Abstract
Background
Many neuroscience and neurology studies have forced a reconsideration of the traditional motor-related scope of cerebellar function, which has now expanded to include various cognitive functions. Spinocerebellar ataxia type 3 (SCA3; the most common hereditary ataxia) is neuropathologically characterized by cerebellar atrophy and frequently presents with cognitive impairment.
Objective
To characterize cognitive impairment in SCA3 and investigate the cerebellum–cognition associations.
Methods
This prospective, cross-sectional cohort study recruited 126 SCA3 patients and 41 healthy control individuals (HCs). Participants underwent a brain 3D T1-weighted images as well as neuropsychological tests. Voxel-based morphometry (VBM) and region of interest (ROI) approaches were performed on the 3D T1-weighted images. CERES was used to automatically segment cerebellums. Patients were grouped into cognitively impaired (CI) and cognitively preserved (CP), and clinical and MRI parameters were compared. Multivariable regression models were fitted to examine associations between cerebellar microstructural alterations and cognitive domain impairments.
Results
Compared to HCs, SCA3 patients showed cognitive domain impairments in information processing speed, verbal memory, executive function, and visuospatial perception. Between CI and CP subgroups, the CI subgroup was older and had lower education, as well as higher severity scores. VBM and ROI analyses revealed volume loss in cerebellar bilateral lobule VI, right lobule Crus I, and right lobule IV of the CI subgroup, and all these cerebellar lobules were associated with the above cognitive domain impairments.
Conclusions
Our findings demonstrate the multiple cognitive domain impairments in SCA3 patients and indicate the responsible cerebellar lobules for the impaired cognitive domain(s).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cerebellum has traditionally been conceptualized in terms of motor function; however, over the past 35 years, a shift has taken place in the understanding of the potential function(s) of the cerebellum, expanding to roles in human cognition and emotion [1,2,3]. The cerebellum is connected to multiple brain regions, and these connections contribute to it participate in cognitive functions [2, 3]. The cerebellum’s roles in cognition could include cognitive control and executive functions, attention and working memory, language and speech, motor learning and skill acquisition, and emotional regulation [2,3,4]. The reconsideration of cerebellum–cognition interrelationships has increased interest in discovering the mechanisms through which the cerebellum exerts its influence on cerebral higher-order functions in many non-hereditary neurodegenerative disorders, including Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis [5,6,7,8,9].
As a noninvasive neuroimaging technique, volumetric MRI allows researchers and clinicians to delineate the neurodegeneration alterations of the brain in vivo, and such data have been used to identify cerebellum–cognition associations. Studies in common non-hereditary neurodegenerative disorders have investigated the role(s) of the cerebellum in cognitive decline, and their findings have to some extent supported the view that cerebellar volume loss involving the posterior lobe can lead to cerebellar cognitive impairment [10,11,12].
Spinocerebellar ataxia type 3 (SCA3) is a common hereditary neurodegenerative disease that is caused by mutation of the ATXN3 gene [13]. SCA3 is neuropathologically defined by the presence of poly(Q)-expanded ataxin-3 aggregates, which lead to neurotoxic degeneration of neurons and neural networks in the cerebellum and brainstem, in basal ganglia, and in cerebral cortex [14,15,16]. Cerebellar atrophy is understood as a “core deficit” in SCA3; and this atrophy is thought to occur during the pre-symptomatic stage of the disease, when the cerebrum volume is relatively preserved [17]. Cognitive impairment is the main non-motor symptom in SCA3 [18]; and impairments in executive functions, verbal fluency, visuospatial abilities, and verbal memory have been reported by studies of SCA3 patients [19]. In addition, expressive and receptive language challenges may arise in some SCA3 patients [20]. Mood alterations, such as depression and anxiety, have been observed in SCA3 patients [21, 22]. SCA3’s monogenic genetic background and well-defined pathogenic mechanism, which predominantly involves cerebellar atrophy, make it a valuable context for cerebellum–cognition investigations.
While several small-sample case–control studies of SCA3 have reported associations between cognitive impairments and neuronal damage in cerebral and cerebellar regions [21, 23], it remains unclear which particular cerebellar lobules may contribute to cognitive impairments, owing largely to the lack of data based on cerebellar segmentation techniques.
In the present cross-sectional study, we evaluated neuropsychological performance in the SCA3 patients, and applied a patch-based multi-atlas segmentation tool called CERES (CEREbellum Segmentation) to automatically segment the cerebellum on T1-weighted MRIs into 26 cerebellar lobules (left and right hemispheres) [24]. The aims of this study were to assess neuropsychological performance in our large SCA3 cohort, to investigate differences in clinical and MRI parameters between the cognitively impaired (CI) and cognitively preserved (CP) subgroups we defined based on performance, and to identify associations between volume loss in specific cerebellar lobules and cognitive impairments in particular cognitive domains in SCA3.
Materials and methods
Standard protocol approvals, registrations and patient consent
The study was approved by the Ethics Committee for Medical Research of the First Affiliated Hospital of Fujian Medical University ([2019]195). The ClinicalTrials.gov identifier is NCT04010214 for this study. All participants gave written informed consent.
Study design and participants
This was a prospective, cross-sectional cohort study to investigate potential associations between cognitive impairment and cerebellar volume loss in genetically confirmed SCA3 patients. All participants were recruited consecutively from the Organization in South-East China for Cerebellar Ataxia Research (OSCCAR) in the department of neurology of the First Affiliated Hospital of Fujian Medical University in China between 1 March 2021 and 31 August 2022. Eligible 126 patients had genetically confirmed SCA3 with cytosine–adenine–guanine (CAG) repeat expansion in alleles of ATXN3 with numbers ranging from 56 to 87 [14]. Forty-one sex-, age-, and education level-matched healthy control individuals (HCs) with normal CAG repeat numbers ranging from 12 to 44 [14] were recruited at the same hospital. Within our cohort study, each participant was required to complete a comprehensive case report form concurrent with their neuropsychological assessment. To ensure the accuracy and reliability of the findings, we excluded individuals with (1) taking drugs that might have effects on cognitive performance, such as antidepressants and antipsychotics, (2) previous or current addiction to substances, including alcohol, (3) a history of other neurologic or systemic diseases, and (4) other causes of structural lesions on MRIs.
