Abstract
Background
Parkinsonian syndromes are characterized by a wide spectrum of non-motor symptoms. A few studies explored cognitive deficits and neuropsychiatric symptoms in atypical parkinsonism compared to Parkinson’s disease (PD). The study was performed to identify cognitive and neuropsychiatric differences between PD, multiple system atrophy (MSA) and progressive supranuclear palsy (PSP) and to evaluate the influence of clinical features, depressive symptomatology and apathy on cognitive performances in the three groups.
Methods
Fifty-five PD, 44 MSA and 42 PSP patients underwent cognitive tests assessing attention, language, memory, visuospatial and executive functions as well as scales assessing depression and apathy. Out of these patients, 20 PD, 20 MSA and 20 PSP patients were selected to be matched for age, education and global cognitive status. Within each whole patients group, correlational analysis was performed between clinical, behavioural and cognitive parameters.
Results
The main difference among the groups matched was on cognitive tests exploring verbal learning, executive and linguistic functions. The PSP group was more impaired than the PD and MSA groups on cognitive tests assessing executive functions. On the other hand, MSA group obtained similar cognitive performance to the PD group. As to behavioural symptoms, in whole PSP and MSA groups, apathy and depression were more severe than in PD group, while apathy (but not depression) were more severe in the PSP group as compared to the MSA group.
Conclusions
The present study underlined the pervasiveness of cognitive deficits, apathy and depressive symptoms in PSP, whereas little cognitive differences were found between PD and MSA. The findings indirectly supported a dysfunction of prefronto-subcortical circuitries (i.e., dorsolateral prefrontal and limbic circuits) in PSP and PD. Cognitive similarities between MSA and PD reinforced the pivotal role of altered basal ganglia and corresponding frontal deafferentation in the occurrence of the cognitive deficits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinsonian syndromes include Parkinson’s disease (PD) as well as atypical parkinsonism such as multiple system atrophy (MSA), progressive supranuclear palsy (PSP) and corticobasal degeneration, all considered proteinopathies with distinctive features [1].
Neuropsychological profile in PD is very heterogeneous and is characterized mainly by frontal-executive dysfunction. Notwithstanding, a subgroup of PD patients more prone to develop dementia shows prominent cholinergic cortical dysfunctions [2]. As opposite to PD, few studies explored cognitive deficits associated with atypical Parkinsonism. The majority of available data focused on the comparison between either MSA or PSP and either PD or healthy subjects, with very few studies examining similarities and differences in cognitive functions by a simultaneous comparison of PD, MSA and PSP subjects [3,4,5,6]. In addition, the examination of language and memory abilities has been largely neglected in favour of the assessment of executive and visuospatial functions [7,8,9,10]. Robbins et al. [10] found impaired executive functions in subjects with Parkinsonism compared with a control group but did not analyse in detail the differences between each group of Parkinsonian subjects. Monza et al. [9] compared small groups of PSP, MSA and PD patients matched for demographic and disease-related variables (although with longer disease duration for PD) and showed ideomotor apraxia, frontal and visuospatial dysfunctions in PSP patients compared to MSA and PD patients. As a drawback, this study lacked of assessments for attention and language domains. Other studies found significant differences comparing PD, MSA and PSP patients on frontal and verbal fluency tasks [7, 8]. These studies were limited by the sample size and the lack of assessments for other cognitive domains besides executive functions.
Neurobehavioural disturbances represent frequent non-motor complains in Parkinsonian syndromes. While an extensive amount of the literature is available for PD, little is known about clinical correlates and nature of the psychopathology (particularly depression and apathy) in atypical Parkinsonism compared to PD [11, 12].
Aims of the present study were to identify differences and similarities in cognitive and neuropsychiatric symptoms of PD and atypical Parkinsonism (i.e., MSA and PSP) and to investigate the possible influence of clinical parameters, depressive symptomatology and apathy on cognitive performances in each of the three patient groups. A better characterization of the behavioural abnormalities and/or cognitive deficits in distinct types of Parkinsonian syndromes can potentially improve the clinical care and management of these patients. Moreover, better neuropsychological profiling in Parkinsonian syndromes might help to provide a basis on which to plan any cognitive remediation interventions.
