Abstract
Pompe disease is an autosomal recessive disorder in which deficiency of the lysosomal enzyme acid alpha-glucosidase results in the accumulation of glycogen mostly in muscle tissues. Several reports suggest a higher incidence of intracranial vascular abnormalities (IVAs) in this condition, as well as brain microbleeds and cerebral vasculopathy. The aim of our study was to evaluate through neuroimaging studies the incidence of these anomalies in our cohort of late-onset Pompe disease (LOPD) patients asymptomatic for cerebrovascular disease, looking for correlations with clinical and genetic data. We studied 18 LOPD patients with brain magnetic resonance angiography (MRA), or contrast-enhanced computed tomography (CECT). Diameters of individual arteries were measured and compared with average values as proposed in the literature. We found IVAs in 13 of the 18 patients, mostly dilatative arteriopathy affecting the vertebrobasilar system. The anterior circle was involved in seven of the 18 patients. The diameter of the basilar artery at 1 cm was found to correlate both with age (spearman rho, p = 0.037) and disease duration (p = 0.004), but no other statistically significant correlation was documented. The incidence of intracranial dilatative arteriopathy in LOPD was higher than in the general population, confirming the literature data. However, we did not find intracranial aneurysms microbleeds or significant cerebrovascular disease. Abnormalities in the anterior and the posterior circle of Willis correlated with age and disease duration, but not with the severity of muscle/respiratory involvement or with genetic data. Further studies in larger cohorts of patients are needed to confirm these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acid alpha-glucosidase (GAA) deficiency is an autosomal recessive disorder in which lack of the lysosomal enzyme results in accumulation of glycogen in several tissues. Also known as Pompe disease (PD) and glycogen storage disease type II (OMIM 232300), the condition has a broad clinical spectrum in terms of age at onset and rate of disease progression [1]. Late-onset forms (LOPD) develop after the age of 1 year, and their predominant clinical feature is muscle involvement, particularly marked at lower limb level and in the scapular girdle [2].
Several studies associated this condition with vacuolar degeneration and glycogen deposits in the vessel walls of cerebral arteries and in smooth muscle cells of other blood vessels [3, 4]. These phenomena lead to arterial wall weakness resulting in vascular abnormalities, such as aneurysms or ectasia, mainly pathologically described in the brain as diffuse or vertebrobasilar dilative arteriopathy [5,6,7]. The presence of intracranial vascular abnormalities (IVAs) could be indicated by cerebrovascular or cerebral compression symptoms, but asymptomatic cases have also been reported [8, 9]. Vertebrobasilar dolichoectasia is usually found in only 0.06% of the general population [8] but its real incidence in the LOPD population remains unknown due to the lack of systematic investigations.
Neuroimaging studies reported evidence of IVAs in LOPD (see Table 1), mainly in sporadic cases [8, 10,11,12,13]. Few systematic studies have been performed in larger LOPD cohorts. Sacconi et al. [9], using magnetic resonance angiography (MRA), identified IVAs in 4 out of 6 patients, specifically basilar arteriopathy, internal carotid dilative arteriopathy, or both. Two of these patients were symptomatic, and all harboured the common mutation c.-32-13T>G [14]. The same group recently published the results of a longitudinal follow-up of 10 years in 5 of these 6 patients underlining as the MRA findings did not show significant changes between the first and last study in all the patients and none of the patient showed major vascular events (ischemic or hemorrhagic strokes and subarachnoid hemorrhages) during the follow-up [15].
Hensel et al. used MRA to investigate IVAs in ten LOPD patients, asymptomatic for cerebrovascular disease, finding a higher than general population incidence of dilative arteriopathy. Distal internal carotid artery (dICA), basilar artery (BA) and vertebral artery (VA) diameters seemed to increase with disease duration [16]. Recently, Montagnese et al. [17] led a study in a larger LOPD cohort in which computed tomography angiography documented IVAs in 13 out of 21 patients; in 62% of the affected patients, these abnormalities were associated with signs of lacunar encephalopathy and correlated with the presence of respiratory impairment. Evidence of microbleeds in brain tissue has also been reported in LOPD [12, 18, 19], but systematic research is lacking.
This study was conducted with a twofold aim: first, to evaluate the incidence of neuroimaging abnormalities in our cohort of LOPD patients, looking for brain microbleeds, signs of cerebral vasculopathy, and IVAs; second, to correlate radiological findings with genetic data to investigate whether IVAs may be related to specific genotypes.