Neuropsychological tests
The Chinese versions of the Mini-Mental State Examination (MMSE) [25] and the Montreal Cognitive Assessment (MoCA) [26] were used to screen for cognitive impairment, and total scores were calculated. We used the modified Minimal Assessment of Cognitive Function in Multiple Sclerosis (MACFIMS) [27, 28] to evaluate five cognitive domains, comprising tests of verbal memory (with tests of the California Verbal Learning Test-Second Edition [CVLT-II]), including short-term storage, consistent long-term retrieval, and delayed recall (DR); visual memory (the Brief Visuospatial Memory Test-Revised [BVMT-R]), including short-term storage and DR; visuospatial perception (the Judgement of Line Orientation Test [JLO]); executive function (the Controlled Oral Word Association Test [COWAT]); and information processing speed (the Symbol Digit Modalities Test [SDMT] and the Paced Auditory Serial Addition Task [PASAT], including PASAT-3 seconds and PASAT-2 seconds). The MACFIMS tests cover various cognitive domains and have been widely applicable, not only for multiple sclerosis but also for other neurological diseases [27], and they acknowledge the potential cognitive function caused by damage to extracerebellar brain regions. Additionally, the MACFIMS has been demonstrated to have good reliability and validity in numerous studies [29]. We also validated the reliability and the validity of the MACFIMS tests in our SCA3 population. In recent years, a valuable cognitive diagnostic tool—cerebellar cognitive affective syndrome (CCAS) scale—is used to perform neuropsychological assessment in SCA3 patients [30]. However, some studies have raised concerns about a high number of false-positive test results of the CCAS scale [31]. The reliability and the validity of the CCAS scale remain to be validated in future studies. The Hamilton Depression Rating Scale (HDRS) [32] and the Hamilton Anxiety Rating Scale (HARS) [33] were used to evaluate emotional performance of depression and anxiety, respectively. To ensure standardization and reliability of data, all neuropsychological tests were performed by one trained team (LLQ, XYC, and CYH); and all participants were tested in a same manner, during daytime, in a quiet room, and in a fixed order. A detailed description of the neuropsychological tests is provided in the supplementary material.
The above neuropsychological test results were reported as mean (± standard deviation, SD). For the modified MACFIMS, the variables that assessed cognitive impairment included test score of CVLT-II total learning (TL; total of five learning trials for short-term storage) and/or CVLT-II DR; test score of BVMT-R total learning (TL; total of three learning trials for short-term storage) and/or BVMT-R DR; test score of JLO; test score of COWAT; and test score of SDMT and/or PASAT-3 seconds and/or PASAT-2 seconds. Impaired function on a single test was defined as –2 SD below the mean of the respective HCs group [34]. SCA3 patients were defined as CI when scoring was outside the normal range in two or more of the evaluated five cognitive domains of the modified MACFIMS; otherwise, SCA3 patients were defined as CP [27].
Due to the extensive time required and intricacy of the process for evaluating all subscales of the modified MACFIMS tests, 6 SCA3 patients were unable to complete one or two subscales. The number of SCA3 patients who successfully completed each subscale of the MACFIMS tests is outlined in Table S1. The missing data were excluded in our further analyses.
Clinical assessment
Clinical profiles of all participants were recorded. “Age” refers to the age at examination for neuropsychological tests and MRI. “Disease duration” denotes the interval from the date of the first symptom onset to the date of the examination for neuropsychological tests and MRI. All participants’ motor functions were assessed using the Scale for the Assessment and Rating of Ataxia (SARA) and the International Cooperative Ataxia Rating Scale (ICARS), which are composite cerebellar ataxia scales [35, 36]. Patients with a SARA score < 3 were defined as pre-symptomatic SCA3; those with SARA score ≥ 3 were defined as symptomatic SCA3 [37]. In the ICARS scale, four subscales of main symptomatology were separately described [36, 38]: (1) postural and gait disturbances; (2) limb ataxia; (3) speech disorders; and (4) oculomotor disorders, which would be used to assess the associations between motor function with the volume of cerebellum.
MRI data acquisition and analysis
Participants underwent 3.0-Tesla brain MRI examinations (Siemens Skyra scanner) at the time of neuropsychological tests. Sagittal anatomical images were acquired using a 3-dimensional T1-weighted magnetization-prepared rapid gradient-echo (MP-RAGE) sequence with the following scan parameters: repetition time = 2300 ms, echo time = 2.3 ms, inversion time = 900 ms, flip angle = 8°, field of view = 240 mm × 256 mm, number of slices = 192, voxel size = 1 × 1 × 1 mm3, total acquisition time = 5.2 min. After completing the MRI scan, all data sets were checked by one experienced radiologist for quality control, including motion correction and cutoffs for head movement.
Structural MRI data analysis was undertaken using Statistical Parametric Mapping software (SPM12, http://www.fl.ion.ucl.ac.uk/spm) running on MATLAB. The T1-weighted images were segmented into gray matter (GM), white matter, and cerebrospinal fluid, and were normalized to standard space with Diffeomorphic Anatomical Registration using the Exponentiated Lie algebra. Modulated GM images were smoothed using an 8-mm full-width at half-maximum Gaussian filter for further voxel-wise statistical analyses [39, 40].
The anatomical automatic labeling brain atlas template was used to quantificationally extract cerebral region volume of the regions of interest (ROIs) based on automated MRI brain volumetry system (www. volbrain.upv.es). The ROIs for cerebral region volume quantization were as follows: (1) bilateral frontal lobes; (2) bilateral parietal lobes; (3) bilateral occipital lobes; (4) bilateral temporal lobes; (5) right middle frontal gyrus; and (6) left caudate. The decision to analyze the left caudate and right middle frontal gyrus as ROIs was guided by the results from voxel-wise statistical analyses. Additionally, cerebellar segmentation was performed to quantify cerebellar lobule volumes based on CERES software. A total of 26 cerebellar lobules were obtained considering left and right hemispheres: white matter and lobules I–II, III, IV, V, VI, Crus I, Crus II, VIIb, VIIIa, VIIIb, IX, and X. The pipeline for cerebellum segmentation and normalization was previously described [24].