Methods
Participants
In the present study, we enrolled consecutive outpatients with clinically probable diagnosis of idiopathic PD, MSA, and PSP according to published clinical criteria (for PD [13]; for MSA [14]; for PSP: [15]). All the participants were recruited at our Center for Neurodegenerative Diseases. We excluded patients affected by (1) radiological structural brain abnormalities not compatible with a diagnosis of a neurodegenerative syndrome, (2) a history of alcohol or substance abuse, (3) previous head trauma with loss of consciousness, with significant neurological or psychiatric comorbidities that might confound the results (4) any diseases causing significant physical disabilities impacting a neuropsychological assessment.
Participants gave their written informed consent to the study which was approved by the appropriate ethics committee and therefore was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Material and procedures
Demographic aspects, disease duration, levodopa equivalent daily dose (LEDD; [16]), functional autonomy in activity of daily living (ADL [17]) and instrumental ADL (IADL [18]), were collected; severity of motor symptoms was evaluated by Unified Parkinson’s Disease Rating Scale part III (UPDRS-III [19]) for PD group, by Unified Multiple System Atrophy Rating Scale (UMSARS [20]) for MSA group, and by Progressive Supranuclear Palsy Rating Scale (PSP-RS [21]) for PSP group.
Neuropsychological assessment
Cognition
All participants underwent the Italian version of the Montreal Cognitive Assessment (MoCA [22]) and standardized neuropsychological tasks for assessment of several frontal/executive functions (by Trail Making Test-B and B-A, TMT, [23] to evaluate set shifting; phonological fluency test [24] to evaluate cognitive flexibility; interference task of Stroop Color-Word Test, Stroop [25] to evaluate inhibitory control; Clock Drawing Test, CDT [26]; immediate and delayed copying tests of Rey–Osterrieth complex figure test [27] to evaluate spatial organization and planning); memory (verbal long-term memory by immediate and delayed recall of the Rey’s auditory 15-word learning test, RAVLT [24]); language (Semantic fluency task [28]; auditory and visual comprehension of single word tasks, and words, non-words and sentence repetition tasks [29]); visuospatial perceptual and constructional functions (by Benton Judgment of Lines Orientation Task, BJLOT [30], and Constructional Apraxia Task, CAT [31]).
Neuropsychiatric assessment
To assess depressive symptomatology and apathy, all patients completed the Italian version of the Beck Depression Inventory-II (BDI-II [32]) and Apathy Evaluation Scale (AES), validated in Parkinsonian syndromes [33].
Statistical analysis
An a priori power analysis was performed with G*Power 3.1 by setting the following parameters: probability level (a) of 0.05, statistical power (1 − b) of 0.80, large effect size Cohen’s f of 0.40 for the Kruskal–Wallis test, and rho of 0.5 for Spearman’s correlation analysis. According to Pitman [34], the sample size required for a nonparametric test is determined by multiplying the sample size calculated for the equivalent parametric test by a correction factor.
Differences in the distribution of categorical variables among groups were assessed by means of Chi square. Group comparisons on demographic, clinical, cognitive and behavioural variables were performed by nonparametric tests (Kruskal–Wallis H test to compare three samples, and the Mann–Whitney U test to compare two samples) to avoid biases due to the small sample size. To avoid type-II errors we used a conservative statistical approach by applying Bonferroni’s correction (p = 0.0026).
The correlations between neuropsychological performances (raw scores) and clinical parameters, depressive symptoms and apathy in each patient group were performed by Spearman’s rank-order correlation. The significance level was set at pre-specified threshold (p < 0.010). Analyses were performed with SPSS version 21 (SPSS Inc. Chicago, IL, USA).
Results
Fifty-five (16 females) PD, 44 (22 females) MSA patients and 42 (17 females) PSP patients were enrolled; these groups differed on age and education but not gender (χ2 = 4.546, p = 0.103): PSP was the oldest while MSA was the youngest group. As for educational level, PD patients had higher educational level than PSP patients (Table 1). All PD patients were on levodopa reporting a significant improvement in motor symptoms. Sixteen/42 PSP and 10/44 MSA patients were not taking levodopa preparations. Functional autonomy was greater in PD compared with PSP and MSA; the PSP group showed the worst functional autonomy score. The mean UPDRS-III score for PD group was 14.6 ± 9.5; the mean PSP-RS score for PSP group was 40.9 ± 17.9; the mean UMSARS-I, II and IV score for MSA group were 22 ± 8.9, 23.2 ± 0.9; 2.7 ± 0.9. Finally, the H test showed significant differences among the three groups on total MoCA (Table 1). The descriptive of demographic, clinical and neuropsychological parameters of each patients group is reported in Supplementary Material 1.
