Abstract
Spinocerebellar ataxia type 2 (SCA2) is an autosomal dominant neurodegenerative disease involving the cerebellum and characterized by a typical motor syndrome. In addition, the presence of cognitive impairment is now widely acknowledged as a feature of SCA2. Given the extensive connections between the cerebellum and associative cerebral areas, it is reasonable to hypothesize that cerebellar neurodegeneration associated with SCA2 may impact on the cerebellar modulation of the cerebral cortex, thus resulting in functional impairment. The aim of the present study was to investigate and quantitatively map the pattern of cerebellar gray matter (GM) atrophy due to SCA2 neurodegeneration and to correlate that with patients’ cognitive performances. Cerebellar GM maps were extracted and compared between SCA2 patients (n = 9) and controls (n = 33) by using voxel-based morphometry. Furthermore, the relationship between cerebellar GM atrophy and neuropsychological scores of the patients was assessed. Specific cerebellar GM regions were found to be affected in patients. Additionally, GM loss in cognitive posterior lobules (VI, Crus I, Crus II, VIIB, IX) correlated with visuospatial, verbal memory and executive tasks, while additional correlations with motor anterior (V) and posterior (VIIIA, VIIIB) lobules were found for the tasks engaging motor and planning components. Our results provide evidence that the SCA2 neurodegenerative process affects the cerebellar cortex and that MRI indices of atrophy in different cerebellar subregions may account for the specificity of cognitive symptomatology observed in patients, as result of a cerebello-cerebral dysregulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cerebellum is a critical node in the distributed neural circuits subserving not only motor but also autonomic, limbic and cognitive functions [1]. Over the years, increasing evidence of the cerebellar involvement in cognition has been reached leading to the description of a clinical condition, referred to as the “Schmahmann’s syndrome” (SS) [2]. Such a condition occurs in the presence of lesions of the cognitive and limbic part of the cerebellum (i.e., posterior lobes; lobules VI, Crus I and II; lobule IX) and is characterized by a complex variety of cognitive deficits [3, 4].
Impaired cognitive performance, involving language, executive, visuospatial and sequencing functions has also been found in patients with cerebellar atrophy [4, 5], a condition characterized by diffuse degeneration of the cerebellar cortex, which is regarded as the central computational integrator of the cerebellar system [6]. Throughout the cerebellar cortex, the information ultimately converges on Purkinje neurons and is then funneled out through the neurons of the deep cerebellar nuclei (DCN), the sole output of the cerebellar cortex. Through DCN, the cerebellum communicates with other parts of central nervous system by extensive excitatory connections. When Purkinje cells are under-functioning, target regions in the cerebral cortex are prevented from receiving an appropriate cerebellar modulation, which is necessary to accomplish functions successfully.
Spinocerebellar ataxia type 2 (SCA2) is an autosomal dominant cerebellar neurodegenerative disease, characterized by a progressive cerebellar syndrome, typically affecting motor functions [7] as well as cognitive performance [8,9,10]. From a neuropathological point of view, patients affected by SCA2 show a pattern of olivo-ponto-cerebellar atrophy (OPCA) combined with neuronal loss in several brainstem and cerebellar nuclei and in the cerebellar cortex alongside a diffuse damage of the brainstem and cerebellar white matter (WM) [11,12,13,14,15,16]. Additionally, cerebral cortical atrophy has been reported at most advanced disease stages [13]. Voxel-based morphometry (VBM) studies have shown cerebellar degeneration to affect supratentorial regions connected with the cerebellum [17], including the right orbitofrontal and temporomesial cortex, and the primary sensorimotor cortex bilaterally [18].
It has been suggested that the cognitive deficits observed in SCA2 patients might result from the disruption of a cerebro-cerebellar circuitry, presumably at the pontine level [19], and also from degeneration of specific cerebellar sites [20]. Consistently, altered inter-nodal connectivity has been recently reported in SCA2 patients between more posterior regions of the cerebellum and regions in the cerebral cortex that are related to cognition and emotion processing [21]. This reduced connectivity suggests that cerebellar dysfunction may affect long-distance cerebral regions, and some clinical symptoms of SCA2 may be due to abnormal connectivity between non-motor cerebello-cortical nodes. Deficits in attention, executive functions, visuo-constructive skills, visual and verbal memory and processing speed have all been reported in SCA2 patients [10]. However, a prevalent involvement of executive and visuospatial skills has been indicated [19, 22, 23] suggesting a fronto-parietal dysfunction, that could be attributed to a disconnection syndrome in the fronto-ponto-cerebello-thalamo-cortical circuits [8]. In line with these observations, the functional topography of the cerebellum posits that distinct regions of the cerebellum contribute to specific functional modules by means of segregated connections with distinct functional zones in the cerebral cortex [24].
