) T2-weighted spin echo (repetition time [TR] = 3000 ms, echo time [TE] = 85 ms, echo train length = 15, flip angle = 90°, matrix size = 512 × 512, field of view [FOV] = 230 × 208 mm2, 46 axial slices with thickness = 3 mm, voxel size 0.449 × 0.449 × 3 mm3); (2) three-dimensional (3D) sagittal T1-weighted fast field echo: TR = 7.1 ms, TE = 3.5 ms, echo train length = 163, flip angle = 8°, matrix size = 256 × 256, FOV = 256 × 204 mm2, 150 axial slices with thickness = 1 mm, voxel size = 1 × 1 × 1 mm3).
Resting state fMRI
(1) T2*-weighted EP imaging (EPI) sequence for “classic” resting state fMRI (TR = 3000 ms, TE = 35 ms, flip angle 90°, matrix = 128 × 128, FOV = 240 × 240 mm2, 30 axial slices with thickness = 4 mm, voxel size 1.875 × 1.875 × 4 mm3; 100 sets of images). During scanning, subjects were instructed to keep their comfortable head position, to remain motionless and to keep their eyes closed; (2) the same T2*-weighted EPI sequence for “sensory trick” resting state fMRI. CD subjects were asked to perform (CD-trick) or to simulate (CD-no-trick) the sensory trick (i.e., slight touch on the cheek/chin/neck) before starting the scan and to maintain it for the entire scanning while remaining motionless with eyes closed. Only when the maximal effect of sensory trick was achieved (when the patients were able to stay motionless with the hand on their face), the technician started to acquire the scan to avoid movements of the head during the acquisition. CD-no-trick patients simulated sensory tricks, matching one by one the CD-trick subjects;
Task-based fMRI
T2*-weighted EPI “sensory trick imagination” task: TR = 2500 ms, TE = 35 ms, flip angle 85°, matrix = 128 × 128, FOV = 240 × 240 mm2, 30 axial slices with thickness = 4 mm, voxel size 1.875 × 1.875 × 4 mm3). A block design (ABAB) was used, in which activation periods (A) alternated with resting periods (B). During activation periods (at the acoustic signal “go”), CD-trick patients were asked to imagine their own effective sensory trick while CD no-trick imagined a simulation of sensory trick. Before scanning, participants were familiarized with the experimental conditions.
MRI analysis
Resting state fMRI: pre-processing
The main pre-processing steps were performed using SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/) and REST software (https://resting-fmri.sourceforge.net/). Resting state fMRI scans were rigid-body realigned to the mean of each session to correct for minor head movements (mean absolute cumulative translation in healthy controls: x = 0.11 mm, y = 0.17 mm, z = 0.18 mm; mean rotation: x = 0.23°, y = 0.11°, z = 0.17°; mean absolute cumulative translation in CD patients during “classic” resting state: x = 0.51 mm, y = 0.53 mm, z = 0.30 mm; mean rotation: x = 0.51°, y = 0.40°, z = 0.51°; mean absolute cumulative translation in healthy controls in CD patients during “sensory trick” resting state: x = 0.47 mm, y = 0.55 mm, z = 0.51 mm; mean rotations: x = 0.57°, y = 0.51°, z = 0.57°). Realigned resting state fMRI images were then normalized to the SPM12 standard Montreal Neurological Institute (MNI) EPI template using a non-linear transformation. Linear detrending and band-pass filtering (0.01–0.08 Hz) were performed to partially remove low-frequency drifts and physiological high-frequency noise. Finally, normalized images were smoothed using a 3D 6-mm Gaussian kernel.
Resting state fMRI: independent component analysis
Resting state functional connectivity (FC) was assessed using independent component analysis (ICA) and the GIFT software (https://mialab.mrn.org/software/gift/) following three main steps: (1) data reduction, (2) group ICA, and (3) back reconstruction, as described in detail elsewhere [31]. The number of independent group components was 40, a dimension determined using the minimum description length criterion [31]. The statistical reliability of the IC decomposition was tested using the ICASSO toolbox [32].
Visual inspection of the spatial patterns, a frequency analysis of the spectra of the estimated ICs and a template-matching procedure allowed removing components clearly related to motion-related artifacts and physiological noise, and to select the resting state sensorimotor network (Fig. 2a), basal ganglia, right and left fronto-parietal networks, default mode network, visual network, visuo-associative network and cerebellum.