Since PD, PSP and MSA patients showed significant differences on age, education and MoCA, we selected eligible cases by scrutiny of these abovementioned parameters to control for the potential bias. Moreover, since the a priori power analysis revealed that at least 60 individuals (20 individuals for each group) for the Kruskal–Wallis test were needed to attain a large effect size at a statistical power of 0.80 and an alpha level of 0.05, we selected 20 patients for each group who were matched as closely as possible between them for demographic features and global cognitive functioning. The three groups of PSP patients, PD patients and MSA patients were compared on cognitive and neuropsychiatric scores.
Neuropsychological assessment
Cognition
The results showed that the three groups matched for age, education and MoCA score had significantly different performance on immediate RAVLT, phonological fluency tests, immediate copy of ROCF, TMT-B, TMT:B-A, time to complete the Stroop test (Table 2). In particular, PSP patients had lower score on all cognitive tests than PD and had poorer performance on Stroop test and TMT than MSA; finally PD patients obtained similar cognitive performance to MSA patients. The percentage of the patients with pathological performance with respect to Italian normative data within each group and between groups was reported in Table 3.
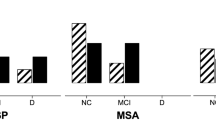
In the three groups, the linguistic and executive domains were the most damaged cognitive domains (Fig. 1). Moreover, as for executive functions, in PD and MSA group spatial planning was the most damaged executive function. In PSP group, both spatial planning and set shifting were the most damaged executive functions (Fig. 2).
Neuropsychiatric assessment
The three groups had significantly different scores on AES and BDI (Table 2). PD patients had lower scores than MSA and PSP patients on depression and apathy scales; MSA patients were less apathetic than PSP patients. Taking into account screening cut-off values of BDI-II and AES, we found that the proportion of depressed MSA patients was higher than that of depressed PSP and PD patients, whereas the proportion of apathetic patients was higher in PSP groups than that in MSA and PD patients. In Fig. 3, pie charts report the percentage of patients with pure apathy, patients with pure depression, patients with apathy and depression, patients without apathy and depression for MSA, PSP and PD groups. We found that the percentage of patients with “pure apathy” was higher in PSP group than in MSA and PD groups, and the percentage of patients with co-occurrence of apathy and depression was similar in PSP and MSA groups.
Correlational results within whole PSP, MSA and PD groups
The a priori power analysis revealed that at least 29 participants for the Spearman’s correlation analysis were needed to attain a large effect size at a statistical power of 0.80 and an alpha level of 0.05. Therefore, on the basis of power-analysis results, we performed correlational analysis on each whole patients group (PD group = 55 patients; MSA group = 44 patients; PSP group = 42 patients).
Correlational results between clinical aspects and cognitive parameters
Whereas in PD and MSA group clinical parameters did not correlate with any cognitive scores, in PSP group, PSP-RS tended to correlate with semantic fluency (rho = − 0.509, p = 0.013) score.
Correlational results between clinical aspects and neuropsychiatric parameters
In PD group, clinical parameters did not correlate with any behavioural scores. In MSA group, we found a significant correlation of UMSARS-I with BDI-II (rho = 0.491, p = 0.008) and ADL (rho = −0.664, p < 0.001), and also a significant correlation of part II and IV of UMSARS-II with ADL scores (rho = − 0.571, p = 0.001; rho = − 0.522, p = 0.004). In PSP group, PSP-RS score correlated with AES (rho = 0.599, p = 0.003), ADL (rho = − 0.616, p = 0.002), and IADL (rho = − 0.644, p = 0.001).
Correlational results between behavioural and cognitive parameters in each patient group
In PD group, AES score significantly correlate with score on phonological fluency task (rho = −0.371, p = 0.008) and number of errors in Stroop test (rho = 0.412, p = 0.004), but not with any remaining cognitive score. BDI-II did not correlate with any cognitive scores.
In MSA group, BDI-II score correlated with ADL (rho = −0.477, p = 0.002) and IADL (rho = −0.445, p = 0.004) but not with any remaining cognitive score. AES score correlated with poorer score on phonological fluency task (rho = −0.420, p = 0.007).