Statistics
Continuous variables were presented as mean (SDs) or median (range); categorical data were presented as percentage (%). Shapiro–Wilk’s test was used to assess normality of the variables. Then, nonparametric continuous variables were compared with the Mann–Whitney U test (for 2 groups); an independent t test was used for continuous variables with a normal distribution. A χ2 test (Fisher’s exact test when the expected value was < 5) was used to compare categorical variables. The strength of correlations between variables was assessed with Pearson’s correlation coefficient. Bonferroni correction was applied to adjust for multiple comparisons of clinical data.
Voxel-based morphometry (VBM) was first performed for comparing GM volume between the two subgroups of CI and CP based on an independent t test, with sex [categorical data], age [continuous variable], and total intracranial volume (TIV) [continuous variable] as covariates. Results were considered significant at P < 0.05 after false discovery rate (FDR) correction at cluster level and cluster size, which must be greater than 100 voxels. Further, multiple regression analysis was performed to compare volume of ROIs between the two subgroups, with sex [categorical data], age [continuous variable], and TIV [continuous variable] as covariates. Lastly, multivariable-adjusted models (adjusted for age [continuous variable], sex [categorical data], years of education [continuous variable], and TIV [continuous variable]) were performed to assess the associations between brain region volume and cognitive function/emotional performance/motor function.
All the statistical analyses were performed using SPSS (version 25; IBM, USA). The P < 0.05 were considered statistically significant.
Results
Participants
A total of 126 SCA3 patients (with CAG repeats ranging in number from 58 to 85) and 41 HCs (CAG repeats from 13 to 43) were included in this study. SCA3 patients and HCs were matched for age, sex, and years of education. Among the SCA3 patients, 76.2% were symptomatic and 23.8% were pre-symptomatic. Demographic, clinical, and genetic characteristics are presented in Table 1.
Cognitive functions
A total of 120 SCA3 patients completed all subscales of the modified MACFIMS tests, and the modified MACFIMS tests showed good reliability (Cronbach’s α > 0.8; Table S2) and good validity (Kaiser–Meyer–Olkin Measure of Sampling Adequacy = 0.8, Bartlett’s Test of Sphericity P < 0.05; Table S3).
Compared to the HCs, the SCA3 patients had lower MMSE and MoCA scores and had higher HARS scores; there were no differences for HDRS values between SCA3 patients and HCs. SCA3 patients showed lower scores in the respective tests of the modified MACFIMS of JLO (P < 0.05), COWAT (P < 0.0001), SDMT (P < 0.0001) and/or PASAT (both P < 0.0001), and CVLT-II (note that excepting the item score for free recall insert, the P values for item scores were all < 0.05) (Fig. 1A). There was no difference in BVMT-R scores between SCA3 patients and HCs. Raw neuropsychological tests scores for single items are shown in Table S4.
Modified Minimal Assessment of Cognitive Function in Multiple Sclerosis (MACFIMS) tests in SCA3 patients. A Test scores of CVLT-II TL (total of five learning trials for short-term storage), CVLT-II DR, BVMT-R TL (total of three learning trials for short-term storage), BVMT-R DR, JLO, COWAT, SDMT, PASAT-3 seconds, and PASAT-2 seconds in SCA3 patients (n = 126, red bar) and healthy control individuals (n = 41, green bar). Bars present the mean value ± SD. The modified MACFIMS test scores were compared by independent sample t test. *P < 0.05; ** P < 0.01; ***P < 0.001; ****P < 0.0001. B Frequency of impairments (red histogram) in the five cognitive domains of information processing speed, verbal memory, visual memory, executive function, and visuospatial perception in SCA3 patients. Green histogram represents preservation in these five cognitive domains. BVMT-R DR = Brief visuospatial memory test-revised, delay recall; BVMT-R TL = brief visuospatial memory test-revised, total learning; COWAT = controlled oral word association test; CVLT-II DR = California verbal learning test-second edition, delay recall; CVLT-II TL = California verbal learning test-second edition, total learning; JLO = judgment line orientation; PASAT 2 s = paced auditory serial addition task-2 s; PASAT 3 s = paced auditory serial addition task-3 seconds; SCA3 = spinocerebellar ataxia type 3; SDMT = symbol digit modalities test
In terms of the five cognitive domains, information processing speed was the most common impaired domain, with 45.9% of SCA3 patients showing decreased PASAT and/or SDMT scores. The ranked order for impairment in the other cognitive domains is as follows: 27.8% of patients had verbal memory impairment showing decreased CVLT-II TL and/or CVLT-II DR scores, 13.2% had visual memory impairment showing decreased BVMT-R TL and/or BVMT-R DR scores, 11.2% had executive function impairment showing decreased COWAT scores, and 6.4% of patients had visuospatial perception impairment showing decreased JLO scores (Fig. 1B).
In general, 30.8% (37 patients) of the 120 fully evaluated SCA3 patients were defined as CI; the other 69.2% SCA3 patients were defined as CP. Compared to the CP subgroup, the CI subgroup was older and had later onset, lower education level, and longer disease duration; the CI subgroup also had higher SARA and ICARS scores and had a higher proportion of motor symptom involvement (all P < 0.05). Notably, only one of the CI subgroup patients was defined as pre-symptomatic; the other 36 patients were defined as symptomatic. No differences were found in sex or the number of CAG repeats between the CI and CP subgroups (Table 2).
VBM-and ROI-based brain MRI comparisons between the CI and CP subgroups
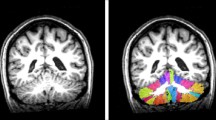
The VBM analysis indicated that the CI subgroup had lower GM volume in brain regions including the cerebellum, left caudate nucleus, and right middle frontal gyrus (peak t value = 4.39, P < 0.05, FDR-corrected at cluster level). The cerebellar lobules with decreased volume in the CI subgroup included bilateral lobule VI, right lobule Crus I, right lobule IV_V, and right lobule III (Fig. 2A and Fig. S1).