Although the cognitive profile of SCA2 has been characterized across several studies [8,9,10], MRI indices of atrophy have never been used to account for the cognitive impairment observed in SCA2 patients.
Aim of the present study was, therefore, to examine and quantitatively map the pattern of cerebellar atrophy in patients with SCA2, to clarify the patho-anatomical basis of their neuropsychological impairment.
Materials and methods
Participants
Nine genetically confirmed patients with SCA2 (mean age/SD: 47.5/10.2; F/M: 6/3), recruited from the Ataxia Lab of Santa Lucia Foundation (Rome, Italy), were enrolled in the present study. At the time of enrollment, all patients had a disease duration longer than 6 months since their genetic confirmation of diagnosis. As part of the inclusion criteria, patients had to present with a selective atrophy of the cerebellum in the absence of any cortical lesion on conventional MRI scans.
Clinical and neurophysiological evaluation revealed that all patients had a pure cerebellar motor syndrome, except for CA-3 who was bilaterally positive for the Babinski sign. A quantification of cerebellar motor deficits was performed using the International Cooperative Ataxia Rating Scale [25], whose global score ranges from 0 (absence of any motor deficit) to 100 (presence of motor deficits at the highest degree).
Additionally, 33 healthy subjects (HS) (mean age/SD: 50.55/6.6; F/M: 21/12) with no history of neurological or psychiatric illness were recruited as control group. A T test comparison ensured that there was no significant difference in the mean age between the two groups (T = − 0.86, p = 0.39).
This research study was approved by the Ethics Committee of Santa Lucia Foundation according to the principles expressed in the Declaration of Helsinki. Written informed consent was obtained from each subject.
Main demographic and clinical characteristics of recruited patients are summarized in Table 1.
Neuropsychological assessment
SCA2 patients first underwent the Wechsler Adult Intelligent Scale-revised (WAIS-R) Intelligent Quotient (IQ) [26,27,28] and the Raven’47 progressive matrices (PM) test [29] to assess their intellectual level. Then they underwent a neuropsychological assessment exploring the domains of visuospatial abilities, verbal memory and executive functions. For each domain, details of single tests and references are summarized here:
-
Visuospatial abilities Rey–Osterrieth Complex Figure Test (recall and copy) [30], forward and backward Corsi [31], and Wechsler Adult Intelligent Scale-revised block design subtest [26,27,28];
-
Verbal memory Forward and backward digit span [32], Short story test (immediate recall) [33] and Rey’s 15 mots short term (immediate recall) [34] for short-term verbal memory; Rey’s 15 mots for the long-term verbal memory (delayed recall) [34];
-
Executive functions Stroop Test (“time effect” and “error effect”) [30], phonological, semantic and verbal fluency [35], Wisconsinn Card Sorting Test (WCST) [36], and tower of London procedure (TOL) [37], Trail Making Test B-A [38].
MRI acquisition protocol
All subjects underwent an MRI examination at 3T (Magnetom Allegra, Siemens, Erlangen, Germany) that included the following acquisitions: (1) dual-echo turbo spin echo [TSE] (TR = 6190 ms, TE = 12/109 ms); (2) fast-FLAIR (TR = 8170 ms, 204TE = 96 ms, TI = 2100 ms); (3) 3D Modified Driven Equilibrium Fourier Transform (MDEFT) scan (TR = 1338 ms, TE = 2.4 ms, matrix = 256 × 224 × 176, in-plane FOV = 250 × 250 mm2, slice thickness = 1 mm). The TSE scans of patients, acquired as part of this research study, were reviewed by an expert neuroradiologist to characterize the brain anatomy and determine the presence of macroscopic structural abnormalities. For the HS, conventional MRI scans were inspected to exclude the presence of any macroscopic brain abnormality.