In PSP group, AES score correlated with score on ADL (rho = −0.491, p = 0.002), IADL (rho = −0.623, p < 0.001), phonological fluency test (rho = −0.563, p < 0.001) and immediate copy of ROCF (rho = −0.523, p = 0.002), whereas BDI-II score did not correlate with any cognitive scores.
Discussion
The present study systematically compared samples of patients with PD, MSA and PSP on a very comprehensive neuropsychological battery to identify cognitive or behavioural differences among Parkinsonian disorders. Since there were significant differences in demographic variables and global cognitive status among the three groups, we performed a comparison on cognitive domain scores achieved by three subgroups of MSA, PD, PSP matched for demographic features and global cognitive functioning (i.e., MoCA score) to control for these potential bias. This procedure revealed significant differences among the three groups on cognitive tests exploring executive functions (i.e., phonological fluency test, TMT-B, and Stroop test) and linguistic functions. The group of patients with PSP was more impaired than the PD and MSA groups on cognitive tests assessing executive functions. On the other hand, the group of patients with MSA obtained similar cognitive performance to the PD group. As to behavioural symptoms, the prevalence of pure apathy (i.e., without co-occurrence of dementia and depression) was higher in patients with PSP (45%) than in patients with MSA (15%) or PD (10%). In the PSP group, apathy and depression were more severe than in the PD group, while apathy (but not depression) were more severe in the PSP group as compared to the MSA group. In patients with PD, symptoms of depression and apathy were less severe than in the MSA group.
Our results that PSP patients are more impaired than PD and MSA patients in some specific executive functions such as cognitive flexibility, set shifting and inhibitory control indicated a marked dysexecutive syndrome in PSP patients when compared to PD or MSA patients, consistently with previous studies [8,9,10]. In particular, in PSP, both spatial planning and set shifting were the most damaged executive functions. Since poor performances on both spatial planning and set shifting tests have been reported as a consequence of a damage of prefrontal cortex in neurodegenerative diseases, our findings support the notion that a consistent group of PSP presents prominent frontal deficits [35]. As a new observation, although the cognitive differences between PD and MSA were statistically not significant, we found that even MSA patients revealed a more marked impairment in executive functions when compared to PD patients supporting the idea frontal-executive dysfunction is an integral part of the disease and the most common presentation in MSA [36]. In particular, in MSA group, spatial planning was the most damaged executive function. This result indicates that deficit of spatial planning is a prominent executive dysfunction in MSA, affecting up to 50% of patients. Taken together, these results indicated that marked frontal cognitive impairment is associated mainly with atypical Parkinsonism and might reflect a prominent subcortical–frontal connection dysfunction [37]. Moreover, the more marked dysexecutive syndrome in PSP patients compared to MSA and PD ones may result from the deafferentation of the prefrontal and premotor areas due to alteration of striato-thalamo-cortical pathway [38].
As regards to memory domain, we found no significant differences between PD and atypical Parkinsonism on tasks assessing long-term memory, a cognitive function mediated mainly by the hippocampus. Although the volume of hippocampus has been found to be more reduced in atypical Parkinsonism compared to PD [39] the absence of a significant difference on long-term memory tests among the patient groups might suggest that long-term memory is equally impaired among PD, MSA and PSP and thus dysfunction in long-term memory does not allow to distinguish several types of basal ganglia pathologies. However, we observed that PSP patients showed poorer performance than PD ones only on verbal learning. To interpret these finding, we should keep in mind that the performance on this cognitive task may be negatively influenced by lapses of attention and working memory which are aspects of a severe dysexecutive syndrome associated with reduced volume of frontal-subcortical gray matter in PSP [40]. Therefore, the difficulties in verbal learning and recall observed in our PSP patients might be due to lapses of attention, deficits in working memory, inability to initiate and maintain a strategic search of stored information. This inability could be related to dysfunctional organizational and temporal aspects of encoding and retrieval mediated by frontal cortex rather than to a loss of stored information. In support of this idea, no difference among the patient groups was found on delayed recall and recognition tests, which assess long-term memory.