Cerebellar volume(s) loss in SCA3 patients with cognitively impaired (CI subgroup) and correlation with impairments of cognitive function/emotional performance/motor function. A Voxel-based morphometry analysis of gray matter volume among the segmented cerebellar lobules of CI subgroup compared with CP subgroup. Results were displayed on a standard cerebellar template; P < 0.05, the false discovery rate corrected at the cluster level. Color bar represents t values. B Pearson’s correlation analysis between cerebellar lobule volumes and test scores of cognitive functions (based on the modified MACFIMS tests), emotional performance (based on the HDRS and HARS), and motor function (based on ICARS), Results were presented with the adjusted r value. Correlations with statistical significance were emphasized with black frames (P < 0.05) and green frames (P < 0.01). CERE = cerebellum; CI = cognitively impaired; COWAT = controlled oral word association test; CP = cognitively preserved; CVLT-II DR = California verbal learning test-second edition, delay recall; CVLT-II TL = California verbal learning test-second edition, total learning; HARS = Hamilton anxiety rating scale; HDRS = Hamilton depression rating scale; ICARS = international cooperative ataxia rating scale; JLO = judgment line orientation; L = left; MACFIMS = minimal assessment of cognitive function in multiple sclerosis; PASAT 2 s = paced auditory serial addition task-2 seconds; PASAT 3 s = paced auditory serial addition task-3 seconds; R = right; SCA3 = spinocerebellar ataxia type 3; SDMT = symbol digit modalities test
Volume quantification bases on ROI measurement indicated that the CI subgroup had lower volumes in the left caudate nucleus (P = 0.010), in the whole cerebellum (P = 0.004), and in several segmented cerebellar lobules, including bilateral lobule VI (P = 0.004 for left and P = 0.005 for right), right lobule crus I (P = 0.040), right lobule III (P = 0.009), and right lobule IV (P = 0.007). No other differences were detected in the ROI-based analysis (Table S5 and Table S6).
Associations between cerebellar volume(s) and cognitive function, emotional performance and motor function
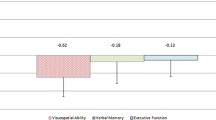
We further examined potential associations between cerebellar volume(s) loss of CI subgroup and cognitive function, emotional performance, and motor function using multivariable-adjusted models with age, sex, years of education, and TIV as covariates. For SCA3 patients, a lower left lobule VI volume was associated with lower test scores including CVLT-II TL (r = 0.52, P < 0.01), JLO (r = 0.44, P < 0.05), COWAT (r = 0.44, P < 0.05), and PASAT-2 s (r = 0.38, P < 0.05). A lower right lobule VI volume was associated with lower test scores including CVLT-II TL (r = 0.44, P < 0.05) and COWAT (r = 0.39, P < 0.05). A lower right lobule Crus I volume was associated with lower test scores including COWAT (r = 0.44, P < 0.05) and PASAT-2 seconds (r = 0.45, P < 0.05). A lower right lobule IV volume was associated with a lower JLO score (r = 0.42, P < 0.05). No associations were detected between cerebellar volumes and emotional performance. Regarding motor function, the SCA3 patient right lobule IV volume was negatively correlated with two subscales of the ICARS test: postural and gait disturbances (r = − 0.21, P < 0.05) and limb ataxia (r = − 0.21, P < 0.05) (Fig. 2B).
Associations between cerebral volume(s) and cognitive function
Multivariable-adjusted models were then performed to examine the associations between cerebral volume(s) and cognitive functions. For SCA3 patients, lower cerebral left caudate nuclear volumes were associated with lower scores including COWAT (r = 0.22, P < 0.05) and SDMT (r = 0.30, P < 0.01), while lower cerebral left parietal and right frontal lobe volumes were associated with lower SDMT scores (r = 0.24, P < 0.05; and r = 0.20, P < 0.05) (Fig. S2).
Discussion
The most frequently impaired cognitive domain detected for the SCA3 patients of our cohort was information processing speed, followed by verbal memory, executive function, and visuospatial perception. SCA3 patients defined as cognitively impaired (CI) had longer disease duration, a lower level of education, and more severe motor function impairment compared to those defined as cognitively preserved (CP). Our MRI data enabled VBM and ROI analyses, which revealed that volume loss in segmented cerebellar lobules (bilateral lobule VI, right lobule Crus I, and right lobule IV, and especially left lobule VI) contributed to cognitive impairments in SCA3.
Previous studies have examined executive functions, verbal fluency, visuospatial abilities, and verbal memory impairments in the SCA3 patients. One study of 38 SCA3 patients reported impairments in visuospatial abilities, attention, and processing speed, as well as executive dysfunction [41]; a study examining 32 SCA3 patients reported impairments in working memory, language, and executive function [21]; and a study examining 8 SCA3 patients reported executive function and verbal memory impairments [42]. Additionally, one study (n = 22) reported that the SCA3 patients had impairments in executive function, visuospatial perception, immediate/delayed recall verbal memory and attention [38]. We here evaluated five cognitive domains (including verbal memory, visual memory, visuospatial perception, executive function, and information processing speed) in our SCA3 patients by the modified MACFIMS, detecting impairments in information processing speed, verbal memory, executive function, and visuospatial perception. While differences exist in the neuropsychological assessment methodologies employed in previous and this studies, all of them indicate that SCA3 patients generally have impaired information processing speed, executive function, verbal memory, and visuospatial perception. Recently, the use of the CCAS scale has been a growing emphasis in cerebellar patients, which potentially provides a more refined and targeted assessment [30, 43]. While the effectiveness of the CCAS scale in its present form remains to be investigated in cerebro-cerebellar diseases, as well as a broader set of neurology and psychiatry disorders, the emergence of CCAS scale poses valuable avenues for future researches.