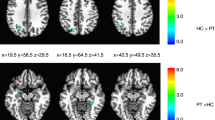
a The resting state fMRI (RS-fMRI) sensorimotor network obtained from the independent component analysis (ICA); b ICA differences between healthy controls (HC) and patients with cervical dystonia without sensory trick (CD-no-trick) during the “classic” RS-fMRI; c ICA differences between the “classic” RS-fMRI and the “sensory trick” RS-fMRI in patients with cervical dystonia with sensory trick (CD-trick); d seed-based functional connectivity of the BA4: differences between CD-trick and HC during a “classic” resting state fMRI; e seed-based functional connectivity of the BA4: differences between CD-no-trick and CD-trick during a “classic” resting state fMRI. Results are shown on axial sections of the Montreal Neurological Institute standard brain. Colour bars denote T values
Resting state fMRI: seed-based functional connectivity analysis
Statistical maps of resting state FC between the left and right primary motor cortex, supplementary motor area and cerebellum, separately, and the remaining voxels of the brain were obtained for each subject using a seed-region correlation approach. We decided to further explore seed-based FC of specific sensorimotor areas with the whole brain because the ICA analysis showed significant results only within the sensorimotor network (see “Results”). Seeds of the primary motor cortex, supplementary motor area and cerebellum were obtained using the masks of L and R Brodmann Areas (BA) 4, 6 and cerebellum included in the WFU PickAtlas toolbox (https://fmri.wfubmc.edu/software/PickAtlas). Then, resting state FC was investigated by calculating the correlation coefficients between the time series extracted from the L and R seeds and any other voxel in the brain. A Fisher’s z transform was used to improve the gaussianity of the obtained correlation coefficients.
Task-based fMRI
Changes in blood oxygenation level dependent (BOLD) contrast associated with the performance of the tasks were assessed on a pixel-by-pixel basis, using the general linear model and the theory of Gaussian fields. FMRI data were analyzed using the statistical parametric mapping (SPM12) software. Prior to statistical analysis, all images were realigned to the first one to correct for subject motion, spatially normalized into the standard space of SPM, and smoothed with a 10-mm, 3D-Gaussian filter. A first-level design matrix, where motion parameters were used as regressors of non-interest, was built. Then, specific effects were tested applying appropriate linear contrasts (i.e., BOLD changes occurring during sensory trick imagination in each subject). Significant hemodynamic changes were assessed using t statistical parametric maps (SPMt).
Statistical analysis
Demographic and clinical data
Demographic and clinical data were compared between groups using the Mann Whitney test or Chi squared test. Within the CD-trick groups, CROM values with and without sensory trick application were compared using the Wilcoxon test.
Resting state fMRI
Individual resting state maps derived from ICA and seed-based FC maps were entered into SPM12 random-effect analysis to assess significant within-group FC (one-sample t test) and between-group differences (two-sample t test). Specifically, one-sample t test (p < 0.05, family wise error [FWE] corrected for multiple comparisons) was used to assess the average fMRI activity during the resting state with and without sensory trick execution. A second-level random-effect analysis was performed to assess differences between CD groups and healthy controls during the “classic” resting state fMRI. A second-level random-effect analysis was performed to assess differences between CD groups (CD-trick vs CD-no-trick) during the resting state fMRI with the sensory trick execution. Within-group changes after the sensory trick application were evaluated using paired t tests (“classic” resting state vs “sensory trick” resting state). Significant results were corrected at the cluster level using small volume correction (SVC) for multiple comparisons (10 mm radius, cut off value for significance p < 0.05).
Task-based fMRI
One-sample t test in SPM was used to assess the average fMRI activity during the “sensory trick imagination” task in each group (p < 0.05, FWE corrected). A second-level random-effect analysis was performed to assess differences between CD groups (CD-trick vs CD-no-trick) during the task. Significant results were corrected at the cluster level using SVC for multiple comparisons (10 mm radius, cut off value for significance p < 0.05).
Clinical-fMRI correlations
Multiple linear regression models were used to assess the correlations between fMRI changes (“classic” vs “sensory trick” resting state) and CROM showing significant changes after sensory trick execution (rotation and laterocollis) in DYT-trick patients.