As for linguistic abilities, although the differences among the groups on the repetition and comprehension tasks did not reach the statistical significance, PSP obtained lower scores than MSA and PD. However, we found a significant difference among groups on semantic fluency task where the atypical Parkinsonism (i.e., PSP) was characterized by more severe impairments when compared to PD. The poor performances on semantic fluency task might be the consequence of speech disorders, such as dysarthria, which are common clinical features of atypical Parkinsonism [41]. Previous evidence of selective impairments of action-verb naming and comprehension in PSP lent to hypothesizing that such linguistic deficits could be due to semantic deficits affecting the conceptual category of actions and could reflect dysfunctions of neural systems in posterior frontal cortical areas critical for processing the conceptual category of actions [42]. Therefore, since we employed comprehensive tests consisting of complex sentences characterized mainly by action verbs, our finding seems to support partially that these deficits in PSP reflect a dysfunctional processing of conceptual category of actions. As for PD patients, although we found a low percentage of patients with impaired performance on linguistic tasks according to Italian normative values (see Table 3), previous evidence demonstrated that PD patients may show impaired performance on tasks assessing naming of verbs [43] and that such impairment in naming verbs may improve after deep brain stimulation of the subthalamic nucleus [44].
As for the behavioural domain, apathy was more severe in PSP than in MSA and PD, confirming the pervasiveness of apathy in PSP [11, 45]. Moreover, the prevalence of pure apathy (i.e., without co-occurrence of dementia and depression) was higher in patients with PSP (45%) than in patients with MSA (15%) or PD (10%). Co-occurrence of apathy and depression was frequent in patients with MSA and PSP.
Correlational analysis, performed in each whole patients group, showed a significant association between apathy and poor performance on frontal tasks in both atypical Parkinsonism and PD patients supporting the frontal origin of apathy [46, 47]. The results of relationship between apathy and poorer scores on executive tests in PD group supported the idea that apathy and executive dysfunctions are both epiphenomena of dysexecutive syndrome related to damaged fronto-subcortical circuitries (see recent meta-analysis [48] and Fig. 4).
Model of the relationship between apathy, depression and different cognitive dysfunctions in patients with MSA, PD and PSP. The figure shows that, despite the type of disease, apathy but not depression was related to executive dysfunctions. In part A, the correlational results of relationship between apathy/depression and cognitive domains were shown. Gray line indicates no correlation in MSA, PD and PSP groups; black line indicates correlation in MSA, PD and PSP groups; black broken line indicates correlation in PD group or in PSP group. In part B, apathy and executive dysfunctions are reported as connected with dysexecutive syndrome and considered as epiphenomena of the dysexecutive syndromes despite the type of disease
Our finding that apathy score and not the depression score significantly correlated with cognitive performance in PSP and in PD evidenced that apathy rather than depression negatively influences cognitive functions in basal ganglia disorders [49] and that apathy and depression are two distinct syndromes [50]. Moreover, our findings support the idea that apathy and cognitive dysfunction in PSP are the consequence of degeneration in shared prefrontal areas or of dysfunction of shared frontal–subcortical connections [51, 52] and are in line with recent studies showing that frontal atrophy in volumetric MRI studies correlates with behavioural changes in PSP [53]. Even in MSA, apathy rather than depression was associated moderately with poorer performance on phonological fluency tasks. The results might suggest the idea that even in MSA apathy and cognitive dysfunctions are non-motor symptoms induced by focal lesions in the basal ganglia, particularly the caudate, which is engaged in controlling affective aspects of behaviour and is characterized by major neuronal loss in MSA [54].
Our finding that motor symptoms were associated with depression in MSA [55] and apathy in PSP, respectively, might suggest that neurodegenerative processes may progress in the two diseases impacting distinct subcortical and cortical regions. Finally, we found a significant association between the severity of motor symptoms and reduced functional autonomy only in MSA and PSP [56] indicating that motor symptoms drastically reduce patients’ autonomy in atypical Parkinsonism rather than in PD.
The present study is characterized by some limitations. First, we did not include healthy control subjects to verify whether cognitive impairments were specific to Parkinsonian syndromes; however, we identified subjects who achieved pathological scores with respect to Italian normative data and provided the percentage of subjects with pathological scores within each patient group. A second limitation of the study might be the unbalanced distribution of the number of cognitive tests in each cognitive domain; in particular, we used many cognitive tests to evaluate the executive domain. However, it allowed us to investigate different types of executive functions such as set shifting, inhibition, cognitive flexibility, spatial organization and planning (for review on executive functions: Diamond [57]). However, another methodological limitation of the study might be the fact that assessment of executive functions did not include any task of problem-solving. Finally, we preferred to apply the National Institute for Neurodegenerative Diseases PSP diagnostic criteria rather than the new Movement Disorders Society (MDS)-proposed diagnostic criteria for PSP [58] since the MDS criteria have just been released and never applied to any prospectically recruited PSP cohort.
