Abstract
Objective
The current clinical measures (ONLS, R-ODS, mRS, and MRC) may not be so sensitive in capturing minimal variations or measuring fatigue in chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). Our aim was to assess if 6-min walk test (6MWT) is able to increase the sensitivity in detecting response to therapy and to capture fatigue in CIDP patients.
Methods
We tested 6MWT in 42 CIDP patients. Using both anchor-based and distribution-based approaches, we estimated the meaningful clinical change after therapy by calculating the minimum improvement cutoff (Minimal Clinically Important Difference Score—MCID) required for considering a patient as responder. We calculated the sensitivity of the 6MWT versus the other clinical outcomes. We analysed fatigue by comparing the velocities between first and sixth minutes of the 6MWT and the effect of treatment on fatigue using an ANOVA model for repeated measures.
Results
MCID resulted equal to 20 m. The combination of 6MWT-MCID cutoff with the other clinical measures led to identify 74% of responders. The sensitivity of the 6MWT was 90% versus 77% of the other clinical measures. The 6MWT was also sensitive in capturing fatigue-related changes, even though fatigue was not influenced by treatment.
Conclusions
The combination of the 6MWT with the other clinical measures increased the chance to detect the quote of responders. We propose to include the 6MWT in the routine assessment of CIDP patients and the MCID cutoff at 20 m could be set for identifying the responders and properly guiding the therapy management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic inflammatory demyelinating polyneuropathy (CIDP) is a chronic-disabling disease with relapsing, progressive, or monophasic course. In addition to motor and sensory symptoms, CIDP patients often complain of fatigue during a relapse or the clinical course of disease.
In daily practice and in clinical trials, the clinicians, to assess the clinical status or the response to therapy, use a battery of clinimetric tests including the modified version of the inflammatory neuropathy cause and treatment (INCAT) scale–sensory subscore (ISS) [1], the Overall Neuropathy Limitation Scale (ONLS) [2], the Rasch-built overall disability scale (R-ODS)[3], the modified Rankin Scale (mRS), and the Medical Research Council scale (MRC) [4, 5]. However, these measures may not be so sensitive in capturing minimal variations or are too operator-dependent.
These issues particularly come out in patients without a clear response to therapy, in whom the self-reported outcome may not be corresponding to the objective clinical evaluation, making challenging for clinicians to assess the real efficacy of therapy and to decide whether to continue with the same treatment or to switch to another.
Moreover, clinical outcome measures now available are not able to measure objectively fatigue, often reported as the main invaliding symptom by CIDP patients.
The 6-min walk test (6MWT) has been proved highly reliable, sensitive, and easy-to-apply for exploring the ambulatory ability as well the fatigue-related changes. Hence, 6MWT has been widely used as clinimetric test in several neurologic disorders comprising Charcot–Marie–Tooth disease [6, 7], multiple sclerosis [8], Pompe disease [9], spinal muscular atrophy [10], stroke [11], facio-scapulo-humeral muscular dystrophy [12], Duchenne/Becker muscular dystrophy and Parkinson disease [13, 14].
Under these premises, we decided to test the 6MWT in a cohort of CIDP patients and to estimate the meaningful clinical change after therapy by calculating the minimum improvement cutoff (Minimal Clinically Important Difference Score—MCID) required to consider a patient as responder.
In fact, the MCID is the smallest difference of score in the domain of interest that the patient perceives as beneficial and that would guide a decision-making in patient management [15, 16].
Methods
We selected fully ambulant patients (Table 1) with a diagnosis of definite CIDP according to the EFNS/PNS criteria and periodically treated with intravenous immunoglobulin (IVIG) or intravenous methylprednisolone (IVMP) [17].
The patients had not changed the type of treatment (IVIG or IVMP) in the last 6 months before the enrolment.
Exclusion criteria were: (a) diagnosis of possible or probable CIDP according to the EFNS/PNS criteria; (b) presence of other possible causes of genetic or acquired demyelinating neuropathy according to the EFNS/PNS criteria [17]; and (c) exclusion of cardiopulmonary co-morbidity potentially affecting 6MWT performances [18].