Results
None of the study participants were excluded from analysis because of motion artifacts. Head translations and rotations were not significant (mean absolute cumulative translation and rotation < 0.6 mm and degrees, respectively) both in healthy controls and CD patients during the fMRI scans.
Clinical data
CD patients and healthy controls were matched for age and sex (Table 1). CD-trick and CD-no-trick patients did not differ in terms of clinical variables (disease duration, therapy duration and number of treatment cycles), except for the TWSTRS values which were higher in the CD-trick group (Table 1). All CD-trick patients showed an effective maneuver ipsilaterally to head rotation, only two patients showed also a contralateral sensory trick but less effective. For this reason, all the patients were evaluated using their ipsilateral sensory trick. The evaluation using CROM showed that CD-trick and CD-no-trick patients were comparable in terms of torticollis severity at rest. As expected, CD-trick patients significantly reduced the rotation and laterocollis CROM during the sensory trick application (Table 1).
Independent component analysis functional connectivity
Only the sensorimotor network showed significant alterations. During the classic resting state fMRI, CD-no-trick patients showed an increased FC of the right premotor/primary motor cortices and supramarginal gyrus relative to healthy controls (Fig. 2b; Table 2). No differences were observed between CD-trick and healthy controls. Only CD-trick subjects showed an effect of sensory trick in terms of reduced FC of the right pre/postcentral areas relative to resting state fMRI without trick (Fig. 2c; Table 2).
Seed-based functional connectivity
Classic resting state fMRI (no sensory trick)
During classic resting state fMRI, CD-trick patients showed a decreased FC of bilateral BA 4 with fronto-parietal areas bilaterally, left superior occipital cortex and right cerebellum 4–5 relative to healthy controls (Fig. 2d; Table 3). CD-no-trick patients showed no differences relative to healthy controls. CD-no-trick relative to CD-trick patients showed an increased FC of left BA 4 with right postcentral gyrus, left precuneus and right superior/inferior temporal gyri and between right BA 4 and bilateral supplementary motor area, right insula and left cerebellum crus I (Fig. 2e; Table 3).
Resting state fMRI with sensory trick
During resting state fMRI with sensory trick, CD-trick showed a decreased FC of bilateral BA 4 with frontal, parietal and occipital areas relative to CD-no-trick patients (Fig. 3a; Table 3).
a Seed-based functional connectivity of the BA4: differences between patients with cervical dystonia with sensory trick (CD-trick) and without sensory trick (CD-no-trick) during “sensory trick” resting state fMRI; b seed-based functional connectivity of the BA4: differences between “classic” and “sensory trick” resting state fMRI in CD-trick patients. Results are shown on axial sections of the Montreal Neurological Institute standard brain. Colour bars denote T values
Resting state fMRI with vs without sensory trick
CD-trick patients, performing sensory trick, showed a reduced FC between bilateral BA 4 and frontal, parietal and occipital areas and an increased FC of left BA 4 with left caudate and amygdala was observed during resting state fMRI with trick relative to resting state without trick (Fig. 3b; Table 3).
CD-no-trick subjects, during resting state with the simulated sensory trick relative to resting without trick, showed a reduced FC of left BA 4 with cerebellar cortex (just few spots in crus II and VIIb) and an increased FC between left BA 4 and right inferior frontal operculum (Table 3).
Task-fMRI: sensory trick imagination task
Figure 4a shows the fMRI patterns of activation in CD patients during the sensory trick imagination fMRI task. CD-trick relative to CD-no-trick patients had an increased recruitment of right (x = 38; y = − 62; z = − 34; T value = 4.05) and left (x = − 30; y = − 64; z = − 36; T value = 5.27) cerebellum crus I (Fig. 4b).
a fMRI patterns of activations in patients with cervical dystonia with and without sensory trick during a “sensory trick imagination” task; b Differences in fMRI patterns of activations between patients with cervical dystonia with sensory trick (CD-trick) and without (CD-no-trick) during a “sensory trick imagination” task. Results are shown on an axial section of the Montreal Neurological Institute standard brain. Colour bar denotes T values
Correlations
ICA fMRI results did not show any correlation with CROM values. Seed-based fMRI analysis showed that CROM improvement during the sensory trick execution was correlated with FC changes between classic and sensory trick resting state in CD-trick subjects. Lower values of rotational CROM (lower severity of dystonic symptoms using sensory trick) correlated with a higher reduction of FC between right BA 4 and left postcentral gyrus (BA = 1; x = − 48, y = − 23, z = 30; T value = 7.32; r = − 0.93), superior frontal gyrus (BA = 10; x = − 20, y = 62, z = 22; T value = 9.79; r = − 0.88) and precuneus (BA = 31; x = − 5, y = − 55, z = 43; T value = 11.48; r = − 0.86).