Patients and methods
Patients
Eighteen late-onset PD patients followed at our institution (median age 52.3 ± 15.26 years, range 28–76 years) voluntarily underwent routine MRI-MRA or CECT, depending on their respiratory status. The patients were in various stages of the disease and the diagnosis of PD was based on muscle biopsy evidence of GAA deficiency and was confirmed by genetic testing (see Table 2 for clinical and genetic data). Informed consent for the use of clinical and radiological data in an anonymized form was obtained from all the patients.
Beyond disease parameters (Walton score, 6-min walking test, vital capacity), we annotated for each patient the presence of the known vascular risk factors (arterial hypertension, metabolic syndrome, hyperglycemia, hyperlipidemia, obesity) and the concomitant presence of peripheral or coronary arterial disease. 4 of 18 patients were not on ERT (two pts with asymptomatic hyperCKemia and muscle pain, but without objective signs of the disease; two patients in moderate and severe Walton stage due to unwillingness to undergo the i.v. treatment). All our patients were asymptomatic for cerebrovascular disease or neurovascular involvement.
Imaging data acquisition
Brain MRI and MRA were performed with a Philips Gyroscan 1.5 T scanner (Koninklijke, the Netherlands) using the following sequences: axial FLAIR [slice thickness (ST) 3 mm; interslice gap (IG) 0 mm; repetition time (TR), 10,000 ms; echo time (TE), 125 ms; voxel size (VS) 1.3 mm], axial T2-weighted fast field echo (FFE) [ST 5 mm; IG 1 mm; TR shortest; TE 23.0138054 ms; VS 0.9], time of flight (TOF) MRA 3D multi-chunk in-flow sequence [IG 10 mm; TR 25 ms; TE 6.9 ms; VS 0.4 mm; field of view (FOV) = 180 × 180 × 80 mm; acquisition matrix = 448 × 256], diffusion weighted imaging [ST 5 mm; IG 1 mm; TR shortest; TE shortest; VS 2 mm]. We also performed a coronal T1-SE sequence at the level of the tongue muscle [ST 5 mm; IS 10 mm; TR 500 ms; TE 10 ms; signal averages 2; VS 1.3 mm]. Total scanning time was usually less than 30 min. Patients who could not undergo MRI underwent brain CT imaging with contrast (Iomeron 400), performed using a Philips Vision MX8000.
Radiological data analysis
Two neuroradiologists (A.P., S.S.) evaluated the brain CT and MRI scans, looking for signs of cerebral vasculopathy (categorized according to the Fazekas scale for white matter lesions) [20]. The final grading was then determined by consensus between the two, and agreement was required. In evaluating the brain MRI scans, consideration was also given to the presence of susceptibility foci indicative of microbleeds on FFE sequences [21].
On raw brain MRA images, the diameters of the cerebral arteries were measured as proposed elsewhere [16], and the results obtained were compared with average control values, as reported in the literature [16] a diameter over three times the standard deviation of control values was defined as dilative arteriopathy.
On brain CECT images, BA ectasia was diagnosed if the external diameter of the artery was greater than 4.5 mm [22, 23]. Moreover, the term “dolicho” was added if, at any point of its course, the artery lay lateral to the margin of the clivus or dorsum sellae, or if it bifurcated above the plane of the suprasellar cistern [22,23,24]. Given that there exist no validated CECT diagnostic criteria for dilative arteriopathy of the carotid circle, vessel caliber was calculated at approximately the same level used for the MRA image analysis.
Genetic analysis
Blood samples for DNA analysis were taken after obtaining the participants’ informed consent, and genomic DNA was isolated using the “GENE ELUTE” kit (Sigma-Aldrich) Mutational analysis was performed as previously reported [24]. The I/D polymorphism in the ACE gene was analyzed as reported by Lindpaintner et al. [25]. The promoter region of MMP3 including the rs 3025058 polymorphism was amplified with the following primers: forward GATTACAGACATGGGTCACG; reverse GAATTCACATCACTGCCACC. The PCR product was then analyzed with Sanger sequencing. All the work was carried out in compliance with the Helsinki Declaration.
