Abstract
Background
123I-MIBG myocardial scintigraphy and clonidine growth hormone test (CGH test) may help to distinguish multiple system atrophy (MSA) from Parkinson’s disease (PD). Their relevance in the first-stage parkinsonism of uncertain etiology is unknown.
Methods
Patients experiencing parkinsonism of ambiguous etiology were clinically classified into the PD group or the MSA group as initial clinical diagnosis (ICD). Then, CGH test and myocardial scintigraphy were performed. Clinical assessment was repeated throughout the disease course until the final clinical diagnosis (FCD) could be established according to the criteria of PD and MSA, respectively.
Results
Twenty-five patients with uncertain diagnosis were included (15 MSA and 10 PD as ICD). At the end of a 6-year follow-up, FCD was MSA in 11/25 patients and PD in 14/25. The CGH test and the scintigraphy showed a sensitivity of 82%, and a specificity of 71 and 93%, respectively, for the diagnosis of MSA. The combination of a normal scintigraphy (i.e., with myocardial MIBG uptake) with genitourinary dysfunction was the most relevant test to diagnose MSA, whereas an abnormal scintigraphy with a levodopa response of > 30% or an abnormal scintigraphy with the absence of OH was the most relevant combinations to diagnose PD. All these combinations had an accuracy superior than 90% and a specificity of 100%.
Conclusion
Combinations of myocardial scintigraphy with genitourinary dysfunction, levodopa response of > 30%, or orthostatic hypotension could be of interest for the distinction between PD and MSA when the clinical diagnosis remains ambiguous at the first stage of the disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is characterized by an isolated, asymmetrical levodopa-responsive parkinsonism (akinesia, rigidity, and optional resting tremor) [1]. Its diagnosis is supported by the UKPDSBB criteria including supportive prospective positive criteria, which may not be taken into account at an early stage of the disease [2]. Multiple system atrophy (MSA) is characterized by poorly levodopa-responsive parkinsonism in addition to dysautonomia, optional cerebellar ataxia and/or pyramidal signs with a more severe outcome including frequent falls, loss of independent walk, and finally death with a median survival of 5–10 years [3]. It may be difficult to differentiate PD from MSA in the first stage of the disease, where MSA may sometimes mimic PD. Dysautonomia may also be encountered in PD and may be lacking in the early stage of MSA and levodopa response may be satisfactory and sustained in some patients affected with MSA [4,5,6]. While PD diagnosis criteria have been recently revised, it is appropriate to provide an early and accurate diagnosis, since the prognosis and complications are more severe in MSA than in PD [7, 8]. 123I-MIBG (123Iode méta-iodobenzylguanidine) myocardial scintigraphy and the clonidine growth hormone test (CGH test) have been reported to be helpful to distinguish PD from MSA [9,10,11,12]. However, these results remain controversial, and to our knowledge, the relevance of their combination in patients experiencing parkinsonism of doubtful etiology has never been assessed [13,14,15,16,17]. We aimed to evaluate the relevance of 123I-MIBG myocardial scintigraphy and/or the CGH test for the distinction between PD and MSA in patients with parkinsonism of uncertain etiology.
Methods
Patients referred between November 2006 and June 2009 to our tertiary care center devoted to movement disorders, who experienced parkinsonism with diagnostic uncertainty between PD and MSA were consecutively included in our study. Inclusion criteria were as follows: (i) parkinsonian symptoms and (ii) diagnostic uncertainty defined by the combination of symptoms that could not fulfill the definite diagnosis of either PD or MSA, or that was consistent with both PD and MSA according to their criteria [2, 3, 18]. Exclusion criteria were (i) patients with other parkinsonian syndromes such as progressive supranuclear palsy and corticobasal degeneration and (ii) patients for whom the diagnosis was clearly suggestive of either PD or MSA. Patients gave written consent and the local ethics committee approved of the study.