All patients underwent clinical evaluation comprehensive of demographic evaluation, clinimetric scales ISS, ONLS, R-ODS, mRS, MRC sum-score, and 6MWT before (baseline) treatment (IVIG or IVMP) and after (follow-up) 2 months. IVIG were administered at the dose of 0.4 g/Kg/day for 5 days and IVMP at the dose of 500 mg/day for 5 days.
To test the reliability of the 6MWT, we repeated it twice within a few days before starting therapy, and thus, we estimated the intraclass correlation coefficients (ICC). The 6MWT was performed according to the American Thoracic Society (ATS) guidelines [18].
At follow-up visit, patients reported whether their condition was stable, improving or getting worse compared to baseline evaluation. The patients also estimated if the improvement was slight or very significant.
Moreover, patients were considered as responders to treatment when at least 1-point increase in ONLS and/or MRC and/or mRS scores was observed or when changes in meters walked at 6MWT were greater than the minimal clinically important difference (MCID) value [19].
Statistical analysis
We applied a simple linear regression for evaluating the correlation at baseline between the 6MWT and the other clinical measures (Table 2).
To calculate the MCID for 6MWT, we used both anchor-based and distribution-based approaches, as suggested by Copay et al. and as yet performed in CIDP patients [16, 20].
Anchor-based methods were based on comparison between variations in outcome measure values and patients reported outcomes (PROs), namely, the self-perception of patient clinical condition changes.
Distribution-based methods instead were based only on mathematical interpolation of baseline and/or follow-up values for each clinical outcome.
The anchor-based method chosen in this study was the “between-patient score change”, based on dividing patients in four different groups according to self-evaluation of clinical changes at follow-up compared to baseline: each patient at follow-up stated if clinical condition was stable, improved (slightly or very significantly), or worsened. MCID came out from the difference of mean value variations between “stable” and “slightly improved” groups. The MCID was obtained by calculating the mean value of the difference in walked meters between “stable” and “slightly improved” groups.
Distribution-based approaches consisted of statistic parameters calculated on the 6MWT at baseline. We took in consideration the minimal detectable change (MDC), [21] calculated as MDC = 1.96 × SD (standard deviation) × SEM, where SEM is the Standard Error of Measurement and correspond to \(\sqrt {1 - ICC}\), and the effect size, considered as small, moderate, or large (respectively, 0.2 × SD, 0.5 × SD, 0.8 × SD) [20].
A possible influence of baseline values of the 6MWT on the MCID was tested performing a simple linear regression corrected for PROs subgroups.
Finally, we compared the sensitivity (the proportion of patients who were identified as responders) between the 6MWT and the other clinical measures (i.e., ONLS, MRC, and mRS combined).
Fatigue was explored performing a comparison between the velocities between the first and sixth minutes of 6MWT at baseline and at follow-up using the t student test for paired sample. Moreover, to evaluate the effect of treatment on fatigue, we applied analysis of variance (ANOVA) for repeated measures with time (two levels: first and sixth minutes) and visit (two levels: baseline and follow-up) as within-subjects factors.
Data availability
No individual patient data have been shared in this article. If requested from any qualified investigator, corresponding authors will share any data not published here in anonymized form.
Patients’ consents
This study was a prospective study and all patients gave written informed consent. The study was approved by the local Ethics Committee (University of Naples “Federico II”, Italy).
Results
We enrolled 42 CIDP patients fulfilling inclusion criteria, whose demographic features are reported in Table 1. Based on 1-point increase in ONLS and/or MRC and/or mRS scores, we identified 24 responders (58%) and 18 non-responders (42%);
Simple linear regression showed a significant correlation between the 6MWT and all the other clinical measures at baseline (Table 2).