Previous meta-analysis of neuroimaging studies reported that lesions to different cerebellar structures/regions affect distinct neurological functions: the anterior lobe (lobules I–IV) and the lobule VIII are engaged during overt movements, whereas posterior and lateral cerebellar regions (lobules VI, VII, Crus I, and Crus II) are active during cognitive tasks [44, 45]. Few studies have employed volumetric neuroimaging to assess cerebellum–motor function associations or cerebellum–cognition associations in SCA3 patients [21, 46]. One study (n = 32) reported that the right cerebellar tonsil was associated with abstract reasoning ability (with decreased scores in Raven’s progressive matrices) [21]; and a study including 5 SCA3 patients reported impaired adaptive motor learning was associated with lower volumes in the right cerebellar lobule VI and left cerebellar Crus I [46]. We used VBM- and ROI-based approaches to evaluate cerebellar lobule volume loss in the CI subgroup, and found that lower volumes in the bilateral lobules VI, right lobule Crus I, and right lobule IV were associated with cognitive impairments.
Beyond SCA3, the associations observed between cerebellum and cognition are common in other cerebellar diseases. Previous studies examining SCA2 patients reported that cerebellar volume loss in lobules VI and Crus I correlated with visuospatial, verbal memory and executive function [47, 48]. In patients with Friedreich’s ataxia (n = 22), structural and functional MRI data showed that the cerebellum lobules VI and Crus I were associated with verbal fluency (executive functioning) [49]. Additionally, studies of other neurodegenerative diseases have demonstrated cerebellar atrophy as a risk factor for disease progression and implicated cerebellar atrophy in cognitive dysfunction [11, 12, 50]. A study examining 53 patients with Alzheimer’s disease reported that atrophy degree in cerebellar lobules VI and Crus I was positively correlated with praxis ability (with decreased scores for copying drawings) [51]. Similarly, studies of multiple sclerosis patients reported that atrophy degree in cerebellar lobules VI, left Crus I, and right VII was associated with information processing speed impairment [52, 53]. In Parkinson’s disease, atrophy degree in cerebellar lobules of bilateral lobules Crus I and left VI was associated with executive function impairment [54]. In familial frontotemporal dementia, cerebellar atrophy was observed in lobules VI and Crus I bilaterally, which were associated with impaired cognitive domains including attention, language, and executive function [55]. Notably, these studies and ours all implicate cerebellar lobules VI and Crus I in cognitive dysfunction in neurodegenerative diseases. Recent intervention studies have utilized both repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) to address neurodegenerative diseases, reporting improvements in cognitive function [6, 56,57,58]. Of particular interest is the observation that modeling studies investigating cerebellar tDCS indicate the highest electric field strengths are centered in Crus I/II, a region associated with cognitive function [59, 60]. These intriguing findings suggest that using rTMS and tDCS to target cerebellar lobules VI and Crus I could confer benefit for SCA3 patients, and should potentially be explored in clinical trials.
Still, it is worth noting that cerebellar atrophy leading to cognitive impairment is not applicable to all cerebellar disorders. For instance, patients with SCA6, where volume loss in lobule VI was identified [61], are often absent of cognitive decline in attention, and executive function [62]. A study utilizing the CCAS scale to assess 30 SCA3 and 14 SCA6 patients reported significant abnormalities in SCA3 but not in SCA6 [31] given that the brain is highly interconnected networks and remarkable capacity for neuroplasticity [63]. For SCA3 and other hereditary ataxias, factors such as the specific genetic mutation, and interactions with brain regions play a complex role in determining the cognitive function.
While cerebellar lobule IV’s primary connections were with motor regions reported in previous studies [3], we found the association between volume loss in lobule IV and visuospatial perception. A recent study (n = 15) reported that lobule IV volume was associated with social cognitive ability in children with autism spectrum disorder [64, 65]. Another study examining 80 probable multiple system atrophy patients reported that the amplitude of low-frequency fluctuation in cerebellar lobule IV was associated with cognitive impairment [66]. These findings indicated that even though lobule IV is primarily connected to motor regions, volume loss in this region could have effects on cognitive functions.
SCA3 is considered as a complex cerebro-cerebellar disease, and pathological ataxin-3 aggregates involved not only the cerebellum but also extracerebellar brain regions, such as the basal ganglia and cerebral cortex [14]. In our study, we used VBM- and ROI-based approaches to assess volume loss in extracerebellar brain regions, and found that lower volumes in left caudate nuclear, left parietal, and right frontal lobe were associated with cognitive impairments among SCA3 patients. Previous studies indicate that executive dysfunction can be attributed to the disruption of neural pathways connecting the cerebellum and the striatum to the frontal lobes, especially in Parkinson’s disease and essential tremor [2, 65]. Moreover, growing evidence has suggested that striato-cortical connections contribute to cognition [67, 68]. These collective findings indicate that cognitive function involves complex interactions across different brain networks in the neurodegenerative diseases, including SCA3.
Several limitations bear consideration. Our findings were based on cross-sectional data, which do not allow an accurate estimation of the time-dependent association and evolution between cognitive function and cerebellar volume. Additionally, although we found that the modified MACFIMS had good reliability and validity for our SCA3 cohort, more studies will likely be needed to assess its applicability for monitoring cognitive domains in SCA3 patients. Finally, as our study was based on neuroimaging of structural MRI, it would be ideal if future investigations can seek confirmation based on postmortem histopathology.
In conclusion, to the best of our knowledge, this is the first cohort study to investigate associations between cognitive impairments and volume loss of responsible cerebellar lobules in SCA3 patients. Our results reveal the features of cognitive domains impairments in SCA3 patients. Together with the findings of previous studies, we suggest that the volumes of cerebellar lobules, especially lobules VI, could serve as a noninvasive, potentially prognostic marker to reflect cognitive function.