First clinical assessment
All patients underwent clinical evaluation [including assessment of rest tremor, symmetrical/asymmetrical parkinsonism, levodopa response, orthostatic hypotension (OH), and genitourinary dysfunction (GUD)], and cerebral MRI (magnetic resonance imaging). To assess the levodopa response, the Unified Parkinson’s disease rating scale (UPDRS) III score was performed in the morning after a 12 h period of treatment withdrawal, before and 1 h following the intake of 250 mg of levodopa. A levodopa response of > 30 or > 50% meant an improvement of the UPDRS III score of > 30 or > 50%, respectively. This evaluation was performed at least 48 h before the CGH test. OH was defined by a reduction of the systolic blood pressure by at least 30 mmHg or of the diastolic blood pressure by at least 15 mmHg after 3 min of standing upright following a 3 min interval in recumbent position [3]. GUD included urinary incontinence (i.e., the inability to control the release of urine from the bladder), dysuria [incomplete bladder emptying (urine residual volume > 100 mL)], and erectile dysfunction in males as recommended by Gilman et al. [3]. Cerebral MRI was performed in all patients and included T1, T2-weighted, T2*-weighted, and FLAIR images. Image acquisition was performed in axial, sagittal, and coronal planes.
The initial clinical diagnosis (ICD) was established based on the first clinical assessment: patients were clinically stratified either to the PD group or the MSA group (even if all the PD or MSA criteria were not fulfilled, since the diagnosis was uncertain according to the inclusion criteria) based on the clinical judgment of the examiner, as commonly applied in clinical practice. The ICD was established without any further investigations. Clinical reassessments were subsequently performed every 6 months throughout the course of the disease. The final clinical diagnosis (FCD) was established when the criteria for either PD or MSA were fulfilled at any time of the follow-up but after a disease duration of at least 5 years [2, 3, 18]. The examiner responsible for both ICD and FCD had no access to the results of 123I-MIBG myocardial scintigraphy and CGH test.
Additional paraclinical investigations
The initial paraclinical assessment of all patients consisted of 123I-MIBG myocardial scintigraphy and the CGH test that were not taken into account to establish the ICD or FCD.
CGH test
The CGH-test protocol was similar to that described in the literature [9]. Following a 12 h night period of treatment withdrawal, the test was performed in the morning fasting after lying supine for at least 30 min. Blood pressure and heart rate were automatically monitored. Blood samples were collected 15 min before injection of clonidine (2 µg/kg of clonidine in 20 mL saline) and every 15 min for 1 h following the clonidine injection to determine the GH (growth hormone) serum level.
GH measurements were all performed in the same laboratory by a radio immunological method (Cis Bio ELSA HGH). The [maximum baseline] values of GH (i.e. maximum increase) were calculated as an outcome measure for both groups. In a normal CGH test, such as in PD, serum GH increases following clonidine injection with a cutoff [maximum baseline] superior of 0.02 ng/mL [19].
123I-MIBG myocardial scintigraphy
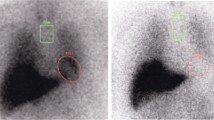
Heart to moscintigraphy was performed 4 h following the intravenous injection of 220 MBq 123I MIBG in supine position and 30 min following the intravenous injection of 740 MBq 99mTc-tetrofosmin by means of a double-head gamma camera (Siemens E-CAM or Simbia T6), high-resolution collimators, matrix size 64 × 64, and zoom 1.45. Planar acquisition was performed following the injection of 123I MIBG to do a semi-quantitative study by quantifying the heart to mediastinum uptake ratio at 1 h (early H/M) and 4 h (late H/M). The MIBG washout rates (WR) were calculated by the following formula: WR = [(early H/M)-(late H/M)] × 100/(early H/M). Furthermore, delayed images were used to perform a qualitative study for each patient. Patients having a normal and homogeneous or a mildly decreased cardiac uptake of MIBG were considered as having an MSA myocardial scintigraphy profile (i.e., normal scintigraphy). Patients presenting with no myocardial uptake were considered as having a PD profile (i.e., abnormal scintigraphy).