Statistical analysis
Statistical analysis was performed using IBM-SPSS Statistics for Windows, version 17. We looked for correlations between radiological data (arterial diameters, the presence of any vascular abnormalities and total vascular score) and clinical data (age, age at onset, disease duration, enzymatic activity, residual vital capacity, motor function stage, the presence of other cardiovascular risk factors, the presence of vascular encephalopathy, BMI), as well as between vascular abnormalities and genetic factors, including (a) GAA mutation, graded as less severe (LS) vs very severe (VS) according to the Pompe mutation database (http://www.pompecenter.nl); (b) genetic polymorphisms in the ACE (I/D polymorphism) and MMP3 (5A/6A polymorphism) genes. Spearman rho and Kruskal–Wallis or Chi-square tests were used for the analysis of continuous and categorical variables, respectively.
Results
Radiological data
Fourteen of the 18 patients investigated underwent brain MRA; in the other four, brain CECT was performed due to respiratory impairment. All the MR and CT images were of adequate quality and coverage to allow assessment of the brain, the arteries of the circle of Willis and the tongue muscle.
The radiological data indicated moderate vascular encephalopathy in two of the 18 patients and non-specific punctate subcortical gliosis in another four. FFE sequences showed no susceptibility foci. Vascular encephalopathy had no clinical consequences (i.e., motor or cognitive impairment). The two patients with moderate vascular encephalopathy (two men aged 58 and 76) had concomitant vascular comorbidities; All 18 patients carried the c.-32-13T>G mutation on one allele and a VS mutation on the other.
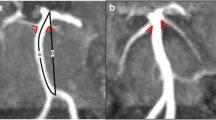
No intracranial aneurysms were found in the anterior or posterior circle. Abnormalities of the intracranial arteries were found in 13 of the 18 patients. In particular, one patient had a dolicho course of the basilar artery, without ectasia, while the remaining 12 showed an arterial diameter greater than control values (see Table 3). Dilative arteriopathy mostly affected the vertebrobasilar arteries (9/12) (Fig. 1) and dolichoectasia was clearly observed in five of these 12 patients
Involvement of the anterior circle was found in seven of the 18 patients, four of whom also showed involvement of the posterior one; the distal internal carotid artery (dICA) and the anterior carotid artery (AcA) were the vessels most frequently involved each in four patients, while only one patient had an abnormal medial carotid artery (MCA) diameter.
Since all the subjects where asymptomatic for cerebrovascular disease or neurovascular involvement no correlation could be performed between radiological findings and related clinical data.
Genetic data
All the patients carried the common mutation c.-32-13T>G on one allele, while data for the second allele allowed them to be divided into subgroups according to the genotype carried (a) c.-32-13T; c.2237G>A (n = 2); (b) c.-32-13T; c.2481+102_2646+31del (n = 2); (c) c.-32-13T; c.784G>A (n = 2) and (d) c.-32-13T>G; c.525delT (n = 5). With regard to the latter group, it is underlined that c.525delT is considered a VS mutation and is more commonly observed in the infantile form of PD than in LOPD [25]. Six of the study patients could not be classified into any subgroup as they presented different mutations from the ones listed above, and in a further patient the second mutation is still to be identified.
Correlations between radiological and clinical-genetic data
The diameter of the BA at 1 cm was found to correlate both with age (spearman rho, p = 0.037) and disease duration (p = 0.004), but no other statistically significant correlations were documented.
It was not possible to look for relationships within the genetic subgroups a, b and c, as they did not show homogeneous radiological data. Within group d, no clear genotype/phenotype/radiological correlations could be detected due to the variable degree of vascular involvement (i.e., ranging from very mild in P12 to more severe in P9 and P10); furthermore, P9 and P10, who belong to the same family, showed similar abnormalities in the vascular system, but very different clinical presentations (muscle strength and respiratory function).
Similarly, analysis of potentially relevant genetic polymorphisms (ACE and MMP3) failed to uncover any correlation with radiological data.
Discussion
We investigated a large cohort of LOPD patients without cerebrovascular symptoms, looking for cerebral vessel abnormalities (in the anterior and posterior circle of Willis) and brain parenchymal abnormalities. We also looked for correlations between radiological parameters and clinical/genetic data. Our patients showed a higher incidence of vertebrobasilar dolichoectasia (VBD) (38%) than is found in the general population, in which the incidence ranges from 0.3 to 4.4% [16, 26, 27]. This result is fairly in line with the values reported in previous LOPD cohorts [16, 17], in which the VBD incidence was 20 and 43%, respectively. Our cohort had larger vessel diameters in both the anterior circle (distal ICA, ACA and, less frequently, MCA) and the posterior circle [BA, intracranial segment of the vertebral artery (V4)] compared with values from healthy controls, and a higher rate of dilative arteriopathy compared with another sample of LOPD patients (66 vs 30%) [16]. However, no intracranial aneurysm was found and the minor vascular abnormalities detected were clinically asymptomatic and not unequivocally associated with parenchymal vascular lesions (the only two pts with mild cerebral vasculopathy had concomitant vascular risk factors, and no pts had brain microbleeds).