Image processing
The cerebellum was pre-processed individually using the Spatially Unbiased Infratentorial Template (SUIT) toolbox [39] implemented in Statistical Parametric Mapping version 8 [Wellcome Department of Imaging Neuroscience; SPM-8 (http://www.fil.ion.ucl.ac.uk/spm/)]. The procedure involved: cropping and isolating the cerebellum from the T1 anatomical images; normalizing each cropped image into SUIT space; reslicing the probabilistic cerebellar atlas into individual subjects’ space using the deformation parameters obtained by normalization. Finally, the images were smoothed using a 8-mm FWHM Gaussian kernel.
Statistical analysis
Neuropsychological assessment
To evaluate the behavioral performance, raw scores were computed (Table 2) and converted into Z scores, according to the following formula: (subject raw score—population mean score)/population standard deviation [SD]).
Published normative data were used for the following tests: Rey–Osterrieth Complex Figure Test, (recall and copy versions), 15 Rey’s mots short- and long-term, Short Story Test (immediate recall), Block Design Test and Trail Making Test. For the remaining tests, raw scores were obtained from specific control groups for each test. Subjects of each group had no history of neurological or psychiatric illness, and were well matched with regards to age and education (independent-sample t test: p = n.s.). See Table 3 for a detailed report of demographic and cognitive data.
For each cognitive function, a single Z score was obtained by calculating the mean Z scores of the tests, grouped according to the relative functional domain. Overall, the performance in the three functional domains listed in Sect. “Neuropsychological assessment” were analyzed (see Table 2).
Voxel-based morphometry
Voxel-based morphometry (VBM) was used to identify differences in regional cerebellar volume between SCA2 patients and HS. This was achieved by performing a voxel-wise two-sample T test in SPM-8 and comparing the gray matter (GM) maps between patients and controls. Age and sex were set as variables of no interest. Results were considered significant at p values < 0.05 after family-wise error (FWE) cluster-level correction (clusters formed with p < 0.005 at uncorrected level). Tto control for the effect of accompanying cortical atrophy in SCA2 patients, a whole-brain VBM was also performed. The cerebellum was set as explicit exclusion mask. Sex, age and intracranial volumes were entered as covariates of no interest. Results were considered significant at p values < 0.05 after FWE cluster-level correction (clusters formed with p < 0.001 at uncorrected level).
Behavioral and motor correlation with regional GM
Based on VBM results, the lobular volumes of significantly reduced GM areas in patients were extracted using FSL command line from the FMRIB software library (FSL, http://www.fmrib.ox.ac.uk/fsl/) and Spearman’s correlations were computed for the relationship between such volumes, expressed in mm3, and neuropsychological performances of patients. For the purpose of these correlations, individual neuropsychological raw scores as reported in Table 2 were used. Additionally, the relation between GM atrophy and ICARS total motor scores of patients was also tested. Correlations significant at p < 0.05 were reported.
Results
Neuropsychological assessment
Total IQ (mean/DS = 84.5/7.7) and Raven’s PM scores (mean/DS = 31.5/2.4) showed that SCA2 patients had a preserved intellectual level.
The evaluation of cognitive profiles revealed that SCA2 patients had negative Z scores for all functional domains explored. A graphical representation of patients’ performances in verbal memory (− 0.17), visuospatial (− 0.62) and executive abilities (− 0.13) is reported in Fig. 1 expressed in Z scores.
Voxel-based morphometry
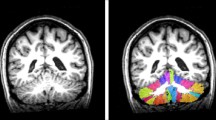
The between-group voxel-wise comparison of the GM maps revealed a statistically significant GM loss in the cerebellar cortex of SCA2 patients compared to controls. More specifically, a large cluster of decreased GM volume (cluster size: 68,396; FWE p = 0.05) included bilateral regions in the anterior cerebellar hemisphere (I–V) as well as in the posterior lobe (VI–IX) and posterior vermis (VI–IX).
As detected by whole brain VBM analysis, only one cluster of reduced GM volume was found in SCA2 patients compared to controls, centered at −15 −100 22 (left occipital pole). No other pattern of GM loss was detected throughout the cerebral cortex of SCA2 patients.