Statistical analysis
The sensitivity and the specificity of the CGH test and the123I-MIBG myocardial scintigraphy alone or combined with clinical signs to diagnose PD and MSA were assessed by statistical analysis. For this purpose, we compared ICD and the diagnosis established by the CGH test and the123I-MIBG myocardial scintigraphy during the first assessment to the FCD. The descriptive analysis of the qualitative variables was done using the frequency of each value and the cumulative frequency and for the quantitative variables, as well as location parameters (mean, median, minimum, maximum, first, and third quartiles), and dispersion parameters (standard deviation, variance, range, and interquartile range). Normality of the distributions was tested using the Shapiro–Wilk test and was assessed graphically using a normal quantile plot. Comparisons between qualitative variables were performed using Chi-squared test or Fisher’s exact test in case of expected values in any of the cells of a contingency table figuring lower than 5. Confidence intervals of proportions were calculated using exact binomial distribution. Comparisons between quantitative and qualitative variables were made using the Student’s t test or Wilcoxon’s test in case of heteroskedasticity or when the variable did not follow a normal distribution. To estimate the potential clinical relevance of covariates, simple and ridge logistic regression models were performed in case of colinearity, and ROC curves were compared with Delong’s test. The significance level was set at 5%. All the analyses were made with the R 3.0.2 software.
Results
Patients
Twenty-five patients with parkinsonism with uncertain diagnosis were included (15 considered as MSA and 10 as PD as ICD). After a mean follow-up of 6 years (corresponding to a mean disease duration of 10 years), 11 patients finally received the diagnosis of MSA, while 14 were diagnosed with PD as FCD (Fig. 1).
Age at disease onset, disease duration at inclusion, and disease duration at the end of follow-up were similar in both groups (Table 1).
All patients had a disease duration longer or equal to 5 years at the end of the study but three patients with MSA and one patient with PD as FCD who died within the 4 years of the follow-up. Patients who were finally diagnosed with PD as FCD had more often a levodopa response of > 30% than patients with MSA, and less frequently OH and GUD at the time of the first clinical assessment (Table 1).
Paraclinical investigations
Considering the FCD as correct diagnosis, CGH test and MIBG scintigraphy had both a sensitivity of 82% and a specificity of 71 and 93%, respectively, for the diagnosis of MSA. We found four significant variables correlating with the FCD: ICD, levodopa response of > 30%, scintigraphy, and CGH test (Table 2). Scintigraphy was superior to both CGH test and ICD with an accuracy of 88%, and an odd ratio of 58.5 (CGH test and ICD had a similar accuracy of 76%).
The combinations of scintigraphy or CGH test with the clinical features (levodopa response, OH or GUD) were significantly correlated with the FCD. The combinations of scintigraphy with GUD, levodopa response of > 30% or OH seemed to be the most relevant for the distinction between MSA and PD with an accuracy of 92%. The combination of scintigraphy with the CGH test was less relevant.
For the diagnosis of MSA, the most relevant test was the combination of a normal scintigraphy (i.e., with myocardial MIBG uptake) with GUD, whereas the most relevant tests for the diagnosis of PD were the combination of an abnormal scintigraphy (i.e., without myocardial MIBG uptake) with a levodopa response of > 30% or the association of an abnormal scintigraphy with the absence of OH (Table 3, supplemental data). All these combinations had an accuracy higher than 90% (91, 93, and 93%, respectively) and a specificity of 100%.
Clonidine GH test
Comparison of basal GH serum level and kinetics of GH serum level in both groups following clonidine injection (p = 0.01 and p = 0.0008 at 45 and 60 mn, respectively) is shown in Fig. 2.
123I-MIBG myocardial scintigraphy
One patient initially diagnosed with PD as ICD had a clinical course leading to MSA as FCD (Fig. 1). He had an abnormal CGH test (i.e., without increase of GH) and a normal scintigraphy, which were both consistent with MSA.
Five patients initially diagnosed with MSA as ICD experienced a clinical course consistent with PD as FCD (Fig. 1). They all had an abnormal scintigraphy and four of them had a normal CGH test, which were consistent with PD.