In conclusion, the present study confirms the pervasiveness of cognitive deficits, mainly executive dysfunctions, apathy and depressive symptoms in PSP. Difficulties in set shifting, inhibitory control and cognitive flexibility (i.e., reduced performance on Stroop test, TMT:B and phonological fluency task, respectively; see Table 3) characterized MSA group rather than PD group. The results indirectly indicated the pivotal role of altered basal ganglia and corresponding frontal deafferentation in the occurrence and maintenance of the cognitive and behavioural disturbances.
References
Gerstenecker A (2017) The neuropsychology (broadly conceived) of multiple system atrophy, progressive supranuclear palsy, and corticobasal degeneration. Arch Clin Neuropsychol 32(7):861–875
Kehagia AA, Barker RA, Robbins TW (2010) Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurol 9(12):1200–1213
Bak TH, Crawford LM, Hearn VC, Mathuranath PS, Hodges JR (2005) Subcortical dementia revisited: similarities and differences in cognitive function between progressive supranuclear palsy (PSP), corticobasal degeneration (CBD) and multiple system atrophy (MSA). Neurocase 11(4):268–273
Brown RG, Lacomblez L, Landwehrmeyer BG et al (2010) Cognitive impairment in patients with multiple system atrophy and progressive supranuclear palsy. Brain 133(Pt 8):2382–2393
Leiguarda RC, Pramstaller PP, Merello M, Starkstein S, Lees AJ, Marsden CD (1997) Apraxia in Parkinson’s disease, progressive supranuclear palsy, multiple system atrophy and neuroleptic-induced parkinsonism. Brain 120(Pt 1):75–90
Pillon B, Gouider-Khouja N, Deweer B et al (1995) Neuropsychological pattern of striatonigral degeneration: comparison with Parkinson’s disease and progressive supranuclear palsy. J Neurol Neurosurg Psychiatry 58(2):174–179
Krishnan S, Mathuranath PS, Sarma S, Kishore A (2006) Neuropsychological functions in progressive supranuclear palsy, multiple system atrophy and Parkinson’s disease. Neurol India 54(3):268–272
Lange KW, Tucha O, Alders GL et al (2003) Differentiation of parkinsonian syndromes according to differences in executive functions. J Neural Transm (Vienna) 110(9):983–995
Monza D, Soliveri P, Radice D et al (1998) Cognitive dysfunction and impaired organization of complex motility in degenerative parkinsonian syndromes. Arch Neurol 55(3):372–378
Robbins TW, James M, Owen AM et al (1994) Cognitive deficits in progressive supranuclear palsy, Parkinson’s disease, and multiple system atrophy in tests sensitive to frontal lobe dysfunction. J Neurol Neurosurg Psychiatry 57(1):79–88
Aarsland D, Litvan I, Larsen JP (2001) Neuropsychiatric symptoms of patients with progressive supranuclear palsy and Parkinson’s disease. J Neuropsychiatry Clin Neurosci 13(1):42–49
Schrag A, Sheikh S, Quinn NP et al (2010) A comparison of depression, anxiety, and health status in patients with progressive supranuclear palsy and multiple system atrophy. Mov Disord 25(8):1077–1081
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55(3):181–184
Gilman S, Wenning GK, Low PA et al (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71(9):670–676
Litvan I, Agid Y, Jankovic J et al (1996) Accuracy of clinical criteria for the diagnosis of progressive supranuclear palsy (Steele–Richardson–Olszewski syndrome). Neurology 46(4):922–930
Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25(15):2649–2653
Katz TF (1963) A.D.L. activities of daily living. JAMA 185:914
Lawton MP, Brody EM (1969) Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 9:179–186
Fahn S, Elton RL, Members of the UPDRS Development Committee (1987) Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Calne D, Goldstein M (eds) Recent developments in Parkinson’s disease. MacMillan, Florham Park, pp 153–304