The pathological basis of dilative arteriopathy and dolichoectasia in the general population is unknown; several studies have shown that the conditions tend to be associated with diffuse atherosclerosis, chronic kidney dysfunction, old age, male sex and cardiovascular risk factors, such as hypertension, smoking and coronary heart disease [26, 29]. Dolichoectasia might be associated with different types of cerebral small-vessel disease, such as lacunar infarcts and white matter lesions [24, 28]; it has been reported that even only a BA diameter increased within the normal range could be a predictor of cardiovascular events [30]. The correlation between LOPD and VBD or other forms of dilative arteriopathy remains to be clarified. It is well known that the arteriopathy in LOPD is histopathologically associated with glycogen accumulation, extensive vacuolar degeneration and necrosis in the vessel wall [5,6,7]. It has recently been suggested that disease duration may, by itself, play a role in the development of cerebral arteriopathy in LOPD and our study seems to corroborate this hypothesis, since we found that BA diameter at 1 cm correlated both with age and disease duration. [16]. Moreover, there could be two additional factors leading to vessel dilation in LOPD: the occurrence of respiratory failure, which could cause more elevated partial pressure of carbon dioxide and consequent vasodilation; the presence of glycogen-filled vacuoles in the vessel walls [7], which could interfere with the production of certain extracellular matrix proteins, such as collagen and elastin [31], or with the production of matrix metalloproteinases and other vasoactive substances such as nitric oxide [32]. In our LOPD cohort, no correlation was found between the incidence of vascular abnormalities and clinical data, including respiratory function and muscle involvement. These results corroborate the hypothesis that changes in cerebral vessels seem to proceed separately and independently of the degree of involvement of striated muscle tissue.
The brain tissue involvement, in the form of cerebral vasculopathy and microbleeds, is reported in the literature on LOPD, mainly in case reports. In a larger sample, Montagnese et al. found signs of lacunar encephalopathy in 13/21 patients (62%), related to respiratory impairment, and speculated that these signs may be due to inadequate cerebral oxygenation resulting from nocturnal hypercapnia and hypoventilation or chronic respiratory failure [17]. In our study, no brain microbleeds were detected and only two of our 18 patients had radiological signs of mild vascular encephalopathy, not related to respiratory impairment, without clinical correlation, and however, possibly related to other vascular risk factor.
Although we did not directly perform serial follow-up imaging, lack of correlation with the severity of motor and respiratory involvement, [8] may be and indirect clue that vascular complications are not a typical marker of disease progression and have distinct risk factors, independent of disease severity. These factors are still unknown although some individual patients with very severe mutations (525delT) seemed to have a higher risk: this may underline the importance of screening for aneurisms, especially among juvenile cases. Up to now the only longitudinal angio-MR follow-up in LOPD patients is the one recently published by Garibaldi et al. [15], on the same cohort of Sacconi et al. [9]. The five patients studied between 2006 and 2016 did not show significant changes between the first and last MRA study, and the authors recommend a neuroradiological follow-up every 2–5 years in the absence of aneurysms.
We suggest to perform at least once in a life time MRA to detect vascular anomalies, even in juvenile/infantile patients, considering the histopathological evidence of glycogen accumulation on cerebral vessels; timing should be decided on individual basis and clinical data. Follow-up of large cohorts of LOPD patients are needed to confirm the suggestion of MRA follow-up every 2–5 years. On the other hand, serial imaging of mild vascular abnormalities does not seem mandatory and is not likely to change disease management: indeed, ERT cannot prevent or influence cerebrovascular pathology due to its inability to cross brain barrier; moreover, cerebrovascular abnormalities do not seem to be substantially influenced by disease severity (Tables 2 and 3).
It is worth underlining that since mutation c.-32-13T>G is very common in LOPD [14], the second allele, whose mutation is different in different patients, may reasonably be assumed to have a greater impact on the clinical presentation. In our cohort of patients, we identified four specific genotypes, but no clear genotype/phenotype/radiological correlation was detectable. A larger sample is needed to identify possible significant effects of mutation type or genetic polymorphisms on cerebral vessel abnormalities in PD.