Finally, among the 6 incorrect ICD, all myocardial scintigraphies, and all but one CGH test were in accordance with the FCD.
Discussion
We aimed to evaluate the relevance of 123I-MIBG myocardial scintigraphy and/or CGH test for the distinction between PD and MSA in patients with parkinsonism of uncertain etiology. We only included patients for whom the distinction between PD and MSA was difficult, since it may be a clinical challenge and as the interest of these 2 tests for typical forms of PD and MSA is known and mostly less useful. We showed that 123I-MIBG myocardial scintigraphy alone was more relevant than the ICD for the distinction between PD and MSA, whereas but CGH test was similar to the ICD.
Our results regarding sensitivity and specificity of CGH test and myocardial scintigraphy for the diagnosis of MSA were similar to those in the literature [20,21,22]. Interestingly, a normal scintigraphy (i.e., with myocardial MIBG uptake) and GUD was the most relevant combination for the diagnosis of MSA, whereas the most relevant combinations for the diagnosis of PD were an abnormal scintigraphy (i.e. without myocardial MIBG uptake) combined to a levodopa response of > 30% or an abnormal scintigraphy combined with the absence of OH, which never has been previously reported. Orthostatic hypotension could be considered as a particularly relevant clinical feature, since the levodopa test is to some extent invasive and time-consuming, whereas GUD such as erectile dysfunction or urinary urgency are less objective or specific. Strikingly, levodopa response of > 50% was not efficient to distinguish PD from MSA probably, since such levodopa response is not common in patients with ambiguous parkinsonism.
Among the 6 incorrect ICD, the scintigraphy always pointed towards the correct diagnosis, and only one CGH test was mistaken. Thus, that we have to be very cautious before diagnosing MSA in the first stage of the disease when both, 123I-MIBG myocardial scintigraphy and CGH test, are suggestive of PD. Interestingly, when concordant, CGH test and scintigraphy predicted the correct final diagnosis in all cases. CGH test and scintigraphy have to be taken into particular consideration when they are concordant, since the 4 patients misdiagnosed with MSA in the first stage in our study had all both CGH test and scintigraphy consistent with PD.
Clonidine is an alpha-2 adrenoceptor agonist that leads to GH release by the activation of hypothalamic adrenoceptors. Clonidine raises GH serum level in healthy patients and in PD patients but not in patients with MSA with central lesions [23]. We calculated the maximum-baseline values of GH (maximum increase) as outcome measure for both groups to alleviate variations in basal concentrations of GH. In our study, mean value of increase for patients with PD was greater than patients with MSA which was in accordance with Kimber et al. [9] and Tranchant et al. [24] who found a significant increase of serum GH following clonidine injection in PD compared with MSA. However, these results remain controversial and were not confirmed by other studies, especially by Clarke et al. [25].
At inclusion in our study, the patients were in the first stage of the disease (≈ 5 years of disease duration for PD and ≈ 3 years for MSA). The distinction between PD and MSA in the first stage could be difficult, since asymmetry of parkinsonism is not rare in MSA and OH is optional in MSA. In the same way, GUD are not uncommon in PD and a levodopa response of > 50% is optional in PD. Dysautonomia is common in MSA, but may sometimes be lacking in the first stage of the disease, whereas it may also be encountered—especially OH—in PD [4,5,6]. Moreover, a levodopa response of > 50% may be absent in the first stage of few PD patients, as it may be encountered in few patients with MSA [4]. This could partially explain why there was no significant difference regarding a levodopa response of > 50% between MSA and PD in our patients considered initially with ambiguous diagnosis. Rest tremor and asymmetry are usually good signs for PD, but they may be also encountered in MSA [26]. Recently, recommendations have been proposed for the management of MSA in clinical practice, especially for aiding the early clinical diagnosis of MSA [27]. Our results could be helpful for this purpose.