Data Availability
The data sets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sereno MI, Diedrichsen J, Tachrount M, Testa-Silva G, d’Arceuil H, De Zeeuw C (2020) The human cerebellum has almost 80% of the surface area of the neocortex. Proc Natl Acad Sci U S A 117:19538–19543
Buckner RL (2013) The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron 80:807–815
Schmahmann JD, Guell X, Stoodley CJ, Halko MA (2019) The theory and neuroscience of cerebellar cognition. Annu Rev Neurosci 42:337–364
Stoodley CJ (2012) The cerebellum and cognition: evidence from functional imaging studies. Cerebellum 11:352–365
Olivito G, Serra L, Marra C, Di Domenico C, Caltagirone C, Toniolo S, Cercignani M, Leggio M, Bozzali M (2020) Cerebellar dentate nucleus functional connectivity with cerebral cortex in Alzheimer’s disease and memory: a seed-based approach. Neurobiol Aging 89:32–40
Yao Q, Tang F, Wang Y, Yan Y, Dong L, Wang T, Zhu D, Tian M, Lin X, Shi J (2022) Effect of cerebellum stimulation on cognitive recovery in patients with Alzheimer disease: a randomized clinical trial. Brain Stimul 15:910–920
Kawabata K, Watanabe H, Bagarinao E, Ohdake R, Hara K, Ogura A, Masuda M, Kato T, Tsuboi T, Maesawa S, Katsuno M, Sobue G (2020) Cerebello-basal ganglia connectivity fingerprints related to motor/cognitive performance in Parkinson’s disease. Parkinsonism Relat Disord 80:21–27
Pasqua G, Tommasin S, Bharti K, Ruggieri S, Petsas N, Piervincenzi C, Pozzilli C, Pantano P (2021) Resting-state functional connectivity of anterior and posterior cerebellar lobes is altered in multiple sclerosis. Mult Scler 27:539–548
Jacobs HIL, Hopkins DA, Mayrhofer HC, Bruner E, van Leeuwen FW, Raaijmakers W, Schmahmann JD (2018) The cerebellum in Alzheimer’s disease: evaluating its role in cognitive decline. Brain 141:37–47
Schmahmann JD, Caplan D (2006) Cognition, emotion and the cerebellum. Brain: J Neurol 129:290–292
Ruet A, Hamel D, Deloire MS, Charre-Morin J, Saubusse A, Brochet B (2014) Information processing speed impairment and cerebellar dysfunction in relapsing-remitting multiple sclerosis. J Neurol Sci 347:246–250
Tabatabaei-Jafari H, Walsh E, Shaw ME, Cherbuin N, Alzheimer’s Disease Neuroimaging I (2017) The cerebellum shrinks faster than normal ageing in Alzheimer’s disease but not in mild cognitive impairment. Hum Brain Mapp 38:3141–3150
Klockgether T, Mariotti C, Paulson HL (2019) Spinocerebellar ataxia. Nat Rev Dis Primers 5:24
Dürr A, Stevanin G, Cancel G, Duyckaerts C, Abbas N, Didierjean O, Chneiweiss H, Benomar A, Lyon-Caen O, Julien J, Serdaru M, Penet C, Agid Y, Brice A (1996) Spinocerebellar ataxia 3 and Machado-Joseph disease: clinical, molecular, and neuropathological features. Ann Neurol 39:490–499
Guimaraes RP, D’Abreu A, Yasuda CL, Franca MC Jr, Silva BH, Cappabianco FA, Bergo FP, Lopes-Cendes IT, Cendes F (2013) A multimodal evaluation of microstructural white matter damage in spinocerebellar ataxia type 3. Mov Disord 28:1125–1132
de Rezende TJ, D’Abreu A, Guimaraes RP, Lopes TM, Lopes-Cendes I, Cendes F, Castellano G, Franca MC Jr (2015) Cerebral cortex involvement in Machado-Joseph disease. Eur J Neurol 22(277–283):e223-274
Rezende TJR, de Paiva JLR, Martinez ARM, Lopes-Cendes I, Pedroso JL, Barsottini OGP, Cendes F, Franca MC Jr (2018) Structural signature of SCA3: from presymptomatic to late disease stages. Ann Neurol 84:401–408
Hengel H, Martus P, Faber J, Giunit P, Garcia-Moreno H, Solanky N, Klockgether T, Reetz K, van de Warrenburg BP, Santana MM, Silva P, Cunha I, de Almeida LP, Timmann D, Infante J, de Vries J, Lima M, Pires P, Bushara K, Jacobi H, Onyike C, Schmahmann JD, Hübener-Schmid J, Synofzik M, Schöls L (2023) The frequency of non-motor symptoms in SCA3 and their association with disease severity and lifestyle factors. J Neurol 270:944–952
Yap KH, Kessels RPC, Azmin S, van de Warrenburg B, Mohamed Ibrahim N (2022) Neurocognitive changes in spinocerebellar Ataxia type 3: a systematic review with a Narrative design. Cerebellum 21:314–327
Ackermann H (2008) Cerebellar contributions to speech production and speech perception: psycholinguistic and neurobiological perspectives. Trends Neurosci 31:265–272
Lopes TM, D’Abreu A, França MC Jr, Yasuda CL, Betting LE, Samara AB, Castellano G, Somazz JC, Balthazar ML, Lopes-Cendes I, Cendes F (2013) Widespread neuronal damage and cognitive dysfunction in spinocerebellar ataxia type 3. J Neurol 260:2370–2379
Silva UC, Marques W Jr, Lourenco CM, Hallak JE, Osorio FL (2015) Psychiatric disorders, spinocerebellar ataxia type 3 and CAG expansion. J Neurol 262:1777–1779
Stefanescu MR, Dohnalek M, Maderwald S, Thürling M, Minnerop M, Beck A, Schlamann M, Diedrichsen J, Ladd ME, Timmann D (2015) Structural and functional MRI abnormalities of cerebellar cortex and nuclei in SCA3, SCA6 and Friedreich’s ataxia. Brain: J Neurol 138:1182–1197
Romero JE, Coupe P, Giraud R, Ta VT, Fonov V, Park MTM, Chakravarty MM, Voineskos AN, Manjon JV (2017) CERES: a new cerebellum lobule segmentation method. Neuroimage 147:916–924
Zeng Y, Feng Q, Hesketh T, Christensen K, Vaupel JW (2017) Survival, disabilities in activities of daily living, and physical and cognitive functioning among the oldest-old in China: a cohort study. Lancet 389:1619–1629