One limitation of our study was that we used both MRA and CECT, the latter being needed in patients with respiratory impairment. MRA has the advantage of not using ionizing radiation or contrast media, and it is comparable to CECT for the diagnosis of VBD [33]. The use of a comparable analysis method allowed us to overcome the above limitation.
In conclusion, our study confirmed a higher incidence of radiological cerebrovascular abnormalities in asymptomatic for cerebrovascular disease LOPD patients compared with the general population, both in the anterior and the posterior circle of Willis, whose pathogenesis is still unknown and which correlated with age and disease duration, but not with genetic data or severity of motor and respiratory involvement. We did not detect significant brain parenchymal vascular abnormalities, which were possibly related to other systemic risk factors. Further studies, also longitudinal, in larger cohorts of patients are needed to confirm these findings and to suggest the appropriate follow-up/management of LOPD patients.
References
Case LE, Beckemeyer AA, Kishnani PS (2012) Infantile Pompe disease on ERT: update on clinical presentation, musculoskeletal management, and exercise considerations. Am J Med Genet C Semin Med Genet 160C:69–79
Carlier RY, Laforet P, Wary C et al (2011) Whole-body muscle MRI in 20 patients suffering from late onset Pompe disease: involvement patterns. Neuromuscul Disord 21(11):791–799
Smith J, Zellweger H, Afifi AK (1967) Muscular form of glycogenosis, type II (Pompe). Neurology 17(6):537–549
Van der Walt JD, Swash M, Leake J, Cox EL (1987) The pattern of involvement of adult-onset acid maltase deficiency at autopsy. Muscle Nerve 10(3):272–281
Makos MM, McComb RD, Hart MN, Bennett DR (1987) Alpha-glucosidase deficiency and basilar artery aneurysm: report of a sibship. Ann Neurol 22(5):629–633
Matsuoka Y, Senda Y, Hirayama M, Matsui T, Takahashi A (1988) Late-onset acid maltase deficiency associated with intracranial aneurysm. J Neurol 235(6):371–373
Kretzschmar HA, Wagner H, Hübner G, Danek A, Witt TN, Mehraein P (1990) Aneurysms and vacuolar degeneration of cerebral arteries in late-onset acid maltase deficiency. J Neurol Sci 98(2–3):169–183
Laforêt P, Petiot P, Nicolino M et al (2008) Dilative arteriopathy and basilar artery dolichoectasia complicating late-onset Pompe disease. Neurology 70:2063–2066
Sacconi S, Bocquet JD, Chanalet S, Tanant V, Salviati L, Desnuelle C (2010) Abnormalities of cerebral arteries are frequent in patients with late-onset Pompe disease. J Neurol 257:1730–1733
Anneser JM, Pongratz DE, Podskarbi T, Shin YS, Schoser BG (2005) Mutations in the acid alpha-glucosidase gene (M. Pompe) in a patient with an unusual phenotype. Neurology 64:368–370
Refai D, Lev R, Cross DT, Shimony JS, Leonard JR (2008) Thrombotic complications of a basilar artery aneurysm in a young adult with Pompe disease. Surg Neurol 70(5):518–520
Renard D, Labauge P (2010) Neurological picture: cerebral microbleeds in Pompe disease. J Neurol Neurosurg Psychiatry 81(11):1217
Quenardelle V, Bataillard M, Bazin D, Lannes B, Wolff V, Echaniz-Laguna A (2015) Pompe disease presenting as an isolated generalized dilative arteriopathy with repeated brain and kidney infarcts. J Neurol 262(2):473–475
Montalvo AL, Bembi B, Donnarumma M et al (2006) Mutation profile of the GAA gene in 40 Italian patients with late onset glycogen storage disease type II. Hum Mutat 27:999–1006
Garibaldi M, Sacconi S, Antonini G, Desnuelle C (2017) Long term follow-up of cerebrovascular abnormalities in late onset Pompe disease (LOPD). J Neurol 264(3):589–590
Hensel O, Hanisch F, Stock K et al (2015) Morphology and function of cerebral arteries in adults with Pompe disease. JIMD Rep 20:27–33
Montagnese F, Granata F, Musumeci O et al (2016) Intracranial arterial abnormalities in patients with late onset Pompe disease (LOPD). J Inherit Metab Dis 39(3):391–398
Brettschneider J, Sperfeld AD, Ludolph AC, Kassubek J (2008) Intracerebral hemorrhage in a patient with glycogenosis type II (Pompe disease): is there a pathophysiological relationship? Muscle Nerve 38(3):1211–1212