Discussion
This study investigates brain fMRI patterns during resting state, sensory trick simulation and sensory trick imagination in CD patients both with and without an effective sensory trick. Results showed that CD patients had an overall increased resting state functional connectivity of the sensorimotor network. However, considering CD-trick and CD-no-trick cases separately, different patterns of resting state brain connectivity alterations were observed: CD-no-trick patients showed not only an increased functional connectivity within the sensorimotor network (premotor and primary motor cortices) compared to healthy controls but also between BA4 and parietal, temporal and cerebellar regions relative to CD-trick patients; on the other hand, CD-trick patients showed a decreased functional connectivity between BA4 and frontal, parietal, occipital and cerebellar areas relative to healthy controls. In CD-trick patients, the sensory trick was associated with a functional connectivity modulation both within the sensorimotor network and between sensorimotor and frontal, parietal and occipital areas. Interestingly, the improvement of dystonic posture during the sensory trick execution in CD-trick patients was correlated with the functional connectivity decrease between BA4 and frontal/parietal areas.
These findings support the hypothesis that CD is a network disorder involving not only the sensorimotor network but also executive and visual circuits [33, 34]. Previous resting state fMRI results have already suggested a functional miscommunication between different brain areas in CD patients showing both increased and decreased functional connectivity alterations [34,35,36]. Particularly, a decreased functional connectivity of the sensorimotor and visual networks and an increased functional connectivity of the executive network were found in CD patients [34,35,36]. Our results are only partially in line with previous findings. Indeed, we found an overall increased connectivity of the sensorimotor network, which was mainly driven by the CD-no-trick group. On the contrary, we found that patients with an effective sensory trick had a reduced connectivity between sensorimotor areas and visual/executive areas, which was further reduced with the sensory trick execution. We can speculate that CD-trick patients have the possibility to frequently modulate the functional connectivity of sensorimotor, visual and executive circuits through sensory trick. It is well known that the sensory trick has a temporary effect, however these patients use the sensory trick very often in their daily life. Thus we can hypothesized that they constantly modulate their brain activity reducing the hyper-connectivity of sensorimotor areas. Also preliminary PET findings suggested that sensory trick reduces the recruitment of the supplementary motor area and primary sensorimotor cortex contralateral to the side of CD and improves the activation of the ipsilateral parietal cortex [27].
Some evidence showed that patients with CD have proprioceptive and somatosensory integration deficits [37,38,39,40], which are less prominent in patients presenting an effective alleviating maneuver [39]. Thus, sensory trick could improve the integration of peripheral sensory input, which could modulate the motor cortex output [41]. Recent evidence confirmed the presence of different proprioceptive processing in CD patients with and without an effective sensory trick, reinforcing the hypothesis about different pathophysiological mechanisms in CD subgroups [42].