Nie K, Zhang Y, Wang L, Zhao J, Huang Z, Gan R, Li S, Wang L (2012) A pilot study of psychometric properties of the Beijing version of montreal cognitive assessment in patients with idiopathic Parkinson’s disease in China. J Clin Neurosci 19:1497–1500
Zhang N, Li YJ, Fu Y, Shao JH, Luo LL, Yang L, Shi FD, Liu Y (2015) Cognitive impairment in Chinese neuromyelitis optica. Mult Scler 21:1839–1846
Dong X, Xu G, Wang J, Yin N, Meng N (2022) Clinical and MRI predictors of cognitive decline in patients with relapsing-remitting multiple sclerosis: a 2-year longitudinal study. Mult Scler Relat Disord 65:103838
Benedict RH, Zivadinov R (2007) Reliability and validity of neuropsychological screening and assessment strategies in MS. J Neurol 254(Suppl 2):II22–II25
Maas R, Killaars S, van de Warrenburg BPC, Schutter D (2021) The cerebellar cognitive affective syndrome scale reveals early neuropsychological deficits in SCA3 patients. J Neurol 268:3456–3466
Thieme A, Faber J, Sulzer P, Reetz K, Dogan I, Barkhoff M, Krahe J, Jacobi H, Aktories JE, Minnerop M, Elben S, van der Veen R, Müller J, Batsikadze G, Konczak J, Synofzik M, Roeske S, Timmann D (2022) The CCAS-scale in hereditary ataxias: helpful on the group level, particularly in SCA3, but limited in individual patients. J Neurol 269:4363–4374
Zheng YP, Zhao JP, Phillips M, Liu JB, Cai MF, Sun SQ, Huang MF (1988) Validity and reliability of the Chinese Hamilton depression rating scale. Br J Psychiatry 152:660–664
Hamilton M (1959) The assessment of anxiety states by rating. Br J Med Psychol 32:50–55
Dusankova JB, Kalincik T, Havrdova E, Benedict RH (2012) Cross cultural validation of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS) and the brief international cognitive assessment for multiple sclerosis (BICAMS). Clin Neuropsychol 26:1186–1200
Schmitz-Hubsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, Giunti P, Globas C, Infante J, Kang JS, Kremer B, Mariotti C, Melegh B, Pandolfo M, Rakowicz M, Ribai P, Rola R, Schols L, Szymanski S, van de Warrenburg BP, Durr A, Klockgether T, Fancellu R (2006) Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology 66:1717–1720
Trouillas P, Takayanagi T, Hallett M, Currier R, Subramony S, Wessel K, Bryer A, Diener H, Massaquoi S, Gomez C, Coutinho P, Ben Hamida M, Campanella G, Filla A, Schut L, Timann D, Honnorat J, Nighoghossian N, Manyam B (1997) International cooperative Ataxia rating scale for pharmacological assessment of the cerebellar syndrome. The Ataxia neuropharmacology committee of the world federation of neurology. J Neurol Sci 145:205–211
Maas RP, van Gaalen J, Klockgether T, van de Warrenburg BP (2015) The preclinical stage of spinocerebellar ataxias. Neurology 85:96–103
Feng L, Chen DB, Hou L, Huang LH, Lu SY, Liang XL, Li XH (2014) Cognitive impairment in native Chinese with spinocerebellar ataxia type 3. Eur Neurol 71:262–270
Lin Q, Liu Y, Ye Z, Hu J, Cai W, Weng Q, Chen W, Wang N, Cao D, Lin Y, Fu Y (2021) Potential markers for sample size estimations in hereditary spastic paraplegia type 5. Orphanet J Rare Dis 16:391
Li M, Chen X, Xu H, Huang Z, Chen N, Tu Y, Gan S, Hu J (2021) Brain structural abnormalities in the preclinical stage of Machado-Joseph disease/spinocerebellar ataxia type 3 (MJD/SCA3): evaluation by MRI morphometry, diffusion tensor imaging and neurite orientation dispersion and density imaging. J Neurol 269:2989–2998
Braga-Neto P, Pedroso JL, Alessi H, Dutra LA, Felicio AC, Minett T, Weisman P, Santos-Galduroz RF, Bertolucci PH, Gabbai AA, Barsottini OG (2012) Cerebellar cognitive affective syndrome in Machado Joseph disease: core clinical features. Cerebellum 11:549–556
Ma J, Wu C, Lei J, Zhang X (2014) Cognitive impairments in patients with spinocerebellar ataxia types 1, 2 and 3 are positively correlated to the clinical severity of ataxia symptoms. Int J Clin Exp Med 7:5765–5771
Hoche F, Guell X, Vangel MG, Sherman JC, Schmahmann JD (2018) The cerebellar cognitive affective/Schmahmann syndrome scale. Brain 141:248–270
Stoodley CJ, Schmahmann JD (2009) Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage 44:489–501
Bushara KO, Wheat JM, Khan A, Mock BJ, Turski PA, Sorenson J, Brooks BR (2001) Multiple tactile maps in the human cerebellum. NeuroReport 12:2483–2486
Bando K, Honda T, Ishikawa K, Takahashi Y, Mizusawa H, Hanakawa T (2019) Impaired adaptive motor learning is correlated with cerebellar hemispheric gray matter atrophy in spinocerebellar ataxia patients: a voxel-based morphometry study. Front Neurol 10:1183
Olivito G, Lupo M, Iacobacci C, Clausi S, Romano S, Masciullo M, Molinari M, Cercignani M, Bozzali M, Leggio M (2018) Structural cerebellar correlates of cognitive functions in spinocerebellar ataxia type 2. J Neurol 265:597–606
D’Agata F, Caroppo P, Boghi A, Coriasco M, Caglio M, Baudino B, Sacco K, Cauda F, Geda E, Bergui M, Geminiani G, Bradac GB, Orsi L, Mortara P (2011) Linking coordinative and executive dysfunctions to atrophy in spinocerebellar ataxia 2 patients. Brain Struct Funct 216:275–288
Dogan I, Tinnemann E, Romanzetti S, Mirzazade S, Costa AS, Werner CJ, Heim S, Fedosov K, Schulz S, Timmann D, Giordano IA, Klockgether T, Schulz JB, Reetz K (2016) Cognition in Friedreich’s ataxia: a behavioral and multimodal imaging study. Ann Clin Transl Neurol 3:572–587