Through the anchor-based method, we found 2 patients who considered themselves as worsened, with an effective mean reduction of 23 m [− 78.65; 124.64] during the 6MWT. In the “stable” group (12 patients), we found an increase of 6.91 m [− 8.41; 22.34]. In the “slightly improved” group, comprehensive of 12 patients, we observed an improvement of 27.17 m [16.54; 37.79], and in the “very significantly improved” group (16 patients), we found an increase of 57.93 m [42.04; 73.83] (Table 3).
By calculating the difference between “stable” and “slightly improved” groups, we obtained a mean MCID value for the 6MWT of 20.26 m [4.07; 30.07].
A preliminary approach to distribution methods was to calculate the ICC (0.983), which has demonstrated a high reliability of the 6MWT, with a SEM of 10.9. Therefore, the minimally detectable change (MDC) had a value of 21.4 m. Moreover, regarding the effect size, we obtained values of 16.96 m (small effect), 42.55 m (moderate effect), and 67.68 m (large effect). Accordingly, we decided to assume a 20-m improvement (corresponding to mean value of anchor-based method, MDC and small effect size) as the cut-off value (our MCID) to consider a patient as 6MWT-responder.
There was no relation between the MCID value and the 6MWT performance at baseline, i.e., the MCID value was not influenced by meters walked at baseline evaluation.
The MCID value was in line with the results of the linear regression between the ONLS and the 6MWT that showed a beta coefficient of − 39.5 [− 54.75; − 24.27], meaning that 1-point variation in the ONLS corresponds to 39-m variation in the 6MWT. Noteworthy, the MCID previously calculated for the ONLS ranged between 0.64 and 0.72 corresponding to a range of 24–28 m for the 6MWT [20].
Therefore, by applying this cutoff for the 6MWT to the cohort of CIDP patients, 7 of 18 patients labelled as non-responders using 1-point increase in ONLS and/or MRC and/or mRS scores shifted to the 6MWT-responder group, while 3 out of 24 patients identified as responders were missed. Six out of the seven patients identified as 6MWT-responders reported also subjective improvement at follow-up visit.
The rate of responders identified by 6MWT was 66% (28 patients), and by combining the 6MWT with the other clinical measures, the rate of overall responders increased up to 74% (31 patients) of our cohort. Overall, the sensitivity of the 6MWT was 90% (28/31) versus 77% (24/31) of the other clinical outcomes (ONLS, MRC, and mRS).
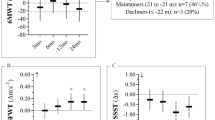
To investigate the fatigue, we calculated at baseline, and at follow-up, the first-minute velocity (FMV) [baseline = 1 m/s (0.93; 1,07); follow-up = 1.09 m/s (1.02;1.17)], the sixth-minute velocity (SMV) [baseline = 0.94 m/s (0.87; 1.02); follow-up = 1.04 m/s (0.96; 1.12)], and the overall mean velocity (OMV) [baseline = 0.95 m/s (0.88; 1.04); follow-up = 1.05 m/s (0.98; 1.13)]. T student test for paired sample showed significant differences between the first and sixth minutes both at baseline (p < 0.00) and follow-up (p < 0.00) visit. 6MWT-responder patients showed a significant improvement at follow-up visit for FMV, SMV, and OMV compared to baseline. Conversely, ANOVA for repeated measures did not show any significant effect of treatment factor on fatigue even in 6MWT-responder group alone and after excluding patients with disability limited to upper limbs.
Discussion
One of the challenges facing the clinicians in managing CIDP patients is whether to consider a patient as responder or not, keeping in mind several variables such as the latency of effect of therapies, the subjective perception of patients and the objective neurological evaluation.
A possible strategy to face the “grey-zone” of patients, whose response is unclear, could be the identification of a scale with a definite cutoff, easily applicable and able to capture minimal important clinical variations (i.e., MCID).
Some authors tried to set MCID for the outcome measures commonly used in CIDP patients, but only the ONLS gained such result to be considered an “ideal” scale,[20, 22] and responder was defined a patient having a MCID ≥ 0.72. However, the ONLS is an ordinal and non-linear scale, and thus, it is not able to capture a change less than 1-point. In other words, if a patient improves more than MCID values but less than 1-point in ONLS, he cannot be considered as responder despite the clinical improvement.