Sandhu D, Rizvi A, Kim J, Reshi R (2014) Diffuse cerebral microhemorrhages in a patient with adult-onset Pompe’s disease: a case report. J Vasc Interv Neurol 7(5):82–85
Fazekas F, Chawluk J, Alavi A, Hurtig H, Zimmerman R (1987) MRI signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJNR Am J Neuroradiol 8:421–426
Mercuri E, Pichiecchio A, Counsell S, Allsop J, Cini C, Jungbluth H et al (2002) A short protocol for muscle MRI in children with muscular dystrophies. Eur J Paediatr Neurol. 6:305–307
Smoker WR, Corbett JJ, Gentry LR, Keyes WD, Price MJ, McKusker S (1986) High-resolution computed tomography of the basilar artery: 2. Vertebrobasilar dolichoectasia: clinical-pathologic correlation and review. AJNR Am J Neuroradiol 7:61–72
Smoker WR, Price MJ, Keyes WD, Corbett JJ, Gentry LR (1986) High-resolution computed tomography of the basilar artery: 1. Normal size and position. AJNR Am J Neuroradiol 7(1):55–60
Pico F, Labreuche J, Touboul PJ, Amarenco P, GENIC Investigators (2003) Intracranial arterial dolichoectasia and its relation with atherosclerosis and stroke subtype. Neurology 61(12):1736–1742
Lindpaintner K, Pfeffer MA, Kreutz R et al (1995) A prospective evaluation of an angiotensin-converting-enzyme gene polymorphism and the risk of ischemic heart disease. N Engl J Med 332(11):706–711
Yu YL, Moseley IF, Pullicino P, McDonald WI (1982) The clinical picture of ectasia of the intracerebral arteries. J Neurol Neurosurg Psychiatry 45:29–36
Ubogu EE, Zaidat OO (2004) Vertebrobasilar dolichoectasia diagnosed by magnetic resonance angiography and risk of stroke and death: a cohort study. J Neurol Neurosurg Psychiatry 75:22–26
Pico F, Labreuche J, Gourfinkel-An I, Amarenco P, GENIC Investigators (2006) Basilar artery diameter and 5-year mortality in patients with stroke. Stroke 37:2342–2347
Ichikawa H, Takahashi N, Mukai M, Katoh H, Akizawa T, Kawamura M (2009) Intracranial dilative arteriopathy is associated with chronic kidney disease and small vessel diseases in the elderly. J Stroke Cerebrovasc Dis 18(6):435–442
Tanaka M, Sakaguchi M, Miwa K et al (2013) Basilar artery diameter is an independent predictor of incident cardiovascular events. Arterioscler Thromb Vasc Biol 33(9):2240–2244
Dobrin PB (1978) Mechanical properties of arteries. Physiol Rev 58:397–460
Loftus IM, Thompson MM (2002) The role of matrix metalloproteinases in vascular disease. Vasc Med 7:117–133
Förster A, Ssozi J, Al-Zghloul M, Brockmann MA, Kerl HU, Groden C (2014) A comparison of CT/CT angiography and MRI/MR angiography for imaging of vertebrobasilar dolichoectasia. Clin Neuroradiol 24(4):347–353
Author information
Authors and Affiliations
Contributions
Anna Pichiecchio, study concept and design, analysis and interpretation of data, study supervision, draft of the manuscript; Simone Sacco, analysis and interpretation of data, draft of the manuscript; Paola De Filippi e Eduardo Caverzasi, acquisition of data, draft of a portion of the manuscript; Sabrina Ravaglia, acquisition of data, draft of a portion of the manuscript, statistical analysis; Stefano Bastianello study concept and design, critical revision of the manuscript; Cesare Danesino study supervision, critical revision of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Pichiecchio received reimbursement for participation in invited lecture by G-enzyme (2015, 2016). Dr. Ravaglia received personal compensation from G-enzyme (2007, 2008).
Funding
There are no funders to report for this submission.
Research involving human participants and/or animals
All human and animal studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 declaration of Helsinki and its later amendments.
Informed consent
Each patient signed an informed consent according to the rules of the hospital caring for him.
Rights and permissions
About this article
Cite this article
Pichiecchio, A., Sacco, S., De Filippi, P. et al. Late-onset Pompe disease: a genetic-radiological correlation on cerebral vascular anomalies. J Neurol 264, 2110–2118 (2017). https://doi.org/10.1007/s00415-017-8601-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-017-8601-1