The cerebellum is highly involved in the modulation of the primary motor cortex output by integrating sensorimotor information, correcting abnormal patterns of movements, supporting executive functions and contributing to the generation of pre-programmed motor patterns [20]. Different studies showed structural, functional and metabolic cerebellar alterations in primary dystonia patients and a miscommunication between cerebellum and basal ganglia in CD subjects [23, 24, 43, 44]. Also evidence from the stroke literature suggested that patients with cerebellar lesions can present dystonic symptoms [45]. Moreover, post-mortem studies confirmed a loss of cerebellar Purkinje cells in CD patients [46]. Thus, there is a growing interest in studying the potential role of the cerebellum in the pathophysiology of dystonia and in the mechanisms underlying sensory trick efficacy [20, 47]. In this study, we found that CD-no-trick patients had an increased connectivity between BA4 and cerebellum at rest relative to CD-trick cases. We also found that the sensory trick imagination elicited an increased activation of the cerebellum in CD-trick patients. To date, it is not clear if the cerebellar involvement in CD subjects is causal, contributory, or compensatory [48]. We hypothesized that the increased recruitment of the cerebellum during the sensory trick imagination might play a compensatory role to inhibit the abnormal motor output and to reduce dystonic manifestations. Accordingly, preliminary studies suggested that non-invasive cerebellar stimulation with TMS could be a promising way to modulate CD symptoms [49, 50]. Probably, CD-trick subjects have the possibility to modulate the activity of the cerebellum during the sensory trick execution and thus they preserve an “adaptation” ability, which is considered a type of motor learning [47]. If this is the case, we can speculate that CD-trick patients might have better chances to respond to rehabilitation approaches [7] such as physiotherapy and TMS relative to CD-no-trick patients. Thus, intensive movement practice together with neuromodulation could enhance motor re-learning particularly in CD subjects with a preserved ability to adapt the sensorimotor circuit such as CD-trick patients. On the other hand, the increased activity of the cerebellum during imagination of sensory trick in the CD-trick group could reflect a higher sensorimotor and working memory ability because these subjects repeat the gesture every day. Future studies should also investigate patients with a partial sensory trick effect or patients who lost the sensory trick effectiveness to better clarify the role of cerebellum.
This study is not without limitations. First, the sample size is small and consequently the fMRI analysis are corrected at a cluster level, but it is important to consider that CD patients fulfilling the criteria to perform fMRI are rare (we excluded patients with important antero-retrocollis and head tremor interfering with the possibility to perform MRI). Moreover, CD was relatively more severe in CD-trick relative to CD-no-trick patients. We think that this heterogeneity could only partially affect our results because, despite the more severe symptoms, CD-trick patients have the potential to ameliorate dystonia and to modulate brain functional connectivity during sensory trick execution. Furthermore, the correlation between fMRI results and CD improvement (objectively evaluated using the CROM assessment) during sensory trick execution supports the specificity of our findings. Second, the lack of standardization of fMRI stimuli is a critical point of our experiment. By definition, the effective maneuvers were different among CD-trick patients. Although sensory tricks simulated (or imagined) by CD-no-trick patients were matched one by one with those of CD-trick subjects, we cannot rule out that the variability of the stimuli had impacted our results. Moreover, the supine position maintained to lying down in the scanner could partially alleviate CD; thus, the occiput touching the bed could work as a sensory trick per se [10]. As we noticed that both CD-trick and CD-no-trick patients showed a slight alleviation of dystonia lying down in the scanner without a complete resolution and with no apparent differences between groups, we can hypothesize that the different fMRI patterns observed in the two patient populations are not directly related with the position during the scan.