Given this limitation, we decided to apply systematically in a group of CIDP patients the 6MWT (before and 2 months after the treatment) that offers continuous values (i.e., meters walked along 6 min) for calculating the MCID. At baseline, the 6MWT correlated with ONLS supporting the sensitivity of our methodological approach in measuring clinical impairment in CIDP. Moreover, ICC analysis demonstrated that 6MWT is also highly reliable.
The MCID resulted equal to 20 m, i.e., the patients walking at least 20 m more than previous assessment (before therapy) could be considered as responders.
Interestingly, the MCID was not influenced by the degree of disability at baseline (meters walked at 6MWT), thus meaning that a higher degree of clinical impairment was not associated with a lower MCID cut-off value.
By applying the MCID for the 6MWT to the cohort of our patients, the rate of responders increased from 58 to 66%. Moreover, the combination of 6MWT with the other clinical measures (i.e., ONLS, MRC, and mRS) led to identify 74% of responders (16% more than classic clinical measures).
Overall, the sensitivity of 6MWT was 13% greater than the other clinical measures (90% vs 77%) in identifying responder patients.
The seven patients identified as responders only through the 6MWT support the sensitivity of a long walk-distance test in assessing globally the impact of neuropathy on gait performance that may be influenced by both sensory and motor involvement.
Conversely, the 6MWT did not identify three patients otherwise classified as responders by other clinical measures. These patients had clinical impairment limited to upper limbs, and thus, it is easily explainable why the 6MWT missed them. As these patients were identified by ONLS, it is conceivable that a minimal clinical data set in future study design may include 6MWT and ONLS.
The 6MWT has been proved to be sensitive not only for exploring the ambulatory ability but also for capturing the fatigue-related changes [23, 24]. We found significant differences in velocities between the first and sixth minutes, both at baseline and follow-up. However, even though FMV, SMV, and OMV were improved after treatment, therapy did not have any significant effect on fatigue even when we analysed separately the cohort of 6MWT-responders, in whom SMV continued to be worse than FMV and by excluding patients, whose disability was limited to upper limbs.
Many factors are associated with performance on 6MWT including sensory and motor involvement, and fatigue. Weakness and sensory disturbances, as well fatigue may depend on conduction blocks or axonal loss. In a short-time follow-up (2 months), only the resolution of conduction blocks may lead to a better performance on 6MWT. This would explain the greater distance walked at follow-up with respect to baseline evaluation.
On the other hand, the missing effect of therapies on fatigue, inferred from the comparable degree of decrease in walking speed in the 6MWT between baseline and follow-up, suggests a different mechanism for fatigue. Thus, the axonal loss might play a main role in developing fatigue and this is poorly influenced by pharmacological effect over the short time (2 months).
In Spinal Muscolar Atrophy type 3 patients, fatigue on 6MWT has been related to a concurrent neuromuscolar junction dysfunction, and as the failure of neuromuscular transmission has been documented in CIDP patients, it is conceivable that the impairment of neuromuscular transmission may have a role in fatigue also in CIDP [25, 26]. Unfortunately, in the present work, we have not tested neuromuscular transmission that would certainly be interesting to evaluate in future studies.
In conclusion, our study supports that the 6MWT is a reliable and sensitive tool for monitoring CIDP patients and underlines that the combination of the 6MWT with the other clinical measures increases the chance to detect the real quote of responders to therapy. We propose to include the 6MWT in the routine neurological examination of CIDP patients and the MCID cutoff at 20 m should be used for identifying the responders and properly guiding the therapy management.