Wenning GK, Tison F, Seppi K et al (2004) Development and validation of the Unified Multiple System Atrophy Rating Scale (UMSARS). Mov Disord 19(12):1391–1402
Golbe LI, Ohman-Strickland PA (2007) A clinical rating scale for progressive supranuclear palsy. Brain 130(Pt 6):1552–1565
Santangelo G, Siciliano M, Pedone R et al (2015) Normative data for the Montreal Cognitive Assessment in an Italian population sample. Neurol Sci 36(4):585–591
Giovagnoli AR, Del Pesce M, Mascheroni S, Simoncelli M, Laiacona M, Capitani E (1996) Trail making test: normative values from 287 normal adult controls. Ital J Neurol Sci 17(4):305–309
Caltagirone C, Gainotti G, Masullo C, Miceli G (1979) Validity of some neuropsychological test in the assessment of mental deterioration. Acta Psychiatr Scand 60:50–56
Barbarotto R, Laiacona M, Frosio R, Vecchio M, Farinato A, Capitani E (1998) A normative study on visual reaction times and two Stroop colour-word tests. Ital J Neurol Sci 19(3):161–170
Siciliano M, Santangelo G, D’Iorio A et al (2016) Rouleau version of the Clock Drawing Test: age- and education-adjusted normative data from a wide Italian sample. Clin Neuropsychol 30(sup1):1501–1516
Caffarra P, Vezzadini G, Dieci F, Zonato F, Venneri A (2002) Rey–Osterrieth complex figure: normative values in an Italian population sample. Neurol Sci 22(6):443–447
Novelli G, Papagno C, Capitani E, Laiacona M, Vallar G, Cappa SF (1986) Tre test clinici di ricerca e produzione lessicale. Taratura su soggetti normali. Arch Psicol Neurol Psichiatria 47(4):477–506
Capasso R, Miceli G (2001) Esame Neuropsicologico per l’Afasia, ENPA. Springer, Milan
Ferracuti S, Sacco R, Cannoni E, Hufty AM, Silvan AB, Hamster KDeS, Varney NR, Spreen O (2000) Contributi per un Assessment Neuropsicologico di Benton AL. Organizzazioni Speciali, Florence
Spinnler H, Tognoni G (1987) Standardizzazione e taratura italiana di una batteria di test neuropsicologici. Ital J Neurol Sci 8:1–120
Ghisi M, Flebus GB, Montano A, Sanavio E, Sica C (2006) Beck Depression Inventory-Second Edition. Adattamento italiano: Manuale. Organizzazioni Speciali, Florence
Santangelo G, Barone P, Cuoco S et al (2014) Apathy in untreated, de novo patients with Parkinson’s disease: validation study of Apathy Evaluation Scale. J Neurol 261(12):2319–2328
Pitman EJG (1948) Lecture notes on nonparametric statistical inference: lectures given for the University of North Carolina. Columbia University, New York
Boxer AL, Yu JT, Golbe LI, Litvan I, Lang AE, Höglinger GU (2017) Advances in progressive supranuclear palsy: new diagnostic criteria, biomarkers, and therapeutic approaches. Lancet Neurol 16(7):552–563
Stankovic I, Krismer F, Jesic A et al (2014) Cognitive impairment in multiple system atrophy: a position statement by the Neuropsychology Task Force of the MDS Multiple System Atrophy (MODIMSA) study group. Mov Disord 29(7):857–867
Houghton DJ, Litvan I (2007) Unraveling progressive supranuclear palsy: from the bedside back to the bench. Parkinson Relat Disord Suppl 3:S341–S346
Rosskopf J, Gorges M, Müller HP et al (2017) Intrinsic functional connectivity alterations in progressive supranuclear palsy: differential effects in frontal cortex, motor, and midbrain networks. Mov Disord 32(7):1006–1015
Messina D, Cerasa A, Condino F et al (2011) Patterns of brain atrophy in Parkinson’s disease, progressive supranuclear palsy and multiple system atrophy. Parkinson Relat Disord 17(3):172–176
Schulz JB, Skalej M, Wedekind D et al (1999) Magnetic resonance imaging-based volumetry differentiates idiopathic Parkinson’s syndrome from multiple system atrophy and progressive supranuclear palsy. Ann Neurol 45(1):65–74
Rusz J, Bonnet C, Klempíř J et al (2015) Speech disorders reflect differing pathophysiology in Parkinson’s disease, progressive supranuclear palsy and multiple system atrophy. J Neurol 262(4):992–1001