Several weaknesses of our study must be raised. First, the pathological confirmation of the diagnosis is lacking; therefore, one may consider that the FCD could be wrong. The main bias of the previous studies dedicated to this topic (which reported the limitation of such paraclinical test) also was the lack of pathological evidence to confirm the diagnosis [13,14,15,16,17, 19, 24]. Indeed, autopsy series indicated that about 24% of patients initially diagnosed during life as having PD with compatible clinical features have pathological evidence of MSA [2, 26, 28, 29]. Herein, given the long-term follow-up of the patients (mean of 6 years), and given the long disease duration (mean of 11 years for PD and 8 years for MSA), that has not been reached by the previous studies, we believe that we were able to adjust the FCD and improve its accuracy despite the lack of pathological confirmation. Our study is also greatly limited by the small number of patients who have been included. Thus, our results need to be confirmed by further larger studies. However, it should be kept in mind that MSA is a rare disease and patients with an ambiguous diagnosis between PD and MSA in the first stage of the disease are still rarer. Moreover, the studies previously published on the same topic included only 18–52 patients [13,14,15,16,17, 19, 24].
Finally, the results of our small sample-size, prospective study need to be confirmed by further studies, especially the potential interest of the combination of scintigraphy with GUD, levodopa response of > 30% or orthostatic hypotension for the distinction between PD and MSA when the clinical diagnosis remains doubtful in the first stage of the disease.
Abbreviations
- CGH test:
-
Clonidine growth hormone test
- FCD:
-
Final clinical diagnosis
- GH:
-
Growth hormone
- GUD:
-
Genitourinary dysfunction
- H/M ratio:
-
Heart-to-mediastinum uptake ratio
- ICD:
-
Initial clinical diagnosis
- 123I-MIBG:
-
123Iode méta-iodobenzylguanidine
- MRI:
-
Magnetic resonance imaging
- MSA:
-
Multiple system atrophy
- OH:
-
Orthostatic hypotension
- OR:
-
Odd ratio
- PD:
-
Parkinson’s disease
- UPDRS III score:
-
Unified Parkinson’s disease rating scale III score
- WR:
-
Washout rate
References
Postuma RB, Berg D, Stern M et al (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord Off J Mov Disord Soc 30:1591–1601
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Gilman S, Wenning GK, Low PA et al (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71:670–676
Hughes AJ, Colosimo C, Kleedorfer B et al (1992) The dopaminergic response in multiple system atrophy. J Neurol Neurosurg Psychiatry 55:1009–1013
Magalhães M, Wenning GK, Daniel SE, Quinn NP (1995) Autonomic dysfunction in pathologically confirmed multiple system atrophy and idiopathic Parkinson’s disease—a retrospective comparison. Acta Neurol Scand 91:98–102
Senard JM, Raï S, Lapeyre-Mestre M et al (1997) Prevalence of orthostatic hypotension in Parkinson’s disease. J Neurol Neurosurg Psychiatry 63:584–589
Berg D, Lang AE, Postuma RB et al (2013) Changing the research criteria for the diagnosis of Parkinson’s disease: obstacles and opportunities. Lancet Neurol 12:514–524
Forjaz MJ, Ayala A, Testa CM et al (2015) Proposing a Parkinson’s disease-specific tremor scale from the MDS-UPDRS: MDS-Updrs tremor scale. Mov Disord 30:1139–1143
Kimber JR, Watson L, Mathias CJ (1997) Distinction of idiopathic Parkinson’s disease from multiple-system atrophy by stimulation of growth-hormone release with clonidine. Lancet Lond Engl 349:1877–1881
Yoshita M (1998) Differentiation of idiopathic Parkinson’s disease from striatonigral degeneration and progressive supranuclear palsy using iodine-123 meta-iodobenzylguanidine myocardial scintigraphy. J Neurol Sci 155:60–67
Braune S, Reinhardt M, Schnitzer R et al (1999) Cardiac uptake of [123I]MIBG separates Parkinson’s disease from multiple system atrophy. Neurology 53:1020–1025