Results of cerebellar VBM are shown in Fig. 2.
Between groups voxel-based comparison of cerebellar GM volume. Cerebellar regions showing patterns of significantly reduced GM in SCA2 compared to TDA are reported and superimposed on the Spatially Unbiased Infratentorial Template (SUIT) [39]. Statistical significance was found at cluster level (FWE = 0.05; cluster size: 68,396) with peak voxel centered in the right lobules V–VI (x = 24 y = − 47 z = 25), left I–IV (x = − 9 y = − 35 z = − 19), and Left Crus II (x = − 14 y = − 89 z = − 29). Regions of reduced GM volumes involved both anterior (red) and posterior (blue) lobules of the cerebellar hemispheres (a) as well as posterior regions of the vermis (green) (b)
Behavioral and motor correlation with Regional GM
In line with VBM results, the correlations between cerebellar regions of reduced GM volumes and neuropsychological raw scores of patients were analyzed. Correlations between GM volumes and cognitive scores were performed separately for left and right lobules, thus accounting for cerebellar functional lateralization. As shown by the Spearman’s correlation coefficients, significant correlations were found between GM volumes in different cerebellar regions and specific cognitive subtests within the distinct functional domains without a clear lateralization. Within the visuospatial domains, significant GM loss in posterior lobules VIIB, VIIIA, Crus I and Crus II, as well as anterior lobules V and vermis, was found to correlate with performances at visuospatial tasks (Rey–Osterrieth Complex Figure, memory and copy, Block Design).
Similarly, within the verbal memory domain, significant GM loss in posterior lobules VI, IX, and Crus I was found to correlate with performances at short- and long-term verbal memory tasks (immediate and delayed recall of Rey’s 15 mots, Digit Span Forward and Backward), with an additional correlation between Digit Span Backward and significant GM loss in anterior lobules V. Finally, within the executive domains, significant GM loss in lobules VI, Crus I, Crus II, and IX was found to correlate with performances at executive tasks (Wisconsin Card Sorting Test, Tower of London, Stroop task, Phonological Fluency) with an additional correlation between Tower of London scores and anterior lobule V. A comprehensive report of results and statistics is summarized in Table 4.
With regards to cerebellar motor impairment, a significant negative correlation was found between the ICARS total score and GM volumes in the left hemispheric lobules I–IV (R: − 0.83; p 0.00) and V (R = 0.75; p 0.01).
Discussion
In the present study, we quantitatively mapped the pattern of cerebellar atrophy in SCA2 patients and assessed its relationship with cognitive profiles. Consistent with the existing literature, SCA2 patients reported negative Z scores in executive, visuospatial and memory domains [10, 20, 22].
As shown by VBM analysis, a specific pattern of GM reduction was found in the cerebellar cortex of SCA2 patients, specifically involving the anterior and posterior hemispheric lobules, and the posterior regions of the vermis.
In line with the well-known cerebellar functional topography [40], significant associations were found between atrophy in the posterior lobules of the cerebellar hemisphere and vermis, and patients’ performances on cognitive tasks with no significant motor component. Interestingly, in our group of patients, a negative correlation emerged also between the severity of cerebellar motor symptoms (as measured by ICARS total score) and GM volumes in the anterior cerebellar lobules.
The functional topography of the cerebellum has been well established by both functional and structural studies in healthy and clinical populations [21, 24, 40,41,42,43,44,45]. A detailed mapping of motor and cognitive dysfunctions linking to specific cerebellar lobules has been proposed in a large cohort of patients with mixed subtypes of cerebellar neurodegenerative disease [45] using the automated cerebellar lobular segmentation proposed by Yang and colleagues [46].
Overall, the findings reported by Kansal and colleagues [45] are consistent with our current results indicating positive associations between anterior lobe and motor and mixed tasks, and posterior lobe with cognitive tasks involving working memory, phonological fluency and immediate and delayed recall. However, due to the heterogeneity of the sample, the study by Kansal and colleagues [45] did not allow to characterize the specific features of a particular cerebellar disease [45] and did not account for the functional lateralization [47], since that left- and right-sided values were combined.
The present study represents a further step forward in the effort to overcome these limitations and going beyond the well-established anterior–posterior distinction of cerebellar functions, characterizing the structural correlates of impaired cognitive performances associated with a particular cerebellar disease, such as SCA2.