In conclusion, this study demonstrates two different patterns of brain FC in CD patients with and without an effective sensory trick. This study contributes to the current knowledge about the mechanisms underlying alleviating maneuvers in CD and could suggest new targets of treatment.
Data sharing
The dataset used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Albanese A, Abbruzzese G, Dressler D, Duzynski W, Khatkova S, Marti MJ, Mir P, Montecucco C, Moro E, Pinter M, Relja M, Roze E, Skogseid IM, Timerbaeva S, Tzoulis C (2015) Practical guidance for CD management involving treatment of botulinum toxin: a consensus statement. J Neurol 262:2201–2213
Girach A, Vinagre Aragon A, Zis P (2019) Quality of life in idiopathic dystonia: a systematic review. J Neurol 266:2897–2906
Avanzino L, Martino D, Marchese R, Aniello MS, Minafra B, Superbo M, Defazio G, Abbruzzese G (2010) Quality of sleep in primary focal dystonia: a case-control study. Eur J Neurol 17:576–581
Molho ES, Stacy M, Gillard P, Charles D, Adler CH, Jankovic J, Schwartz M, Brin MF (2016) Impact of cervical dystonia on work productivity: an analysis from a patient registry. Mov Disord Clin Pract 3:130–138
Castelao M, Marques RE, Duarte GS, Rodrigues FB, Ferreira J, Sampaio C, Moore AP, Costa J (2017) Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst Rev 12:CD003633
Delnooz CC, Horstink MW, Tijssen MA, van de Warrenburg BP (2009) Paramedical treatment in primary dystonia: a systematic review. Mov Disord 24:2187–2198
Prudente CN, Zetterberg L, Bring A, Bradnam L, Kimberley TJ (2018) Systematic review of rehabilitation in focal dystonias: classification and recommendations. Mov Disord Clin Pract 5:237–245
Ramos VF, Karp BI, Hallett M (2014) Tricks in dystonia: ordering the complexity. J Neurol Neurosurg Psychiatry 85:987–993
Poisson A, Krack P, Thobois S, Loiraud C, Serra G, Vial C, Broussolle E (2012) History of the 'geste antagoniste' sign in cervical dystonia. J Neurol 259:1580–1584
Schramm A, Reiners K, Naumann M (2004) Complex mechanisms of sensory tricks in cervical dystonia. Mov Disord 19:452–458
Wissel J, Muller J, Ebersbach G, Poewe W (1999) Trick maneuvers in cervical dystonia: investigation of movement- and touch-related changes in polymyographic activity. Mov Disord 14:994–999
Greene PE, Bressman S (1998) Exteroceptive and interoceptive stimuli in dystonia. Mov Disord 13:549–551
de Vries PM, Johnson KA, de Jong BM, Gieteling EW, Bohning DE, George MS, Leenders KL (2008) Changed patterns of cerebral activation related to clinically normal hand movement in cervical dystonia. Clin Neurol Neurosurg 110:120–128
Lokkegaard A, Herz DM, Haagensen BN, Lorentzen AK, Eickhoff SB, Siebner HR (2016) Altered sensorimotor activation patterns in idiopathic dystonia-an activation likelihood estimation meta-analysis of functional brain imaging studies. Hum Brain Mapp 37:547–557
Opavsky R, Hlustik P, Otruba P, Kanovsky P (2011) Sensorimotor network in cervical dystonia and the effect of botulinum toxin treatment: a functional MRI study. J Neurol Sci 306:71–75
Prell T, Peschel T, Kohler B, Bokemeyer MH, Dengler R, Gunther A, Grosskreutz J (2013) Structural brain abnormalities in cervical dystonia. BMC Neurosci 14:123
Prudente CN, Stilla R, Singh S, Buetefisch C, Evatt M, Factor SA, Freeman A, Hu XP, Hess EJ, Sathian K, Jinnah HA (2016) A functional magnetic resonance imaging study of head movements in cervical dystonia. Front Neurol 7:201
Lozeron P, Poujois A, Richard A, Masmoudi S, Meppiel E, Woimant F, Kubis N (2016) Contribution of TMS and rTMS in the understanding of the pathophysiology and in the treatment of dystonia. Front Neural Circ 10:90
Hallett M (2004) Dystonia: abnormal movements result from loss of inhibition. Adv Neurol 94:1–9
Kaji R, Bhatia K, Graybiel AM (2018) Pathogenesis of dystonia: is it of cerebellar or basal ganglia origin? J Neurol Neurosurg Psychiatry 89:488–492
Ganos C, Ferre ER, Marotta A, Kassavetis P, Rothwell J, Bhatia KP, Haggard P (2018) Cortical inhibitory function in cervical dystonia. Clin Neurophysiol 129:466–472
Burciu RG, Hess CW, Coombes SA, Ofori E, Shukla P, Chung JW, McFarland NR, Wagle Shukla A, Okun MS, Vaillancourt DE (2017) Functional activity of the sensorimotor cortex and cerebellum relates to cervical dystonia symptoms. Hum Brain Mapp 38:4563–4573