References
Merkies IS, Schmitz PI, van der Meché FG, van Doorn PA (2000) Psychometric evaluation of a new sensory scale in immune-mediated polyneuropathies. Inflammatory Neuropathy Cause and Treatment (INCAT) Group. Neurology 54(4):943–949
Graham RC, Hughes RA (2006) A modified peripheral neuropathy scale: the overall neuropathy limitations scale. J Neurol Neurosurg Psychiatry 77(8):973–976 (Epub 2006 Mar 30)
van Nes SI, Vanhoutte EK, van Doorn PA et al (2011) Rasch-built Overall Disability Scale (R-ODS) for immune-mediated peripheral neuropathies. Neurology 76(4):337–345. https://doi.org/10.1212/WNL.0b013e318208824b
Bamford JM, Sandercock PA, Warlow CP, Slattery J (1989) Interobserver agreement for the assessment of handicap in stroke patients. Stroke 20(6):828 (No abstract available)
Kleyweg RP, van der Meché FG, Schmitz PI (1991) Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain–Barré syndrome. Muscle Nerve 14(11):1103–1109
Padua L, Pazzaglia C, Pareyson D et al (2016) CMT-TRIAAL Group. Novel outcome measures for Charcot-Marie-Tooth disease: validation and reliability of the 6-min walk test and StepWatch(™) Activity Monitor and identification of the walking features related to higher quality of life. Eur J Neurol 23(8):1343–1350. https://doi.org/10.1111/ene.13033 (Epub 2016 May 10)
Pazzaglia C, Camerota F, Germanotta M, Di Sipio E, Celletti C, Padua L (2016 Jul) Efficacy of focal mechanic vibration treatment on balance in Charcot-Marie-Tooth 1A disease: a pilot study. J Neurol 263(7):1434–1441. https://doi.org/10.1007/s00415-016-8157-5 (Epub 2016 May 13)
Bennett SE, Bromley LE, Fisher NM, Tomita MR, Niewczyk P (2017) Validity and reliability of four clinical gait measures in patients with multiple sclerosis. Int J MS Care 19(5):247–252. https://doi.org/10.7224/1537-2073.2015-006
Angelini C, Semplicini C, Ravaglia S et al (2012) Italian Group on GSDII. New motor outcome function measures in evaluation of late-onset Pompe disease before and after enzyme replacement therapy. Muscle Nerve 45(6):831–834. https://doi.org/10.1002/mus.23340
Dunaway Young S, Montes J, Kramer SS et al (2016) Six-minute walk test is reliable and valid in spinal muscular atrophy. Muscle Nerve 54(5):836–842. https://doi.org/10.1002/mus.25120 (Epub 2016 May 13)
Fulk GD, Echternach JL, Nof L, O’Sullivan S (2008) Clinometric properties of the six-minute walk test in individuals undergoing rehabilitation poststroke. Physiother Theory Pract 24(3):195–204. https://doi.org/10.1080/09593980701588284
Eichinger K, Heatwole C, Heininger S et al (2017) FSHD Clinical Trials Research Network. Validity of the 6 minute walk test in facioscapulohumeral muscular dystrophy. Muscle Nerve 55(3):333–337. https://doi.org/10.1002/mus.25251 (Epub 2016 Dec 5)
McDonald CM, Henricson EK, Han JJ et al (2010) The 6-minute walk test in Duchenne/Becker muscular dystrophy: longitudinal observations. Muscle Nerve 42(6):966–974. https://doi.org/10.1002/mus.21808
Kobayashi E, Himuro N, Takahashi M (2017) Clinical utility of the 6-min walk test for patients with moderate Parkinson’s disease. Int J Rehabil Res 40(1):66–70. https://doi.org/10.1097/MRR.0000000000000205
Jaeschke R, Singer J, Guyatt GH (1989) Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials 10(4):407–415
Copay AG, Subach BR, Glassman SD, Polly DW Jr, Schuler TC (2007) Understanding the minimum clinically important difference: a review of concepts and methods. Spine J 7(5):541–546 (Epub 2007 Apr 2. Review)
Joint Task Force of the EFNS and the PNS. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of paraproteinemic demyelinating neuropathies (2010) Report of a Joint Task Force of the European Federation of Neurological Societies and the Peripheral Nerve Society–first revision. J Peripher Nerv Syst 15(3):185–195. https://doi.org/10.1111/j.1529-8027.2010.00278.x
ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories (2002) ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 166(1):111-117 (No abstract available. Erratum in: Am J Respir Crit Care Med. 2016 May 15;193(10):1185)
Nobile-Orazio E, Cocito D, Jann S et al (2015) IMC Trial Group. Frequency and time to relapse after discontinuing 6-month therapy with IVIg or pulsed methylprednisolone in CIDP. J Neurol Neurosurg Psychiatry 86(7):729–734. https://doi.org/10.1136/jnnp-2013-307515 (Epub 2014 Sep 22)
Merkies IS, van Nes SI, Hanna K, Hughes RA, Deng C (2010) Confirming the efficacy of intravenous immunoglobulin in CIDP through minimum clinically important differences: shifting from statistical significance to clinical relevance. J Neurol Neurosurg Psychiatry 81(11):1194–1199. https://doi.org/10.1136/jnnp.2009.194324 (Epub 2010 Jul 20)
Wright A, Hannon J, Hegedus EJ, Kavchak AE (2012) Clinimetrics corner: a closer look at the minimal clinically important difference (MCID). J Man Manip Ther 20(3):160–166. https://doi.org/10.1179/2042618612Y.00000000001
Hughes RA, Lunn MP (2016) Regarding the past, what is the trial you have always been dreaming of in CIDP? Rev Neurol (Paris) 172(10):620–626. https://doi.org/10.1016/j.neurol.2016.07.020 (Epub 2016 Sep 13. Review)
Montes J, McDermott MP, Martens WB et al (2010) Muscle Study Group and the Pediatric Neuromuscular Clinical Research Network. Six Minute Walk Test demonstrates motor fatigue in spinal muscular atrophy, Neurology 74(10):833–838. https://doi.org/10.1212/WNL.0b013e3181d3e308
Goldman MD, Marrie RA, Cohen JA (2008) Evaluation of the six-minute walk in multiple sclerosis subjects and healthy controls. Mult Scler 14(3):383–390 (Epub 2007 Oct 17)
Pera MC, Luigetti M, Pane M et al (2017) 6MWT can identify type 3 SMA patients with neuromuscular junction dysfunction. Neuromuscul Disord 27(19):879–882. https://doi.org/10.1016/j.nmd.2017.07.007 (Epub 2017 Jul 14)
Gilmore KJ, Allen MD, Dhoerty TJ, Kimpinski K, Rice CL (2017) Electrophysiological and neuromuscolar stability of persons with chronic inflammatory demylinating polyneuropathy. Muscle Nerve 56(3):413–420. https://doi.org/10.1002/mus.25516 (Epub 2017 Mar 23)
Funding
No funding sources.
Author information
Authors and Affiliations
Contributions
ES: study design, acquisition of data, analysis and interpretation of data, and drafting the manuscript, ES: statistical analysis was performed. AT: study design, acquisition of data, and interpretation of data, revising the manuscript. RI: acquisition and interpretation of data, revising the manuscript; ST: acquisition and interpretation of data, revising the manuscript; LR: acquisition and interpretation of data, revising the manuscript; RD: acquisition and interpretation of data, revising the manuscript; ME: acquisition and interpretation of data, revising the manuscript; PD: analysis and interpretation of data; LS: study conceptualization, interpretation of data, and revising the manuscript; FM: study design, acquisition and interpretation of data, and drafting and revising the manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
Dr. Spina reports no disclosures; Dr. Topa reports no disclosures; Dr. Iodice reports no disclosures; Dr. Tozza reports no disclosures; Dr. Ruggiero reports no disclosures; Dr. Dubbioso reports no disclosure; Dr. Esposito reports no disclosure; Dr. Dolce reports no disclosure; Dr. Santoro reports no disclosure; and Dr. Manganelli reports no disclosures. The authors declare that they have no conflict of interest.
Ethical standard
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Spina, E., Topa, A., Iodice, R. et al. Six-minute walk test is reliable and sensitive in detecting response to therapy in CIDP. J Neurol 266, 860–865 (2019). https://doi.org/10.1007/s00415-019-09207-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09207-1