Yang H, Wang N, Luo X, Lv H, Liu H, Li Y, Fan G (2019) Cerebellar atrophy and its contribution to motor and cognitive performance in multiple system atrophy. Neuroimage Clin 23:101891
Toniolo S, Serra L, Olivito G, Marra C, Bozzali M, Cercignani M (2018) Patterns of cerebellar gray matter atrophy across Alzheimer’s disease progression. Front Cell Neurosci 12:430
Moroso A, Ruet A, Lamargue-Hamel D, Munsch F, Deloire M, Coupé P, Ouallet JC, Planche V, Moscufo N, Meier DS, Tourdias T, Guttmann CR, Dousset V, Brochet B (2017) Posterior lobules of the cerebellum and information processing speed at various stages of multiple sclerosis. J Neurol Neurosurg Psychiatry 88:146–151
Fritz NE, Edwards EM, Ye C, Prince J, Yang Z, Gressett T, Keller J, Myers E, Calabresi PA, Zackowski KM (2022) Cerebellar contributions to motor and cognitive control in multiple sclerosis(✰✰✰). Arch Phys Med Rehabil 103:1592–1599
Morelli N (2022) Patients with Parkinson’s disease and a history of falls have decreased cerebellar grey matter volumes in the cognitive cerebellum. Rev Neurol (Paris) 178:924–931
Chen Y, Landin-Romero R, Kumfor F, Irish M, Dobson-Stone C, Kwok JB, Halliday GM, Hodges JR, Piguet O (2022) Cerebellar integrity and contributions to cognition in C9orf72-mediated frontotemporal dementia. Cortex 149:73–84
Benussi A, Cantoni V, Manes M, Libri I, Dell’Era V, Datta A, Thomas C, Ferrari C, Di Fonzo A, Fancellu R, Grassi M, Brusco A, Alberici A, Borroni B (2021) Motor and cognitive outcomes of cerebello-spinal stimulation in neurodegenerative ataxia. Brain 144:2310–2321
Chen XY, Lian YH, Liu XH, Sikandar A, Li MC, Xu HL, Hu JP, Chen QL, Gan SR (2022) Effects of repetitive transcranial magnetic stimulation on cerebellar metabolism in patients with spinocerebellar ataxia type 3. Front Aging Neurosci 14:827993
Maas R, Teerenstra S, Toni I, Klockgether T, Schutter D, van de Warrenburg BPC (2022) Cerebellar transcranial direct current stimulation in spinocerebellar ataxia type 3: a randomized, double-blind, sham-controlled trial. Neurotherapeutics 19:1259–1272
D’Mello AM, Turkeltaub PE, Stoodley CJ (2017) Cerebellar tDCS modulates neural circuits during semantic prediction: a combined tDCS-fMRI study. J Neurosci 37:1604–1613
Maas R, Faber J, van de Warrenburg BPC, Schutter D (2023) Interindividual differences in posterior fossa morphometry affect cerebellar tDCS-induced electric field strength. Clin Neurophysiol 153:152–165
Yacoubi B, Casamento-Moran A, Burciu RG, Subramony SH, Vaillancourt DE, Christou EA (2020) Temporal invariance in SCA6 is related to smaller cerebellar lobule VI and greater disease severity. J Neurosci 40:1722–1731
Suenaga M, Kawai Y, Watanabe H, Atsuta N, Ito M, Tanaka F, Katsuno M, Fukatsu H, Naganawa S, Sobue G (2008) Cognitive impairment in spinocerebellar ataxia type 6. J Neurol Neurosurg Psychiatry 79:496–499
Grady C (2012) The cognitive neuroscience of ageing. Nat Rev Neurosci 13:491–505
Kumar M, Hiremath C, Khokhar SK, Bansal E, Sagar KJV, Padmanabha H, Girimaji AS, Narayan S, Kishore MT, Yamini BK, JacFredo AR, Saini J, Bharath RD (2023) Altered cerebellar lobular volumes correlate with clinical deficits in siblings and children with ASD: evidence from toddlers. J Transl Med 21:246
Torres K (2023) Comparison of core and process scores on the California verbal learning test-3 for Parkinson’s disease and essential tremor patients. J Clin Exp Neuropsychol 28:1–15
Li Y, Liu H, Yu H, Yang H, Guo M, Cao C, Pang H, Liu Y, Cao K, Fan G (2023) Alterations of voxel-wise spontaneous activity and corresponding brain functional networks in multiple system atrophy patients with mild cognitive impairment. Hum Brain Mapp 44:403–417
Monchi O, Petrides M, Strafella AP, Worsley KJ, Doyon J (2006) Functional role of the basal ganglia in the planning and execution of actions. Ann Neurol 59:257–264
Anderkova L, Barton M, Rektorova I (2017) Striato-cortical connections in Parkinson’s and Alzheimer’s diseases: relation to cognition. Mov Disord 32:917–922
Acknowledgements
The authors would like to thank the kind patients and families who participated in this research.
Funding
This work has been supported by the grants 82230039 (N.W.), 81870902 (N.W.), U21A20360 (Y.F.), 81971082 (S.R.G.) from the National Natural Science Foundation of China.
Author information
Authors and Affiliations
Consortia
Contributions
YF, NW, and SRG formulated and designed the study concept. ZXY, JB, LLQ, XYC, XYC, YSQ, RYY, XTY, CYH, WL, and WJC enrolled the patients and conducted clinical assessments and neuropsychological tests. ZXY, MCL, and JPH conducted imaging data collection. XYC, XTY, BC, and WL performed laboratory determination. ZXY, JB, LLQ, NW, YF, and SRG analyzed the data, and interpreted the results. All authors were involved in drafting/revising the manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ye, ZX., Bi, J., Qiu, LL. et al. Cognitive impairment associated with cerebellar volume loss in spinocerebellar ataxia type 3. J Neurol 271, 918–928 (2024). https://doi.org/10.1007/s00415-023-12042-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-12042-0