Filip P, Gallea C, Lehericy S, Bertasi E, Popa T, Marecek R, Lungu OV, Kasparek T, Vanicek J, Bares M (2017) Disruption in cerebellar and basal ganglia networks during a visuospatial task in cervical dystonia. Mov Disord 32:757–768
Jinnah HA, Hess EJ (2006) A new twist on the anatomy of dystonia: the basal ganglia and the cerebellum? Neurology 67:1740–1741
Quartarone A, Morgante F, Sant'angelo A, Rizzo V, Bagnato S, Terranova C, Siebner HR, Berardelli A, Girlanda P (2008) Abnormal plasticity of sensorimotor circuits extends beyond the affected body part in focal dystonia. J Neurol Neurosurg Psychiatry 79:985–990
Amadio S, Houdayer E, Bianchi F, Tesfaghebriel Tekle H, Urban IP, Butera C, Guerriero R, Cursi M, Leocani L, Comi G, Del Carro U (2014) Sensory tricks and brain excitability in cervical dystonia: a transcranial magnetic stimulation study. Mov Disord 29:1185–1188
Naumann M, Magyar-Lehmann S, Reiners K, Erbguth F, Leenders KL (2000) Sensory tricks in cervical dystonia: perceptual dysbalance of parietal cortex modulates frontal motor programming. Ann Neurol 47:322–328
Albanese A, Sorbo FD, Comella C, Jinnah HA, Mink JW, Post B, Vidailhet M, Volkmann J, Warner TT, Leentjens AF, Martinez-Martin P, Stebbins GT, Goetz CG, Schrag A (2013) Dystonia rating scales: critique and recommendations. Mov Disord 28:874–883
Capuano-Pucci D, Rheault W, Aukai J, Bracke M, Day R, Pastrick M (1991) Intratester and intertester reliability of the cervical range of motion device. Arch Phys Med Rehabil 72:338–340
Williams MA, Williamson E, Gates S, Cooke MW (2012) Reproducibility of the cervical range of motion (CROM) device for individuals with sub-acute whiplash associated disorders. Eur Spine J 21:872–878
Calhoun VD, Adali T, Pearlson GD, Pekar JJ (2001) A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp 14:140–151
Himberg J, Hyvarinen A, Esposito F (2004) Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage 22:1214–1222
Battistella G, Termsarasab P, Ramdhani RA, Fuertinger S, Simonyan K (2017) Isolated focal dystonia as a disorder of large-scale functional networks. Cereb Cortex 27:1203–1215
Li Z, Prudente CN, Stilla R, Sathian K, Jinnah HA, Hu X (2017) Alterations of resting-state fMRI measurements in individuals with cervical dystonia. Hum Brain Mapp 38:4098–4108
Delnooz CC, Pasman JW, Beckmann CF, van de Warrenburg BP (2013) Task-free functional MRI in cervical dystonia reveals multi-network changes that partially normalize with botulinum toxin. PLoS ONE 8:e62877
Delnooz CC, Pasman JW, Beckmann CF, van de Warrenburg BP (2015) Altered striatal and pallidal connectivity in cervical dystonia. Brain Struct Funct 220:513–523
Abbruzzese G, Marchese R, Buccolieri A, Gasparetto B, Trompetto C (2001) Abnormalities of sensorimotor integration in focal dystonia: a transcranial magnetic stimulation study. Brain 124:537–545
Antelmi E, Erro R, Rocchi L, Liguori R, Tinazzi M, Di Stasio F, Berardelli A, Rothwell JC, Bhatia KP (2017) Neurophysiological correlates of abnormal somatosensory temporal discrimination in dystonia. Mov Disord 32:141–148
Patel N, Hanfelt J, Marsh L, Jankovic J, members of the Dystonia C (2014) Alleviating manoeuvres (sensory tricks) in cervical dystonia. J Neurol Neurosurg Psychiatry 85:882–884
Tinazzi M, Fiorio M, Fiaschi A, Rothwell JC, Bhatia KP (2009) Sensory functions in dystonia: insights from behavioral studies. Mov Disord 24:1427–1436
Kagi G, Katschnig P, Fiorio M, Tinazzi M, Ruge D, Rothwell J, Bhatia KP (2013) Sensory tricks in primary cervical dystonia depend on visuotactile temporal discrimination. Mov Disord 28:356–361
Brugger F, Peters A, Georgiev D, Kagi G, Balint B, Bhatia KP, Day BL (2019) Sensory trick efficacy in cervical dystonia is linked to processing of neck proprioception. Parkinsonism Relat Disord 61:50–56
Delmaire C, Vidailhet M, Elbaz A, Bourdain F, Bleton JP, Sangla S, Meunier S, Terrier A, Lehericy S (2007) Structural abnormalities in the cerebellum and sensorimotor circuit in writer's cramp. Neurology 69:376–380
Calderon DP, Fremont R, Kraenzlin F, Khodakhah K (2011) The neural substrates of rapid-onset dystonia-parkinsonism. Nat Neurosci 14:357–365
Kojovic M, Parees I, Kassavetis P, Palomar FJ, Mir P, Teo JT, Cordivari C, Rothwell JC, Bhatia KP, Edwards MJ (2013) Secondary and primary dystonia: pathophysiological differences. Brain 136:2038–2049
Zoons E, Tijssen MA (2013) Pathologic changes in the brain in cervical dystonia pre- and post-mortem—a commentary with a special focus on the cerebellum. Exp Neurol 247:130–133