Daniele A, Barbier A, Di Giuda D et al (2013) Selective impairment of action-verb naming and comprehension in progressive supranuclear palsy. Cortex 49(4):948–960
Rodríguez-Ferreiro J, Menéndez M, Ribacoba R, Cuetos F (2009) Action naming is impaired in Parkinson disease patients. Neuropsychologia 47(14):3271–3274
Silveri MC, Ciccarelli N, Baldonero E, Piano C, Zinno M, Soleti F, Bentivoglio AR, Albanese A, Daniele A (2012) Effects of stimulation of the subthalamic nucleus on naming and reading nouns and verbs in Parkinson’s disease. Neuropsychologia 50(8):1980–1989
Litvan I, Mega MS, Cummings JL, Fairbanks L (1996) Neuropsychiatric aspects of progressive supranuclear palsy. Neurology 47(5):1184–1189
Santangelo G, Vitale C, Trojano L et al (2015) Relationship between apathy and cognitive dysfunctions in de novo untreated Parkinson’s disease: a prospective longitudinal study. Eur J Neurol 22(2):253–260
Santangelo G, D’Iorio A, Maggi G et al (2018) Cognitive correlates of “pure apathy” in Parkinson’s disease. Parkinson Relat Disord. https://doi.org/10.1016/j.parkreldis.2018.04.023
D’Iorio A, Maggi G, Vitale C, Trojano L, Santangelo G (2018) “Pure apathy” and cognitive dysfunctions in Parkinson’s disease: a meta-analytic study. Neurosci Biobehav Rev 94:1–10. https://doi.org/10.1016/j.neubiorev.2018.08.004
Santangelo G, Vitale C, Picillo M et al (2015) Apathy and striatal dopamine transporter levels in de-novo, untreated Parkinson’s disease patients. Parkinson Relat Disord 21(5):489–493
Levy ML, Cummings JL, Fairbanks LA, Masterman D, Miller BL, Craig AH, Paulsen JS, Litvan I (1998) Apathy is not depression. J Neuropsychiatry Clin Neurosci 10(3):314–319
Cordato NJ, Halliday GM, Caine D et al (2006) Comparison of motor, cognitive, and behavioral features in progressive supranuclear palsy and Parkinson’s disease. Mov Disord 21:632–638
Lee CN, Kim M, Lee HM, Jang JW, Lee SM, Kwon DY, Park KW, Koh SB (2013) The interrelationship between non-motor symptoms in Atypical Parkinsonism. J Neurol Sci 327(1–2):15–21
Cordato NJ, Pantelis C, Halliday GM et al (2002) Frontal atrophy correlates with behavioural changes in progressive supranuclear palsy. Brain 125:789–800
Wenning GK, Ben-Shlomo BY, Megalhães M et al (1994) Clinical features and natural history of multiple system atrophy: an analysis of 100 cases. Brain 117:835–845
Tison F, Yekhlef F, Chrysostome V (2006) Depression and self-reported depressive symptoms in multiple system atrophy compared to Parkinson’s disease. Mov Disord 21(7):1056–1057
Duff K, Gerstenecker A, Litvan I, Investigators and Coordinators of the ENGENE-PSP Study Group (2013) Functional impairment in progressive supranuclear palsy. Neurology 80(4):380–384
Diamond A (2013) Executive functions. Annu Rev Psychol 64:135–168
Höglinger GU, Respondek G, Stamelou M et al (2017) Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord 32(6):853–864
Acknowledgements
The authors thank Dr. Maria Francesca Tepedino, Renzo Manara, Marianna Amboni, Carmine Vitale, Autilia Cozzolino, Giovanna Dati, Pietro Siano, Massimo Squillante, Annamaria Vallelunga, Giampiero Volpe for supporting the enrollment of patients. Moreover, the authors thank Dr. Arianna Cappiello and Immacolata Carotenuto for their help in collecting neuropsychological data.
Funding
The study did not receive any source of funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All authors declared no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Santangelo, G., Cuoco, S., Pellecchia, M.T. et al. Comparative cognitive and neuropsychiatric profiles between Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy. J Neurol 265, 2602–2613 (2018). https://doi.org/10.1007/s00415-018-9038-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-9038-x