Cognitive deficits have been reported in SCA2 patients [9, 10, 22] as a result of the disruption of a cerebro-cerebellar circuitry [18] and as influenced by the specific site of cerebellar degeneration [20]. To our knowledge this is the first study that attempts to investigate the relationship between SCA2 cerebellar degeneration (measured by regional atrophy) and functional outcomes of the patients.
In our cohort of SCA2 patients, performances at executive tasks variably correlated with GM loss in posterior cerebellar lobules as well as anterior cerebellar lobules in the case of tasks that engaged planning and motor components (i.e., ToL, see Table 4 for details). In line with the proposed link between structural and functional connectivity [48], a functional disconnection has been previously reported in SCA2 patients between posterior cerebellar lobules and cortical prefrontal regions which have been implicated in a wide range of executive tasks with both verbal and visuospatial stimuli [21].
It is worth noting that phonological fluency scores correlated with reduced cerebellar GM volume while no correlation was found with semantic fluency. This fits with the frequent observation of cerebellar patients being selectively impaired in phonological fluency, a function that requires an unusual word searching strategy (compared to semantic strategy) thus reflecting the role of the cerebellum in strategy formation [49].
Within the verbal and visuospatial domains, working memory measures were variably correlated with hemispheric and vermal regions in the posterior cerebellum, either including cerebellar Crus I and more sensorimotor lobules such as lobule VIII [50]. Overall, the lobular pattern of correlations largely overlaps with the initial description of SS, suggesting that in cerebellar patients cognitive impairment is associated with posterior damage of the cerebellum, specifically Crus I and Crus II [2], and is consistent with neuroimaging functional studies [51], showing that sensorimotor lobule VIII is also engaged during working memory tasks.
Although the majority of cerebello-cerebral connections are contralateral [52,53,54], correlations between cerebellar volumes and visuospatial and verbal scores did not show specific pattern of lateralization.
In line with the functional lateralization of the cerebellum [40, 55], this could be somewhat unexpected. However, it has to be considered that anatomical and functional studies have shown connections between cerebellum and cerebral cortex to be also ipsilateral [56, 57], passing through the Superior Cerebellar Peduncle and reaching the ipsilateral thalamus [58]. Consistently, evidence that patients with left or right cerebellar damage presented with a similar cognitive profile has been previously reported [4].
Another issue that deserves to be discussed is the negative correlation emerging between spatial span scores and right cerebellar lobule VIIIB, suggesting that lower GM volumes are associated with better spatial span scores. Although these findings seem to be counter-intuitive, in line with Stoodley and colleagues [44], they further emphasize the idea that, in the case of the cerebellum, the specific lesion location rather than size may be an important factor for the clinical outcome [44]. Although the cerebellar focal lesion may represent a more interesting model to map the lobular functional organization of the cerebellum for motor versus cognitive functions, here we provided evidence of a lobular functional subdivision in a diffuse neurodegenerative disorder of the cerebellum by showing that a specific pattern of cerebellar atrophy is associated to SCA2 with a clear anterior–posterior distinction. As showed by the whole-brain VBM, SCA2 patients did not show a significant pattern of cortical atrophy. Indeed, no significant GM reductions were detected throughout the cerebral cortex of SCA2 patients, except for only one cluster centered in the left occipital pole. Thus, it is reasonable to think that this finding is not specific for SCA2 and may be due to the close anatomical proximity between the cerebellum and the occipital pole. In line with the evidence that SCA2 patients in the present study did not have significant cortical GM changes, we assume that the cerebellar atrophy may have altered functional connectivity patterns within relevant cerebello-cerebral networks and reduced the cerebellar modulation of cerebral cortex regions, thus resulting in functional depression of such regions and accounting for the various clinical dysfunctions typically observed [5, 21]. Specifically, in line with Fancellu and colleagues [9], a functional disconnection of the fronto-ponto-cerebello-thalamo-cortical pathway may result in a fronto-parietal dysfunction and be responsible for the pattern of executive, visuospatial and verbal impairment observed in SCA2 patients.
The main limitation of the study is the small sample size. However, it has to be considered that the strict inclusion criteria (see Sect. “Participants”) clearly affects the inclusion rate. In spite of this, the statistically high significance of our data and the consistence with the existent literature strongly reinforce the relevance of the results.