Sadnicka A, Patani B, Saifee TA, Kassavetis P, Parees I, Korlipara P, Bhatia KP, Rothwell JC, Galea JM, Edwards MJ (2014) Normal motor adaptation in cervical dystonia: a fundamental cerebellar computation is intact. Cerebellum 13:558–567
Shakkottai VG, Batla A, Bhatia K, Dauer WT, Dresel C, Niethammer M, Eidelberg D, Raike RS, Smith Y, Jinnah HA, Hess EJ, Meunier S, Hallett M, Fremont R, Khodakhah K, LeDoux MS, Popa T, Gallea C, Lehericy S, Bostan AC, Strick PL (2017) Current opinions and areas of consensus on the role of the cerebellum in dystonia. Cerebellum 16:577–594
Koch G, Porcacchia P, Ponzo V, Carrillo F, Caceres-Redondo MT, Brusa L, Desiato MT, Arciprete F, Di Lorenzo F, Pisani A, Caltagirone C, Palomar FJ, Mir P (2014) Effects of two weeks of cerebellar theta burst stimulation in cervical dystonia patients. Brain Stimul 7:564–572
Franca C, de Andrade DC, Teixeira MJ, Galhardoni R, Silva V, Barbosa ER, Cury RG (2018) Effects of cerebellar neuromodulation in movement disorders: a systematic review. Brain Stimul 11:249–260
Funding
None.
Author information
Authors and Affiliations
Contributions
ES: substantial contributions to the conception of the work. Acquisition, analysis and interpretation of data. Drafting the work and revising it critically for important intellectual content. Final approval of the version published. Agreement to be accountable for all aspects of the work. FA: substantial contributions to the conception and design of the work. Interpretation of data. Revising the work critically for important intellectual content. Final approval of the version published. Agreement to be accountable for all aspects of the work. NP: substantial contributions to the conception of the work. Analysis of data. Revising the work critically for important intellectual content. Final approval of the version published. Agreement to be accountable for all aspects of the work. FB and CB: substantial contributions to the conception of the work. Acquisition of data. Revising the work critically for important intellectual content. Final approval of the version published. Agreement to be accountable for all aspects of the work. RG: substantial contributions to the conception of the work. Revising the work for important intellectual content. Final approval of the version published. Agreement to be accountable for all aspects of the work. SA: substantial contributions to the conception of the work. Acquisition of data. Revising the work critically for important intellectual content. Final approval of the version published. Agreement to be accountable for all aspects of the work. UDC substantial contributions to the conception of the work. Acquisition of data. Revising the work critically for important intellectual content. Final approval of the version published. Agreement to be accountable for all aspects of the work. MF: substantial contributions to the conception and design of the work. Interpretation of data. Revising the work critically for important intellectual content. Final approval of the version published. Agreement to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflicts of interest
E. Sarasso, N. Piramide, F. Bianchi, C. Butera, R. Gatti, S. Amadio, U. Del Carro report no disclosures. F. Agosta is Section Editor of NeuroImage: Clinical; has received speaker honoraria from Philips, Novartis and Biogen Idec; and receives or has received research supports from the Italian Ministry of Health, AriSLA (Fondazione Italiana di Ricerca per la SLA), and the European Research Council. Prof. Filippi is Editor-in-Chief of the Journal of Neurology; received compensation for consulting services and/or speaking activities from Biogen Idec, Merck-Serono, Novartis, Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Merck-Serono, Novartis, Teva Pharmaceutical Industries, Roche, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA).
Ethical standards
All procedures performed in the study involving human participants are in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All participants provided written informed consent prior to study inclusion.
Rights and permissions
About this article
Cite this article
Sarasso, E., Agosta, F., Piramide, N. et al. Sensory trick phenomenon in cervical dystonia: a functional MRI study. J Neurol 267, 1103–1115 (2020). https://doi.org/10.1007/s00415-019-09683-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09683-5