Druschky A, Hilz MJ, Platsch G et al (2000) Differentiation of Parkinson’s disease and multiple system atrophy in early disease stages by means of I-123-MIBG-SPECT. J Neurol Sci 175:3–12
Raffel DM, Koeppe RA, Little R et al (2006) PET measurement of cardiac and nigrostriatal denervation in Parkinsonian syndromes. J Nucl Med Off Publ Soc Nucl Med 47:1769–1777
Chung EJ, Lee WY, Yoon WT et al (2009) MIBG scintigraphy for differentiating Parkinson’s disease with autonomic dysfunction from Parkinsonism-predominant multiple system atrophy. Mov Disord Off J Mov Disord Soc 24:1650–1655
Kimpinski K, Iodice V, Burton DD et al (2012) The role of autonomic testing in the differentiation of Parkinson’s disease from multiple system atrophy. J Neurol Sci 317:92–96
Köllensperger M, Seppi K, Liener C et al (2007) Diffusion weighted imaging best discriminates PD from MSA-P—a comparison with tilt table testing and heart MIBG scintigraphy. Mov Disord Off J Mov Disord Soc 22:1771–1776
Nagayama H, Ueda M, Yamazaki M et al (2010) Abnormal cardiac [(123)I]-meta-iodobenzylguanidine uptake in multiple system atrophy. Mov Disord Off J Mov Disord Soc 25:1744–1747
Gilman S, Low PA, Quinn N et al (1999) Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci 163:94–98
Zhang K, Zeng Y, Song C et al (2010) The comparison of clonidine, arginine and both combined: a growth hormone stimulation test to differentiate multiple system atrophy from idiopathic Parkinson’s disease. J Neurol 257:1486–1491
Pellecchia MT, Pivonello R, Colao A, Barone P (2006) Growth hormone stimulation tests in the differential diagnosis of Parkinson’s disease. Clin Med Res 4:322–325
Orimo S, Suzuki M, Inaba A, Mizusawa H (2012) 123I-MIBG myocardial scintigraphy for differentiating Parkinson’s disease from other neurodegenerative parkinsonism: a systematic review and meta-analysis. Parkinsonism Relat Disord 18:494–500
Treglia G, Cason E (2012) Meta-analysis on MIBG scintigraphy in differential diagnosis between Parkinson’s disease and neurodegenerative parkinsonism. Parkinsonism Relat Disord 18:805; author reply 806
Thomaides TN, Chaudhuri KR, Maule S et al (1992) Growth hormone response to clonidine in central and peripheral primary autonomic failure. Lancet Lond Engl 340:263–266
Tranchant C, Guiraud-Chaumeil C, Echaniz-Laguna A, Warter JM (2000) Is clonidine growth hormone stimulation a good test to differentiate multiple system atrophy from idiopathic Parkinson’s disease? J Neurol 247:853–856
Clarke CE, Ray PS, Speller JM (1999) Failure of the clonidine growth hormone stimulation test to differentiate multiple system atrophy from early or advanced idiopathic Parkinson’s disease. Lancet Lond Engl 353:1329–1330
Colosimo C, Albanese A, Hughes AJ et al (1995) Some specific clinical features differentiate multiple system atrophy (striatonigral variety) from Parkinson’s disease. Arch Neurol 52:294–298
Walsh RR, Krismer F, Galpern WR et al (2018) Recommendations of the global multiple system atrophy research roadmap meeting. Neurology 90:74–82
Rajput AH, Rozdilsky B, Rajput A (1991) Accuracy of clinical diagnosis in parkinsonism—a prospective study. Can J Neurol Sci J Can Sci Neurol 18:275–278
Wenning GK, Ben-Shlomo Y, Magalhães M et al (1995) Clinicopathological study of 35 cases of multiple system atrophy. J Neurol Neurosurg Psychiatry 58:160–166
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
No competing or conflicts of interest and no funding sources declared.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alves Do Rego, C., Namer, I.J., Marcel, C. et al. Prospective study of relevance of 123I-MIBG myocardial scintigraphy and clonidine GH test to distinguish Parkinson’s disease and multiple system atrophy. J Neurol 265, 2033–2039 (2018). https://doi.org/10.1007/s00415-018-8941-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-8941-5