Overall, our data suggest that MRI indices of atrophy, in relation to cognitive performances in patients with cerebellar degeneration, might differentiate between different SCA or cerebellar atrophy subtypes. In light of the important clinical implication for patients, this issue merits to be deeply investigated in the future by comparing larger populations affected by cerebellar pathology of different etiology.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Schmahmann JD, Caplan D (2006) Cognition, emotion and the cerebellum. Brain 129(Pt 2):290–292
Schmahmann JD, Sherman JC (1998) The cerebellar cognitive affective syndrome. Brain 121:561–579
Clausi S, Bozzali M, Leggio MG, Di Paola M, Hagberg GE, Caltagirone C, Molinari M (2009) Quantification of gray matter changes in the cerebral cortex after isolated cerebellar damage: a voxel-based morphometry study. Neuroscience 162(3):827–835
Tedesco AM, Chiricozzi FR, Clausi S, Lupo M, Molinari M, Leggio MG (2011) The cerebellar cognitive profile. Brain 134:3672–3686
Olivito G, Lupo M, Iacobacci C, Clausi S, Romano S, Masciullo M, Molinari M, Cercignani M, Bozzali M, Leggio M (2017) Microstructural MRI basis of the cognitive functions in patients with Spinocerebellar ataxia type 2. Neuroscience 366:44–53
Chopra R, Shakkottai VG (2014) Translating cerebellar Purkinje neuron physiology to progress in dominantly inherited ataxia. Futur Neurol 9:187–196
Takahashi T, Katada S, Onodera O (2010) Polyglutamine diseases: where does toxicity come from? What is toxicity? Where are we going? J Mol Cell Biol 2:180–191
Klinke I, Minnerop M, Schmitz-Hübsch T, Hendriks M, Klockgether T, Wüllner U, Helmstaedter C (2010) Neuropsychological features of patients with Spinocerebellar ataxia (SCA), types 1,2,3, and 6. Cerebellum 9:433–442
Fancellu R, Paridi D, Tomasello C, Panzeri M, Castaldo A, Genitrini S, Soliveri P, Girotti F (2013) Longitudinal study of cognitive and psychiatric functions in spinocerebellar ataxia types 1 and 2. J Neurol 260:3134–3143
Moriarty A, Cook A, Hunt H, Adams ME, Cipolotti L, Giunti P (2016) A longitudinal investigation into cognition and disease progression in spinocerebellar ataxia types 1, 2, 3, 6, and 7. Orphanet J Rare Dis 11:82
Della Nave R, Ginestroni A, Tessa C, Cosottini M, Giannelli M, Salvatore E, Sartucci F, De Michele G, Dotti MT, Piacentini S, Mascalchi M (2008) Brain structural damage in spinocerebellar ataxia type 2. A voxel-based morphometry study. Mov Disord 23(6):899–903
Durr A, Smadja D, Cancel G, Lezin A, Stevanin G, Mikol J et al (1995) Autosomal dominant cerebellar ataxia type I in Martinique (French West Indies), Clinical and neuropathological analysis of 53 patients from three unrelated SCA2 families. Brain 118:1573–1581
Estrada R, Galarraga J, Orozco G, Nodarse A, Auburger G (1999) Spinocerebellar ataxia 2 (SCA2),: morphometric analyses in 11 autopsies. Acta Neuropathol (Berl) 97:306–310
Gilman S, Sima AA, Junck L, Kluin KJ, Koeppe RA, Lohman ME et al (1996) Spinocerebellar ataxia type1 with multiple system degeneration and glial cytoplasmic inclusions. Ann Neurol 39:241–255
Pang JT, Giunti P, Chamberlain S, An SF, Vitaliani R, Scaravilli T, Martinian L, Wood NW, Scaravilli F, Ansorge O (2002) Neuronal intranuclear inclusions in SCA2: a genetic, morphological and immunohistochemical study of two cases. Brain 125:656–663
Iwabuchi K, Tsuchiya K, Uchihara T, Yagishita S (1999) Autosomal dominant spinocerebellar degenerations. Clinical, pathological, and genetic correlations. Rev Neurol (Paris) 155:255–270
Dayan M, Olivito G, Molinari M, Cercignani M, Bozzali M, Leggio M (2016) Impact of cerebellar atrophy on cortical gray matter and cerebellar peduncles as assessed by voxel-based morphometry and high angular resolution diffusion imaging. Funct Neurol 31(4):239–248
Brenneis C, Bosch SM, Schocke M, Wenning GK, Poewe W (2003) Atrophy pattern in SCA2 determined by voxel-based morphometry. NeuroReport 14:1799–1802
Bürk K, Globas C, BöschS Klockgether T, ZühlkeI C, Daum J, Dichgans J (2003) Cognitive deficits in spinocerebellar ataxia type 1, 2, and 3. J Neurol 250:207–211
Kawai Y, Suenaga M, Watanabe H, Sobue G (2009) Cognitive impairment in spinocerebellar degeneration. Eur Neurol 61:257–268
Olivito G, Cercignani M, Lupo M, Iacobacci C, Clausi S, Romano S, Masciullo M, Molinari M, Bozzali M, Leggio M (2017) Neural substrates of motor and cognitive dysfunctions in SCA2 patients: a network based statistics analysis. Neuroimage Clin 14:719–725
Le Pira F, Zappala G, Saponara R, Domina E, Restivo AD, Reggio E, Nicoletti A, Giuffrida S (2002) Cognitive findings in spinocerebellar ataxia type 2: relationship to genetic and clinical variables. J Neurol Sci 201:53–57
Storey E, Forrest SM, Shaw JH, Mitchell P, Gardner RJ (1999) Spinocerebellar ataxia type 2: clinical features of a pedigree displaying prominent frontal-executive dysfunction. Arch Neurol 56(1):43–50
Stoodley CJ, Schmahmann JD (2010) Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 46(7):831–844
Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, Bryer A, Diener HC, Massaquoi S, Gomez CM, Coutinho P, Ben Hamida M, Campanella G, Filla A, Schut L, Timann D, Honnorat J, Nighoghossian N, Manyam B (1997) International cooperative ataxia rating scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci 145:205–211
Wechsler D (1981) Scala di intelligenza Wechsler per adulti rivisitata (WAIS-R), Manuale, Firenze Organizzazioni Speciali
Orsini A, Laicardi C (2003) WAIS-R e terza età, Firenze Organizzazioni Speciali
Orsini A, Laicardi C (1997) WAIS-R-Contributo alla Taratura Italiana, Firenze Organizzazioni Speciali
Raven JC (1949) Progressive matrices, Sets A, Ab, B: Board and Book forms. Lewis, London
Caffarra P, Vezzadini G, Dieci F, Zonato F, Venneri A (2002) Rey–Osterrieth complex figure: normative values in an Italian population sample. Neurol Sci 22:443–447
Corsi PM (1972) Human memory and the medial temporal regions of the brain. Dissert Abst Int 34:891
Monaco M, Costa A, Caltagirone C, Carlesimo GA (2013) Forward and backward span for verbal and visuo-spatial data: standardization and normative data from an Italian adult population. Neurol Sci. https://doi.org/10.1007/s10072-012-1130-x
Spinnler H, Tognoni G (1987) Standardizzazione e taratura italiana di test neuropsicologici. Ital J Neurol Sci 8(Suppl):1–120
Carlesimo GA, Caltagirone C, Gainotti G (1996) The mental deterioration battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The Group for the Standardization of the Mental Deterioration Battery. Eur Neurol 36(6):378–384
Borkowsky JG, Benton AL, Spreen O (1967) Word fluency and brain-damage. Neuropsychologia 5:135–140
Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G (2000) WCST: Wisconsin Card Sorting Test. Forma completa revisionata. Adattamento italiano a cura di Hardoy MC, Carta MG, Hardoy MJ e Cabras PL. Ed. It. O.S. Organizzazioni Speciali. Firenze
Krikorian R, Bartok J, Gay N (1994) Tower of London procedure: a standard method and developmental data. J Clin Exp Neuropsychol 16(6):840–850
Giovagnoli AR, Del Pesce M, Mascheroni S, Simoncelli M, Laiacona M, Capitani E (1996) Trail making test: normative values from 287 normal adult controls. Ital J Neurol Sci 17:305–309
Diedrichsen J, Balsters JH, Flavell J, Cussan SE, Ramnani N (2009) A probabilistic atlas of the human cerebellum. Neuroimage 46:39–46
Stoodley CJ, Schmahmann JD (2009) Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage 44:489–501
Krienen FM, Buckner RL (2009) Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex 19(10):2485–2497
Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD (2009) Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci 29:8586–8594
Bernard JA, Seidler RD, Hassevoort KM, Benson BL, Welsh RC, Wiggins JL, Jaeggi SM, Buschkuehl M, Monk CS, Jonides J, Peltier SJ (2012) Resting state functional connectivity networks: a comparison of anatomical and self-organizing map approaches. Front Neuroanat 10:6–31
Stoodley CJ, MacMore JP, Makris N, Sherman JC, Schmahmann JD (2016) Location of lesion determines motor vs. cognitive consequences in patients with cerebellar stroke. Neuroimage Clin 12:765–775
Kansal K, Yang Z, Fishman AM, Sair HI, Ying SH, Jedynak BM, Prince JL, Onyike CU (2017) Structural cerebellar correlates of cognitive and motor dysfunctions in cerebellar degeneration. Brain 140(3):707–720
Yang Z, Ye C, Bogovic JA, Carass A, Jedynak BM, Ying SH et al (2016) Automated cerebellar lobule segmentation with application to cerebellar structural analysis in cerebellar disease. Neuroimage 127:435–444
King M, Hernandez-Castillo C, Diedrichsen J (2017) Towards a multi-function mapping of the cerebellar cortex. Brain 140(3):522–524
van den Heuvel MP, Hulshoff Pol HE (2010) Exploring the brain network, a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol 20:519–534
Leggio MG, Silveri MC, Petrosini L, Molinari M (2000) Phonological grouping is specifically affected in cerebellar patients: a verbal fluency study. J Neurol Neurosurg Psychiatry 69:102–106
Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT (2011) The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 106:2322–2345
Keren-Happuch E, Chen SH, Ho MH, Desmond JE (2014) A meta-analysis of cerebellar contributions to higher cognition from PET and fMRI studies. Hum Brain Mapp 35:593–615
Evarts EV, Thach WT (1969) Motor mechanisms of the CNS: cerebrocerebellar interrelations. Annu Rev Physiol 31:451–498
Kemp JM, Powell TP (1971) The connexions of the striatum and globus pallidus: synthesis and speculation. Philos Trans R Soc Lond B Biol Sci 262:441–457
Schmahmann JD, Pandya DN (1997) The cerebrocerebellar system. Int Rev Neurobiol 41:31–60
Wang D, Buckner RL, Liu H (2013) Cerebellar asymmetry and its relation to cerebral asymmetry estimated by intrinsic functional connectivity. J Neurophysiol 109(1):46–57
Middleton FA, Strick PL (2001) Cerebellar projections to the prefrontal cortex of the primate. J Neurosci 21:700–712
Allen G, McColl R, Bernard H, Ringe WK, Fleckenstein J, Cullum CM (2005) Magnetic Resonance Imaging of cerebellar-prefrontal and cerebellar parietal functional connectivity. Neuroimage 28:39–48
Milardi D, Arrigo A, Anastasi G, Cacciola A, Marino S, Mormina E, Calamuneri A, Bruschetta D, Cutroneo G, Trimarchi F, Quartarone A (2016) Extensive direct subcortical cerebellum-basal ganglia connections in human brain as revealed by constrained spherical deconvolution tractography. Front Neuroanat 10:29
Acknowledgements
This work was supported by the Ministry of Education, Universities and Research (MIUR) (Grant Number C26A1329AR) to ML, and Ministry of Health (Grant Number RF-2011-02348213) to MM and (Grant Number GR-2013-02354888) to SC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
This study has been approved by the appropriate ethical committee and has, therefore, been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
All individuals participating in the study gave their informed consent prior to their inclusion in the study.
Conflicts of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Olivito, G., Lupo, M., Iacobacci, C. et al. Structural cerebellar correlates of cognitive functions in spinocerebellar ataxia type 2. J Neurol 265, 597–606 (2018). https://doi.org/10.1007/s00415-018-8738-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-8738-6